Abstract
Chitosan as a natural polysaccharide derived from chitin of arthropods like shrimp and crab, attracts much interest due to its inherent properties, especially for application in biomedical materials. Presently, biodegradable and biocompatible chitosan nanoparticles are attractive for drug delivery. However, some physicochemical characteristics of chitosan nanoparticles still need to be further improved in practice. In this work, chitosan nanoparticles were produced by crosslinking chitosan with 3-methoxy-4-hydroxybenzaldehyde (vanillin) through a Schiff reaction. Chitosan nanoparticles were 200–250 nm in diameter with smooth surface and were negatively charged with a zeta potential of − 17.4 mV in neutral solution. Efficient drug loading and drug encapsulation were achieved using 5-fluorouracil as a model of hydrophilic drug. Drug release from the nanoparticles was constant and controllable. The in vitro cytotoxicity against HT-29 cells and cellular uptake of the chitosan nanoparticles were evaluated by methyl thiazolyl tetrazolium method, confocal laser scanning microscope and flow cytometer, respectively. The results indicate that the chitosan nanoparticles crosslinked with vanillin are a promising vehicle for the delivery of anticancer drugs.
Introduction
Nanoparticles made from natural biopolymers have been widely used as drug carriers due to their significant advantages in effective delivery (Gupta & Jabrail, Citation2006; Gu et al., Citation2007). Among these, chitosan is one of the biopolymers which can form nanoparticles with unique properties (Prabaharan et al., Citation2006; Li et al., Citation2011a,b; Bharmoria et al., Citation2013; Rubert et al., Citation2013).
The presence of many functional groups such as amino groups and hydroxyl groups makes chitosan amenable to form nanoparticles by crosslinking with polyanionic polymers. Chitosan nanoparticles have been extensively studied as drug delivery system in recent years (Hua et al., Citation2011; Jana et al., Citation2013; Ali et al., Citation2014). At present, the most common methods for the preparation of chitosan particles were covalent crosslinking (Zheng et al., Citation2011; Almalik et al., Citation2013), ion crosslinking (Pan et al., Citation2002; Sang et al., Citation2013), sedimentation (Mao et al., Citation2001) and self-assembly (Baek et al., Citation2008). However, some concerns have been raised regarding to the safety of chitosan nanoparticles due to the use of toxic crosslinking reagent such as glutaraldehyde, aldehydes and glyoxal, which can inactivate macromolecule drugs (Fürst and Banerjee, Citation2005; Gupta & Jabrail, Citation2006; Ajit et al., Citation2007) and restrict their wide application.
To avoid these potential side effects, some physical crosslinking reagent like sodium tripolyphosphate or sodium sulfate have been used to form nanoparticles with chitosan; however, the mechanical strength of these nanoparticles was not high enough and “burst release” was often accompanied these nanoparticles (Gupta & Jabrail, Citation2006; Rathna, Citation2008; Pramila & Brahmeshwar, Citation2014). Therefore, the selection of appropriate crosslinking reagent is crucial for the preparation of chitosan particles. 3-Methoxy-4-hydroxybenzaldehyde (vanillin) is a flavouring agent which has been widely used in cosmetics, drink and food (Ho et al., Citation2011). In addition, it has been reported that vanillin exhibits many bioactive properties (Zhang, Citation2004; Makni et al., Citation2011; Tai et al., Citation2011). There were some reports on the preparation of chitosan microspheres using vanillin as crosslinking reagent in references (Li et al., Citation2006), but few studies on the preparation of chitosan nanoparticles by crosslinking with vanillin.
Therefore, the objective of this work is to develop novel drug-loaded chitosan–vanillin nanoparticles and investigate its physicochemical properties. In addition, in vitro drug release profile was studied in simulated digestive fluid and cellular uptake and cytotoxicity against HT-29 cells were also evaluated.
Materials and methods
Materials
Chitosan was purchased from Guoyao Group (Shanghai, China), the deacetylation degree was ≥90%. Vanillin, 5-fluorouracil (5-FU), methyl thiazolyl tetrazolium (MTT) and sorbitan sesquioleate were purchased from Sigma Aldrich (Shanghai, China). Fluorescein was obtained from Yuanye Biotech. Co. Ltd (Shanghai, China). RPMI1640 medium, calf serum, penicillin, streptomycin, trypsin and ethylenediaminetetra acetic were purchased from Invitrogen (Shanghai, China). Liquid paraffin and magnesium stearate were provided by Zhanjiang Xingmao Chemical-Glass Co. Ltd (Zhanjiang, China). All other chemicals were of analytical grade.
Preparation of chitosan nanoparticles
Chitosan nanoparticles were prepared by an emulsion-solvent evaporation method with minor modification (Peng et al., Citation2010; Hongyan et al., Citation2011). Chitosan was dissolved in 10 mL aqueous acetic acid solution (1%) with a concentration of 2% (w/v) to form a water phase. 5-FU (50 mg) was dissolved in chitosan solution. Liquid paraffin (50 mL) containing 2% sorbitan sesquioleate and 1% magnesium stearate was used as the oil phase. Then, the water phase solution with or without drugs was added dropwise into the oil phase to form a W/O emulsion under mechanical stirring, and this emulsion was stirred continuously with a three-blade propeller at 1000 rpm for 1 h. Afterwards vanillin dissolved in acetone (50 mL, 25 mg/mL) was added dropwise into the W/O emulsion under mechanical agitation. Stirring was continued until the complete evaporation of organic solvent. Chitosan nanoparticles were collected by centrifugation at 12 500g for 10 min and washed three times with petroleum ether and isopropanol, separately. Finally, samples were freeze-drying at −50 °C for 24 h.
Characterization of nanoparticles
The morphology of the chitosan nanoparticles was examined by scanning electron microscopy (SEM, S-4800, Hitachi, Tokyo, Japan) and transmission electron microscopy (TEM, JEM 2100, Japan). Samples were dispersed in aqueous solution under ultrasonication and one drop of the nanoparticle suspension was cast onto a glass plate. Samples were dried at room temperature and sputter-coated with gold before being observed by SEM. Zeta potential of the nanoparticles was measured by photon correlation spectroscopy (Nano-zs & MPT-2, Malvern, UK). XRD analysis was conducted using an X-ray diffractometer (D8 Advance, Bruck, Saarbrucken, Germany) with Cu as target. The measurement was carried out at 40 kV and 2θ range from 0° to 60°.
Determination of encapsulation efficiency and loading capacity
Drug-loaded chitosan nanoparticles (50 mg) were accurately weighed and ground sufficiently with a mortar. The samples were carefully transferred into a beaker and treated with ultrasonification for 3 min (Sonics & Materials, Newtown, USA) in distilled water. Then, they were centrifuged at 15 000g for 10 min. The supernatant was adjusted to 50 mL. The optical density (OD) value was measured with an ultraviolet spectrophotometer (Lambda35, Perkin-Elmer, Waltham, USA) at 265 nm. The encapsulation efficiency (EE) and loading capacity (LC) were obtained by measuring the ultraviolet absorption of the supernatant. EE and LC were calculated using the following equations (Li et al., Citation2011a,Citationb; Wang et al., Citation2011):
(1)
(2)
Evaluation of in vitro release
The drug release profile of the chitosan nanoparticles was evaluated in a simulated gastric fluid (HCl solution, pH 1.2), simulated colonic fluid (phosphate buffer saline (PBS), pH 6.8) and simulated intestinal fluid (PBS, pH 7.4). Chitosan nanoparticles (50 mg) were placed in a dialysis bag (MWCO 5 kDa) and incubated in 100 mL release medium. The system was maintained at 37 ± 0.5 °C in a temperature controlled oscillator. At prescheduled time intervals, samples (3 mL) were withdrawn and measured with an ultraviolet spectrophotometer (Lambda35, Perkin-Elmer) at 265 nm.
In vitro cytotoxicity study
HT-29 cells were cultured continuously at 37 °C in a humidified atmosphere containing 5% CO2 in 1640 RPMI medium supplemented with 10% (v/v) calf serum. Penicillin (100 U/mL) and streptomycin (100 µg/mL) were also used in a 96-well plate. After that, cell lines were digested with 0.25% (w/v) trypsin and the cell concentration was adjusted to 1 × 105 cells/mL by medium. Then, 100 µL cells suspension was added into each well of a 96-well plate. After 24 h incubation, another 100 µL medium containing drug-loaded chitosan nanoparticles with different concentrations (2, 1, 0.5, 0.25, 0.125, 0.0625 mg/mL) was added into the 96-well plate. After incubation for 24 h, the medium and the particles were removed and displaced by 180 µL fresh medium and 20 µL MTT solutions (5 mg/mL). Four hours later, the supernatant was removed and 200 µL DMSO was added into each well of the plate and the OD was measured at 492 nm by using enzyme mark instrument (Multiskan MK3, Thermo, Waltham, USA). The inhibition ratio was obtained by the following equation:
(3)
Cellular uptake of nanoparticles
HT-29 cells in logarithmic growth phase were cultured in a confocal dish for the cellular uptake of fluorescein-loaded chitosan nanoparticles (Campos et al., Citation2004). After 24-h incubation, fresh RPMI1640 medium containing fluorescein-loaded chitosan nanoparticles at different concentrations (0.0625 and 2 mg/mL) was added into each dish. Six hours later, the supernatant was removed and the cells were observed under confocal laser scanning microscope (CLSM) (TCS SP5 II, Leica, Wetzlar, Germany) after being washed with PBS five times. To further quantify the cellular uptake of chitosan nanoparticles, flow cytometer (BD FACSCanto II) was used. HT-29 cells in logarithmic growth phase were cultured in a six-well plate. After incubation for 24 h, fresh RPMI1640 medium containing fluorescein-loaded chitosan nanoparticles with different concentrations (2, 1, 0.5, 0.25, 0.125, 0.0625 mg/mL) was added into each well. Six hours later, the supernatant was removed and the cells were washed with PBS for five times. The suspended cells in 500 µL PBS were analyzed by the flow cytometer after being digested by 0.25% (w/v) trypsin.
Results and discussion
Preparation of chitosan nanoparticles
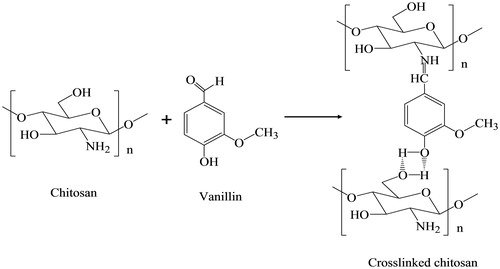
The preparation of chitosan nanoparticles was based on Schiff reaction between the amino group of chitosan and the aldehyde group of vanillin as well as hydrogen bonding between chitosan and vanillin. As shown in , the carbon of the carbonyl group in vanillin was positively charged due to the electronegativity of the oxygen of the carbonyl group. Therefore, the carbon of the carbonyl group was sensitive to be attacked by a nucleophilic reagent such as chitosan because of the existence of its electron-ion pairs. Therefore, the Schiff reaction between the amino group of the chitosan and the aldehyde group can occur easily. In addition, the oxygen existing in the hydroxyl group which is opposite to the aldehyde in the vanillin molecule and the oxygen of the hydroxyl group in methylene are both intensely electronegative. The atomic radius of oxygen is small and possesses valence electrons, which generates the hydrogen bonding between chitosan and vanillin.
XRD analysis
The physical state of 5-FU, before and after being encapsulated in the chitosan nanoparticles, was studied by XRD analysis. As shown in , intense peaks are observed at 2θ = 16.5°, 19.3°, 20.7°, 28.7°, 31.48°, 32.3°, 33.5°, 59.4° in the diffraction pattern of pure 5-FU, indicating that 5-FU is a crystalline powder (Li et al., Citation2011a,Citationb). No diffraction peaks are observed after 5-FU was encapsulated into chitosan nanoparticles (), which implies that 5-FU is distributed in the chitosan–vanillin nanoparticles in an amorphous state.
Figure 2. XRD spectra of (a) 5-FU, (b) chitosan and (c) chitosan nanoparticles and (d) drug-loaded chitosan nanoparticles (the amount of 5-FU encapsulated into chitosan nanoparticles is 38.5 mg, and the EE and drug LC of chitosan nanoparticles is 77% and 4.2%, respectively).
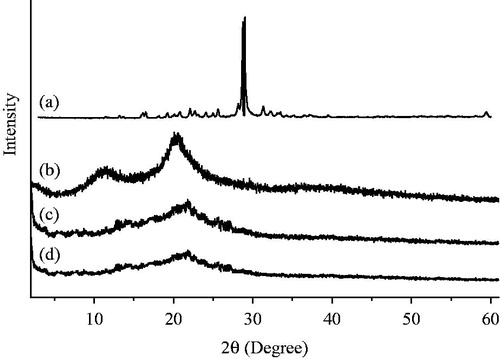
The crosslinking between chitosan and vanillin was based on a Schiff reaction. XRD analysis also proves that the degree of crystallinity of chitosan nanoparticles made by crosslinking with vanillin declines significantly when compared with that of chitosan. As shown in , intense diffraction peaks are observed at 2θ = 20.28° and 11.34°. The diffraction peak at 2θ = 11.34° of chitosan becomes weak and is shifted to 2θ = 13.44° after crosslinking with vanillin (in ), and the intense peak at 2θ = 20.28° is shifted to 21.54°. This can be explained by the generation of micro-crystals during the dehydration and crosslinking between the amino group of chitosan and the aldehyde group of vanillin, which limits the movement of the molecular chain of chitosan nanoparticles.
Morphological and zeta potential of chitosan nanoparticles
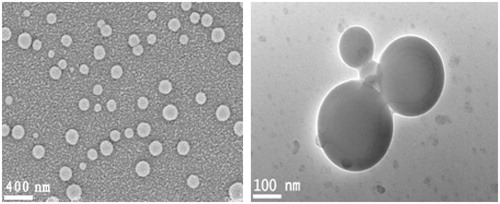
The release of the drug from nanoparticles was more controllable when the particles were compact and uniform in size. As shown in , the chitosan nanoparticles made by crosslinking with vanillin have smooth surfaces and are mainly 200–250 nm in diameter with a narrow size distribution. The zeta potential of the chitosan nanoparticles in distilled water is − 17.4 mV. It was demonstrated in the previous work of our group that the preparation of nanoparticles was affected by many factors including emulsifier, stirring speed and the ratio of water to oil phase. When span 80 and tween 80 (1:1, v/v) was used as emulsifier particles with microsize were obtained (Peng et al., Citation2010). When sorbitan sesquioleate was used as emulsifier, particle with nanosize was obtained (Wang et al., Citation2011).
Drug loading capacity and in vitro release profile
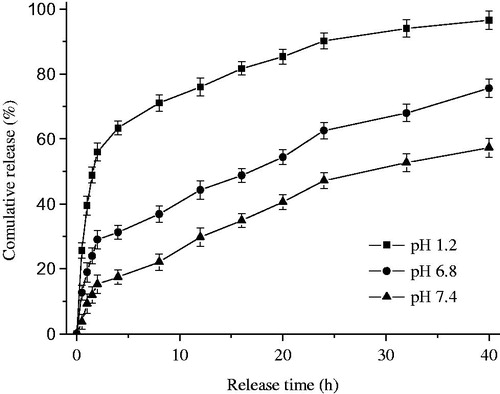
The in vitro release profile in three different medium shown in , and before the release test we have got the EE and drug LC in chitosan nanoparticles at 77% and 4.2%, respectively. From the profile we find that the burst release phenomenon is also present (56.6%) in acidic conditions (pH 1.2) during the first 2 h, although it significant decreased compared with nanoparticles crosslinking with tripolyphosphate (Li et al., Citation2011a,Citationb). However, the cumulative release in simulated colonic fluid (pH 6.8) and simulated intestinal fluid (pH 7.4) are 28.9% and 14.5% in first 2 h, respectively. After 40 h, the drug release completely from nanoparticles in simulated gastric fluid. Nevertheless, only 72% and less than 60% of drugs release from simulated colonic fluid and simulated intestinal fluid at the end of experiment and the cumulative release increases slowly during the whole process.
Cytotoxicity assay and cellular uptake of fluorescein-loaded chitosan nanoparticles
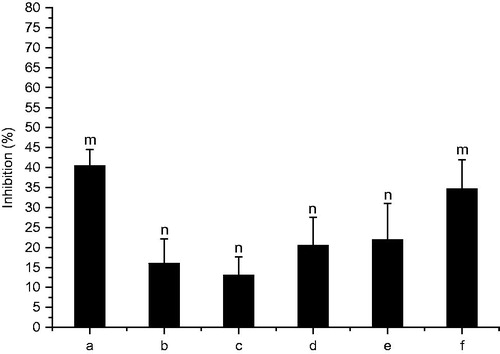
In order to further investigate the cytotoxicity of drug-loaded nanoparticles, MTT method was used to explore the inhibition effects of nanoparticles at different concentrations on HT-29 cells. As shown in , groups with the concentration at 0.0625 and 2 mg/mL have significant effects on the inhibition ratio against HT-29 cells when compared with other groups (p < 0.001). Interestingly, nanoparticles at low concentration (0.0625 mg/mL) also show high inhibitory effect like them at high concentration (2 mg/mL) after 24-h incubation, the difference in relative concentration of drug released from nanoparticles lead to the difference of absorption mechanism may be the main factor.
Figure 5. Inhibition ratio of drug-loaded chitosan nanoparticles: (a) 2 mg/mL, (b) 1 mg/mL; (c) 0.5 mg/mL; (d) 0.25 mg/mL; (e) 0.125 mg/mL; (f) 0.0625 mg/mL. Data were given as mean ± SD (n = 5), m indicates significant differences in group a and f (p < 0.001) when compared with other groups. n indicates significant differences in group b–e when compared with other groups (p < 0.001).
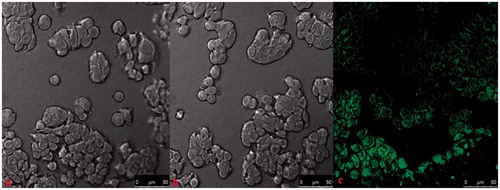
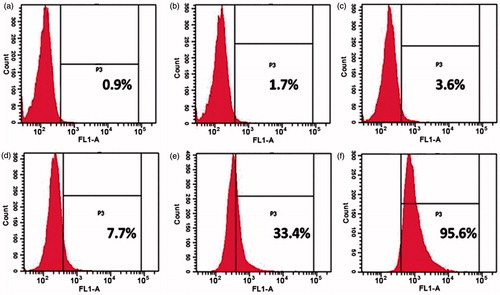
To study the cellular uptake of the chitosan nanoparticles, fluorescein-loaded chitosan nanoparticles were formed. According to the previous results of cytotoxicity, chitosan nanoparticles at 0.0625 and 2 mg/mL were used to study the cellular uptake of the nanoparticles. Fluorescence images () demonstrate that within 6 h, fluorescence is not observed when the nanoparticle concentration is 0.0625 mg/mL, whereas the green fluorescence is observed in group with concentration of 2 mg/mL. In contrast with the release profile, the release of drugs within 6 h is extremely rare when the nanoparticle concentration is 0.0625 mg/mL. To further quantify the uptake of the chitosan nanoparticles in the cancerous cells, flow cytometric analysis was applied. As shown in , the positive rate of chitosan nanoparticles increases with the increase of the nanoparticle concentration. When the nanoparticle concentration reaches 2 mg/mL, the positive rate is 95.6%, while it is only 0.9% when the concentration is 0.0625 mg/mL, which is consistent with the result obtained in .
Conclusions
Chitosan nanoparticles for drug delivery were successfully developed by crosslinking chitosan with vanillin. These chitosan–vanillin nanoparticles were 200–250 nm in size. 5-FU was efficiently encapsulated into the chitosan nanoparticles via electrostatic interaction and existed in the nanoparticles in amorphous state. Efficient EE was obtained for 5-FU. In vitro release demonstrated that a constant and controlled release of 5-FU from vanillin–chitosan nanoparticles was achieved and chitosan–vanillin nanoparticles exhibit excellent drug release profile. The cytotoxicity study showed that the uptake mechanisms of drug were relative to its concentration. Under the appropriate concentration, the drug can cross the biologic barrier by facilitating passive diffusion with the help of carrier. However, when the concentration is too high, the passive diffusion would be the main way to cross the barrier. Interestingly, we noted that the uptake of particles at 0.0625 mg/mL was very low during the first 6 h according to the uptake experiment while its efficacy was almost the same as that at high concentration after 24 h. Combined with these novel properties, the chitosan nanoparticles crosslinked with vanillin are a promising vehicle for the controlled delivery of anticancer drugs to colon.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgements
The authors acknowledge financial support by the Natural Science Foundation of Hainan Province (Nos 512106, 513151), and the Fundamental Research Funds for Rubber Research Institute, CATAS (Nos 1630022013019, 1630022012007).
References
- Ajit PR, Namdev BS, Sangamesh AP, Tejraj MA. (2007). Novel interpenetrating polymer network microspheres of chitosan and methycellulose for controlled release of theophylline. Carbohydr Polym 69:678–87
- Ali F, Elina EG, Mahnaz A, et al. (2014). Preparation and characterization of self-assembled chitosan nanoparticles for the sustained delivery of streptokinase: an in vivo study. Drug Deliv 19:593–7
- Almalik A, Donno R, Christopher J, et al. (2013). Hyaluronic acid-coated chitosan nanoparticles: molecular weight-dependent effects on morphology and hyaluronic acid presentation. J Control Release 172:1142–50
- Baek SH, Kim B, Suh KD (2008). Chitosan particles/multiwall carbon nanotube composites by electrostatic interactions. Coll Surfaces A Physicochem Eng Aspects 316:292–6
- Bharmoria P, Singh T, Kumar A. (2013). Complexation of chitosan with surfactant like ionic liquids: molecular interactions and preparation of chitosan nanoparticles. J Coll Interface Sci 407:361–9
- Campos AM, Diebold Y, Carvalho ELS, et al. (2004). Chitosan nanoparticles as new ocular drug delivery systems: in vitro stability, in vivo fate, and cellular toxicity. Pharm Res 21:803–10
- Fürst W, Banerjee A. (2005). Release of glutaraldehyde from an albumin-glutaraldehyde tissue adhesive causes significant in vitro and in vivo toxicity. Ann Thorac Surg 79:1522–8
- Gu FX, Karnik R, Wang AZ, et al. (2007). Targeted nanoparticles for cancer therapy. Nano Today 2:14–21
- Gupta KC, Jabrail FH. (2006). Glutaraldehyde and glyoxal cross-linked chitosan microspheres for controlled delivery of centchroman. Carbohydr Res 341:744–56
- Ho K, Yazan LS, Ismail N, Ismail M. (2011). Toxicology study of vanillin on rats via oral and intra-peritoneal administration. Food Chem Toxicol 49:25–30
- Hongyan Z, Fei L, Jing G, et al. (2011). Folate-modified chitosan micelles with enhanced tumor targeting evaluated by near infrared imaging system. Carbohydr Polym 86:1118–29
- Hua DB, Jiang JL, Kuang LJ, et al. (2011). Smart chitosan-based stimuli-responsive nanocarriers for the controlled delivery of hydrophobic pharmaceuticals. Macromolecules 44:1298–302
- Jana S, Maji N, Nayak AK, et al. (2013). Development of chitosan-based nanoparticles through inter-polymeric complexation for oral drug delivery. Carbohydr Polym 98:870–6
- Li PW, Wang YC, Peng Z, et al. (2011a). Development of chitosan nanoparticles as drug delivery systems for 5-fluorouracil and leucovorin blends. Carbohydr Polym 85:698–704
- Li PW, Wang YC, Zeng FB, et al. (2011b). Synthesis and characterization of folate conjugated chitosan and cellular uptake of its nanoparticles in HT-29 cells. Carbohydr Res 346:801–6
- Li QX, Song BZ, Yang ZQ, Fan HL. (2006). Preparation and characterization of chitosan microcapsules crosslinked by vanillin. Chin J Process Eng 6:608–13
- Makni M, Chtourou Y, Fetoui H, et al. (2011). Evaluation of the antioxidant, anti-inflammatory and hepatoprotective properties of vanillin in carbon tetrachloride-treated rats. Eur J Pharmacol 668:133–9
- Mao HQ, Roy K, Troung-Le VL, et al. (2001). Chitosan DNA nanoparticles as gene delivery carriers: synthesis, characterization and transfection efficiency. J Control Release 70:399–421
- Pan Y, Li Y, Zhao H, et al. (2002). Chitosan nanoparticles improve the intestinal absorption of insulin in vivo. Int J Pharm 249:139–47
- Peng HL, Xiong H, Li JH, et al. (2010). Vanillin cross-linked chitosan microspheres for controlled release of resveratrol. Food Chem 121:23–8
- Prabaharan M, Borges JP, Godinho MH, Mano JF. (2006). Liquid crystalline behaviour of chitosan in formic, acetic, monochloroacetic acid solutions. Mater Sci Forum 514–516:1010–4
- Pramila C, Brahmeshwar M. (2014). Mannose-conjugated chitosan nanoparticles loaded with rifampicin for the treatment of visceral leishmaniasis. Carbohydr Polym 101:1101–8
- Rathna GVN. (2008). Gelatin hydrogels: enhanced biocompatibility, drug release and cell viability. J Mater Sci Mater Med 19:2351–8
- Rubert ND, Lorena T, Montserrat M, et al. (2013). In vitro antitumor activity of methotrexate via pH-sensitive chitosan nanoparticles. Biomaterials 34:2758–72
- Sang NV, Hiep DM, Zung NA. (2013). Study on chitosan nanoparticles on biophysical characteristics and growth of Robusta coffee in green house. Biocatal Agric Biotechnol 2:289–94
- Tai A, Sawano T, Yazama F, Ito H. (2011). Evaluation of antioxidant activity of vanillin by using multiple antioxidant assays. Biochim Biophys Acta 1810:170–7
- Wang G, Li PW, Peng Z, et al. (2011). Formulation of vanillin cross-linked chitosan nanoparticles and its characterization. Adv Mater Res 335–336:474–7
- Zhang ZJ. (2004). Therapeutic effects of herbal extracts and constituents in animal models of psychiatric disorders. Life Sci 75:1659–99
- Zheng AP, Liu HX, Yuan L, et al. (2011). Comprehensive studies on the interactions between chitosan nanoparticles and some live cells. J Nanopart Res 13:4765–76