Abstract
Objective: This investigation deals with the development and evaluation (in vitro and in vivo) of pH triggered Eudragit-coated chitosan microspheres of curcumin (CUR) for treating ulcerative colitis.
Methods: CUR-loaded chitosan microspheres were initially prepared by emulsion cross linking method followed by coating with Eudragit S-100. The pharmacodynamics of the developed formulation was analyzed in mice by acetic acid induced colitis model.
Results: The developed microspheres were of uniform spherical shape with high entrapment efficiency. CUR-chitosan microspheres showed less intense peaks compared to free CUR confirming inclusion of drug within microspheres as revealed by X-ray diffractogram. Uncoated CUR-chitosan microspheres exhibited burst release within initial 4 h while microspheres coated with Eudragit S-100 prevented premature release of CUR and showed controlled release up to 12 h following Higuchi model. In vivo organ biodistribution study showed negligible amount of CUR in stomach and small intestine confirming integrity of microsphere in upper gastrointestinal tract (GIT). In vivo study revealed significant reduction in severity and extent of colonic damage with CUR-loaded microspheres as compared to pure CUR which was further confirmed by histopathological study.
Conclusion: In vitro and in vivo studies proved the developed formulations as a promising system for pH-dependent delivery of drug to colon in ulcerative colitis.
Introduction
Inflammatory bowel diseases (IBDs) are a group of debilitating inflammatory disorders specially influencing colonic tissue in which full long term remission with current standardized treatments is yet unidentifiable. Side effects and therapeutic efficacy considerations with conventional systems demands the development of more effective systems which can reduce the required drug doses, systemic adverse effects and can target the drug specifically to the desired site of action in colon (Talaei et al., Citation2013). IBD mainly includes ulcerative colitis and Crohn’s disease (Ahmad et al., 2012b). Ulcerative colitis is mostly detected in adults between 30–40 years of age irrespective of any sex. The treatment regimen for IBD mainly includes salicylates, antimicrobials, corticosteroids and immunosuppressant, associated with number of adverse effect (Mehrabani et al., Citation2011). Therefore, it is the need of the hour to develop a system with maximum therapeutic outcomes with fewer side effects. Curcumin, a yellow natural polyphenol derived from rhizome of herb Curcuma longa which has been subject to multiple of investigations over the last few decades that indicated numerous health benefits (Khatik et al., Citation2013). Curcumin having safety and tolerance up to a dose of 12 g/day
The pharmacological efficacy and safety of curcumin makes it a suitable candidate for treatment and prevention of various human diseases and in recent years this molecule has been utilized by various researchers for site specific delivery to colon (Lao et al., Citation2006; Wu et al., Citation2011; Sareen et al., 2013b). The anti-inflammatory activity of curcumin involves inactivation of both the lipoxygenase and cyclooxygenase enzyme systems (Duvoix et al., Citation2005). The objective of this study was to develop a multiparticulate system of curcumin for colon targeting in the treatment of ulcerative colitis. Multiparticulate system was opted as it has numerous merits over the single unit system like slower transit through colon, higher surface area for localized action, less inter-subject variation, uniform drug absorption, homogeneous drug delivery and reduced local irritation (Chandran et al., Citation2009).
Chitosan is a polysaccharide obtained by N-deacetylation from chitin and has been widely investigated as a carrier for novel delivery systems owing to its biodegradability, biocompatibility and safety. Chitosan is also prone to degradation by the colonic micro flora and therefore it can be used for colon specific drug delivery incorporated in pH sensitive polymer (Rabiskova et al., Citation2012).
Eudragit S-100 is pH-sensitive polymer having threshold pH above 7, which may release the drug to the distal ileum as in ulcerative colitis the distal potion of colon is mainly affected (Lamprecht et al., Citation2004; Sareen et al., 2013a).
In this study, Eudragit-coated chitosan microspheres capable of delivering curcumin into the colon were prepared. Chitosan microspheres were prepared using emulsion cross linking method and subsequently coated with Eudragit S100 coatings to prevent dissolution of chitosan microspheres in acidic environment. Prepared microspheres were characterized and compared for in vitro release profiles. Microspheres with promising results were evaluated for pharmacodynamic activity in acetic acid-induced colitis in mice (Li et al., Citation2006; Singh, Citation2007).
To study the pharmacodynamic effects, acetic acid-induced colitis model in mice was used (Barollo et al., Citation2011) as it provides the same experimental conditions after intra-rectal administration as that in humans’ IBD.
Experimental
Materials
Curcumin was purchased from Sigma Aldrich, Mumbai, India. Chitosan (low mol. wt., viscosity 20–200 cP) was received as a gift sample from V-Laboratory, Solan, India. The deacetylation degree of chitosan according to the specifications from the provider was higher than 80%. Eudragit S100 (Viscosity 50–200 mPa s, Mol. wt. 135 000) was obtained from Evonik Rohm Pharma, Darmstadt, Germany. Span 80 (Mol. wt. 428.6, viscosity 1200–2000 mPa s) and liquid paraffin were purchased from SD Fine Chemicals Mumbai, India. All chemicals and reagents used were of analytical grade.
Preparation of microspheres
CUR-loaded chitosan microspheres were prepared by emulsion cross-linking method. Chitosan was dissolved in 10 mL of aqueous solution of acetic acid (1%) and CUR was dispersed in the polymeric solution in different ratios (1:1, 1:2, 1:3 and 1:4; ). This solution was incorporated into 100 mL of liquid paraffin containing Span 80 (1% v/v) followed by stirring at 1500 rpm for 2 h with the help of a mechanical stirrer (Remi Motors, Mumbai, India). Then 0.5 mL of glutaraldehyde was added to the emulsion and kept for 1 h. The excess amount of glutaraldehyde was removed by stirring under vacuum. Finally, microspheres were obtained by filtration and washed with petroleum ether and dried in hot air oven at 40 °C (Jose et al., Citation2011). All the studies were carried out in amber-colored glass containers under dark conditions.
Table 1. Composition of colon targeted CUR microspheres.
Polymeric coating of chitosan microspheres of CUR
The CUR chitosan microspheres were coated with Eudragit S100 by emulsification-solvent evaporation method. The developed CUR-loaded chitosan microspheres were transferred to 10 ml of ethanolic solution of Eudragit S100 (10% w/v). Then emulsification was achieved by adding 70 ml of light paraffin and Span 80 (1% v/v) followed by stirring for 2 h at 1500 rpm with a mechanical stirrer. Finally, Eudragit S100-coated microspheres were collected by filtration, washing with petroleum ether and dried in hot air oven at 50 °C for 3 h (Perge et al., Citation2012).
HPLC analysis of CUR
Analysis of CUR was performed by using a reverse phase HPLC column (eclipse XBD, C18, 5 μm, 150 mm × 4.6 mm; Agilent Technology 1200, Waldbronn, Germany). The mobile phase was composed of a mixture of methanol and acetonitrile in a ratio of 50:50 v/v and passed through the column with a flow rate of 0.8 ml/min. The detection wavelength was set at 435 nm and the retention time of the CUR was found to be 9.1 min. The assay was linear (r2 = 0.9991) in the concentration range of 0.1–30 μg/ml with the lowest detection limit at 0.003 μg/ml. All samples were filtered through 0.22 μm nylon filter prior to analysis. shows all validation parameters of developed method of CUR.
Table 2. Validation Parameters for HPLC method of CUR.
In vitro characterization of microspheres
Determination of particle size, shape and surface morphology
Malvern Mastersizer (Malvern Instruments, Mastersizer 2000, Malvern, UK) was used to determine the particle size of CUR microspheres (Tsai et al., Citation2013). The results obtained are the average of three analyses. The values (d50) were denoted for all formulations as average size range.
The prepared microspheres were characterized for shape and surface morphology using scanning electron microscope (SEM-QUANTA 250, FEI Makers, Singapore). The microspheres were mounted on an aluminum stub with carbon-glue and coated with gold using a gold sputter module in a high vacuum evaporator. The photographs were taken at an excitation voltage of 10 kV. The magnifications selected were sufficient to depict the detail morphology of the samples under study (Yadav et al., Citation2013).
X-ray diffraction
Qualitative powder X-ray diffraction of CUR and its microspheres was determined by X-ray diffractometer (Bruker Axs D8 Advance, Karlsruhe, Germany). The instrument was operated at a voltage of 40 kV and a current of 30 mA, with copper as the tube anode material (Philip et al. Citation2009). The samples were run over a range of 2θ angles from 2° to 80°.
Determination of percent yield, drug loading and entrapment efficiency
The percent yield (PY) was calculated based on mass of CUR and polymer added, using following equation:
About 100 mg of prepared microspheres were first crushed with the help of a glass mortar pestle followed by dispersion in 100 ml of methanol and kept overnight for the extraction of drug. The supernatant was diluted with methanol after centrifugation at 2500 rpm for 10 min and drug content was measured at 435 nm after filtration through 0.22μm syringe filter in a HPLC system. The drug loading was calculated for each batch in triplicate (Singh & Pathak, Citation2011; Ahmad et al., 2012c; Zhao et al., Citation2013).
Using the following equation, drug loading (DL) and entrapment efficiency (EE) were determined:
In vitro swelling
Swelling was determined by placing 100 mg of chitosan microspheres and Eudragit-coated chitosan microspheres without drug in a cellophane membrane dialysis bag (D9402, Sigma–Aldrich, Mumbai), containing phosphate buffer (pH 7.4). Then microspheres were allowed to swell for a period of 8 h. The changes in weight were measured by removal of the samples and blotted with a filter paper for 10 s to absorb excess solvent on surface. The degree of swelling was determined using the following equation:
where Sα represents the degree of swelling, Wt and W0 represent weights of the sample at equilibrium swelling and the original dry weight, respectively (Ruiz-Caro et al., Citation2009).
In vitro drug release
In vitro release study of developed formulations was performed in USP Type-I (basket type, Electrolab, EDT-08 LX, Mumbai, India) dissolution apparatus using various simulated GI fluids. The simulation of variation in GI pH was achieved by altering the pH of the dissolution medium at different time intervals. The dissolution was performed at pH 1.2 with 0.1 N HCl for 2 h, pH 6.4 for next 2 h with phosphate buffer and then at pH 7.4 up with phosphate buffer to 12 h. The pH of dissolution medium was altered from pH 1.2 to 6.4 by adding KH2PO4 and Na2HPO4·2H2O and adjusted with 1.0 M NaOH. Then after 4 h, the pH was adjusted to 7.4 with 1.0 M NaOH. At regular time intervals, 5 ml of samples were withdrawn and replaced with equal amount of the fresh medium to maintain sink condition. The samples were analyzed by HPLC after filtration through 0.22 μm syringe filter. Further the in vitro release of selected microspheres formulation was carried out in the presence of 0.32% w/v pepsin for first 4 h to predict the stability of Eudragit S-100 and then in the presence of 10% w/v of rat caecal content from fifth hour, to analyze the biodegradability of chitosan in presence of colonic microflora (Paharia et al., Citation2007, Singh & Pathak, Citation2013).
Determination of kinetic model for release of CUR-loaded chitosan microspheres
The in vitro drug release data of the Eudragit-coated chitosan microspheres was applied to different kinetic models, i.e. zero order, first order, the Higuchi and Peppas model and on the basis of the results (Correlation co-efficient R2 value), and the best model was selected (Christoper et al., Citation2013).
In vivo organ biodistribution study
All the in vivo studies were conducted with prior approval of Institutional Animal Ethical Committee (IAEC/SU-PHARM/13/009). For this study, CUR-loaded microspheres were orally administered to mice in 1%w/v carboxymethyl cellulose (CMC). After 12 h the mice were sacrificed and the stomach, small intestine and colon were isolated. Then the organs were homogenized by using Tissue Homogenizer with 2 ml of phosphate buffer (pH 7.4). To this homogenate 2 ml of ethanol was added and kept for 1 h. The drug content was determined by using HPLC method (Agilent Technology 1200, Germany) after appropriate dilution (Ahmad et al., 2012a).
In vivo study using acetic acid-induced colitis
In vivo study was performed by using acetic acid-induced experimental ulcerative colitis model. Swiss Mice (body weight 18–22 g), n = 6 were selected and caged individually provided with food and water ad libitum. Animals were randomly distributed in three groups, i.e. Control, CUR and CUR-Microspheres, each consisting six animals. The mice were first anesthetized with ether and 0.2 ml (4%) (v/v) of acetic acid was administered through intra-rectal route to induce ulcerative colitis in mice which resembles the IBD condition. For 3 days, mice were kept without any treatment for the development of full IBD model. The animal of standard and test groups received oral CUR and CUR-microspheres in 1%w/v CMC (0.5 ml) daily for 3 days. The control group received 1%w/v CMC orally. Animals were sacrificed after 24 h of last drug administration. The colon portion of mice was isolated and washed with saline, photographed and scored by blinded observer using the scoring system of Wallace and Keenan: (i) 0 – no damage, (ii) 1 – localized hyperemia without ulcers, (iii) 2 – linear ulcer with significant inflammation, (iv) 3 – linear ulcer with inflammation at one site, (v) 4 – two or more sites of ulceration and/or inflammation and (vi) 5 – two or more major sites of inflammation and ulceration extending more than 1 cm along the colon (Tahan et al., Citation2011). The colonic portions were also assessed for the weight/length ratio (Kandhare et al., Citation2012). Further. the histopathological study was performed on sections of colon tissues. The colonic tissues about 2 cm long after isolation were preserved in formalin (10% v/v) solution to prevent autolysis. The preserved colonic sections were fixed on paraffin slide, stained and analyzed by microscope (Azad et al., Citation2011).
Results
Eudragit-coated chitosan microspheres of curcumin were prepared by emulsion cross-linking method as stated above. The developed formulations were subjected to various evaluation parameters. shows the SEM image of CUR-loaded chitosan microspheres and shows Eudragit-coated chitosan microspheres. On varying the drug–polymer ratio from 1:1 to 1:4, the mean diameter of microspheres was in the range between 36.84 and 77.25 μm (). Further Eudragit S100 coating showed increase in the size and increase in the weight (15–25%). X-ray diffractogram of CUR exhibited several peaks of different intensities between 2θ = 2° and 40° while X-ray diffractogram of CUR-loaded microspheres showed less intense peaks in the same range (). shows values of drug loading, percentage yield and encapsulation efficiency of different batches of CUR-loaded chitosan microspheres. The PY values ranged from 81.25% to 88.87%. The studied formulations showed EE values varied from 73.88% to 82.50%. The in vitro swelling studies of developed microspheres as performed in phosphate buffer pH 7.4 was found to be increased with increase in chitosan concentration, as shown in .
Figure 1. (a) SEM image of CUR-chitosan microspheres, (b) SEM image of Eudragit-coated chitosan microspheres.
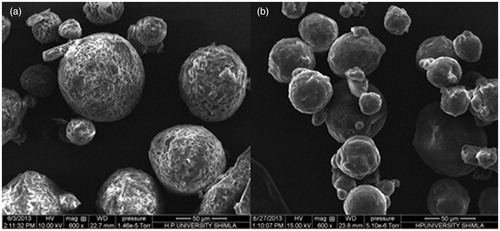
Figure 2. X-ray diffractogram of (a) CUR showing more intense peaks, (b) CUR-loaded chitosan microspheres showing less intense peaks, (c) CUR with blank chitosan microspheres.
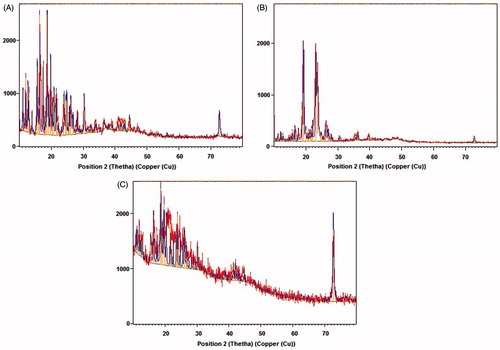
Table 3. Compilation of the evaluation parameters of CUR-loaded microspheres.
In vitro release studies of CUR-loaded microspheres were performed in simulated GI fluids using USP dissolution (type-I) test apparatus in 900 ml of the dissolution medium, stirred at 50 rpm at 37 ± 0.5 °C. The in vitro release profiles of various batches were performed using three different dissolution media to mimic the GI conditions. show the in vitro release profiles of drug from chitosan microspheres and Eudragit-coated chitosan microspheres. The in vitro release studies of F6 formulation were also performed in presence of rat caecal contents and pepsin to simulate the GIT environment and the results are shown in . Drug release data was subjected to different kinetic models ().
Figure 3. In vitro release profile of (a) CUR-loaded chitosan microspheres showing burst release at stomach pH (F1–F4), (b) Eudragit S-100-coated CUR-chitosan microspheres showing delayed release at colonic pH (F5–F8).
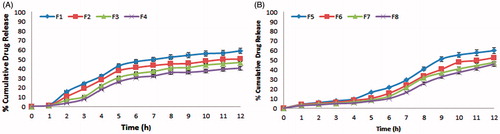
Figure 4. Comparative in vitro release profile of F6 with and without pepsin and rat caecal content (RCC) showing significant influence of RCC on drug release at colonic pH.
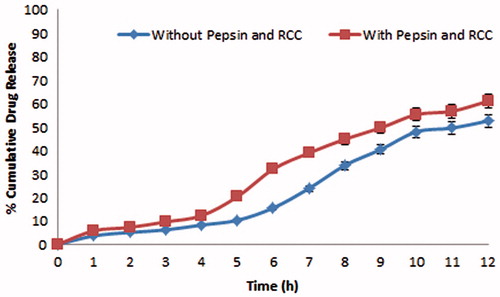
Table 4. Release kinetics for dissolution data of different formulations (F5–F8).
In vivo organ biodistribution study showed negligible amount of CUR in stomach (1.12 ± 0.64%) and small intestine (2.63 ± 0.87%) while high concentration (51.23 ± 2.53%) was observed in colon after 12 h.
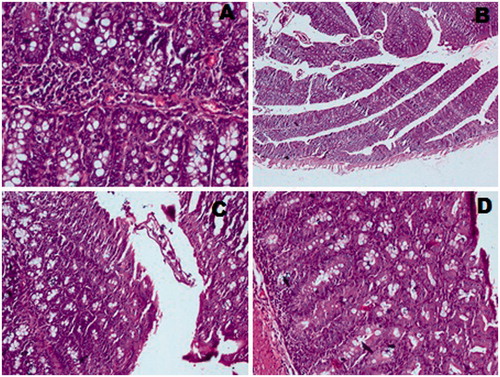
CUR and its CUR-loaded microspheres were analyzed by acetic acid-induced ulcerative colitis in mice. Acetic acid-induced colitis resulted in stiff and thick walls of colon due to erosion, edema, necrosis of epithelium and inflammatory reactions. Ulcers were associated with bleeding and redness along the colon wholly or partially in control group. CUR-loaded microspheres treated group exhibited mild lesions and inflammation while free CUR treated group showed moderate lesions and inflammatory reactions ( and ).
Figure 5. Evaluation of experimental colitis in colon of mice in (A) control group (severe lesions and inflammation) (B) CUR-treated group (moderate lesions) (C) CUR microsphere-treated group (mild lesions).
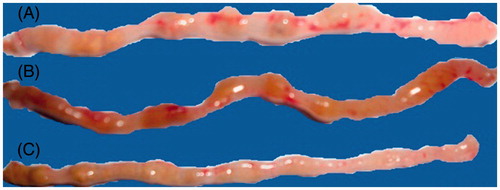
Table 5. Macroscopic evaluation of colonic lesions of mice.
The colonic portions were also assessed for the weight/length ratio and this study revealed that weight/length ratio of colon was significantly higher (0.256 ± 0.018) in acetic acid-treated control group as compared to CUR-treated (0.125 ± 0.031) and CUR-chitosan microsphere-treated groups (0.074 ± 0.012). The results showed a significant difference between control, CUR-treated and CUR-chitosan microsphere-treated groups (p < 0.05). Further, the histopathological studies of colonic portion of mice were performed and results are shown in
Discussion
For an effective delivery of drug specifically to colon, the drug release should be kept minimum while its transit through the upper GIT so as to ensure the maximum dose of drug reaches to colon. The pH conditions of the dissolution studies were selected so as to simulate the GI conditions. In vitro drug release of CUR from the chitosan microspheres was 17.97–32.12% in the initial 4 h, indicating burst release of drug. These types of dissolution profiles are not appropriate for colon-targeted drug delivery system. Burst release of CUR from chitosan microspheres might be due to solubilization of chitosan at acidic pH. This problem was overcome by coating of chitosan microspheres with Eudragit S-100 as it exhibits pH-dependent solubility with a threshold pH of 7.0. The Eudragit coating of chitosan microspheres was attained using emulsification solvent evaporation method. SEM photomicrograph of chitosan microspheres indicated that the cross-linked chitosan microspheres exhibited rough surface and spherical shape while SEM photomicrograph of Eudragit-coated chitosan microspheres revealed smooth surface and spherical shape. The size of microspheres was found to increase with increase in chitosan concentration. Further coating with Eudragit also showed significant increase in the size of microspheres. X-ray diffractogram revealed that after the entrapment of drug into chitosan polymer, the intensity of peaks markedly decreased. This suggested the inclusion of CUR into microsphere formulation. Analysis of the diffractograms revealed higher crystallinity of the CUR as compared with the microspheres. The EE was found to increase with increase in the drug–polymer ratio and F4 formulation exhibited the highest EE consisting drug–polymer ratio of 1:4. Eudragit coating of chitosan microspheres prevented the premature release of CUR in the upper GIT. The release rate of CUR from Eudragit-coated microspheres was found to increase after 4 h, due to the exposure of microspheres to pH 7.4, which is more than the solubilizing pH of Eudragit S-100 polymer. In the presence of pepsin, Eudragit S-100 coating was found to be protected and exhibited unchanged in vitro release profile up to 4 h. The mechanism of release from the developed microspheres was supposed to follow the following pattern: (a) dissolution of Eudragit coating above pH 7.0, (b) swelling of chitosan and (c) diffusion of CUR from swollen chitosan gel. The release rate of drug from the microspheres was also affected by the chitosan concentration as with the increase in the concentration of chitosan, the release rate was found to decrease. This may be due to the fact that drug has to travel more path length in case of higher concentration of chitosan. For colon targeted drug delivery, the conventional dissolution study does not predict accurately the in vivo performance of targeted system as the release of drug is triggered by colonic microflora. Therefore, the release study of developed formulation was carried out in the presence of rat caecal contents from the fifth hour in the simulated colonic fluid. The release of CUR was increased significantly in the presence of rat caecal contents, which suggested biodegradability of chitosan in the presence of colonic microflora. A drug release of 60.95% was found with rat caecal contents after 12 h, while it was 52.53% in the absence of rat caecal contents. On the basis of best correlation co-efficient, the in vitro release data seems to fit better with the Higuchi model, thus suggesting diffusion as the main mechanism for drug release. Among the various Eudragit-coated microsphere formulations, the F6 exhibited best pattern of release with highest value of r2 (0.9992) and the release exponent of the Peppas model revealed super case II diffusion kinetics indicating swelling and relaxation of polymer.
In vivo organ distribution study of optimized formulation was carried out in order to analyze its targeting potential in the colon and results suggested protection of microspheres in upper GIT to release drug after reaching colon due to solubilization of Eudragit coating and microbial degradation of chitosan.
In vivo study using acetic acid-induced colitis model in the control group showed that the cecum, colon and rectum had evidence of mucosal erosion, congestion and hemorrhagic ulceration. The treatment with pure CUR showed insignificant reduction in severity and extent of the colonic damage as compared to the control group, while significant reduction in severity and extent of colonic damage was observed with CUR-loaded microspheres. The elevated weight to length ratio of colon was reduced by CUR-loaded microspheres, indicating its healing property. The results were also supported by histopathological studies, which showed extensive necrosis of colonic epithelium and hemorrhagic lesions of the entire colonic mucosa in acetic acid-treated control group. The CUR-treated group showed negligible reduction in the severity of colitis, whereas these disturbances were healed significantly in CUR-loaded microsphere-treated group.
Conclusion
A pH-triggered delivery of CUR to colon was successfully achieved using Eudragit S-100-coated microspheres. Encapsulation of CUR in microspheres enhanced its delivery to the large bowel. The colon-specific delivery of CUR from microspheres exhibited a distinct increase of the pharmacological effect as compared to free CUR. The developed formulation of curcumin utilizing pH triggered delivery may seem to be a promising approach for ulcerative colitis.
Acknowledgements
The authors thank Central Research Institute, Kasauli for cooperation in the laboratory and for providing the facilities to carry out parts of this investigation. They would also like to offer highest regard and gratitude to the reverend Prof. P. K. Khosla (Vice-Chancellor Shoolini University) for their perennial guidance, inspiration and encouragement to complete this paper.
Declaration of interest
The authors report no declarations of interest and have received no financial support for this study.
References
- Ahmad MZ, Akhter S, Ahmad I, et al. (2012a). In-vitro and in-vivo evaluation of Assam Bora rice starch-based bioadhesive microsphere as a drug carrier for colon targeting. Expert Opin Drug Deliv 9:141–9
- Ahmad MZ, Akhter S, Anwar M, Ahmad FJ. (2012b). Assam Bora rice starch based biocompatible mucoadhesive microsphere for targeted delivery of 5-fluorouracil in colorectal cancer. Mol Pharm 9:2989–94
- Ahmad MZ, Akhter S, Anwar M, et al. (2012c). Colorectal cancer targeted Irinotecan-Assam Bora rice starch based microspheres: a mechanistic, pharmacokinetic and biochemical investigation. Drug Dev Ind Pharm 39:1936–43
- Azad S, Sood N, Sood A. (2011). Biological and histological parameters as predictors of relapse in ulcerative colitis: a prospective study. Saudi J Gastroenterol 17:194–8
- Barollo M, Medici V, D’Incà R, et al. (2011). Antioxidative potential of a combined therapy of anti TNFα and Zn acetate in experimental colitis. World J Gastroenterol 17:4099–103
- Chandran S, Sanjay KS, Asghar A. (2009). Microspheres with pH modulated release: design and characterization of formulation variables for colonic delivery. J Microencapsul 26:420–31
- Christoper GVP, Raghavan CV, Siddharth K, et al. (2013). Formulation and optimization of coated PLGA-Zidovudine nanoparticles using factorial design and in-vitro in-vivo evaluations to determine brain targeting efficiency. Saudi Pharm J 22:133–40
- Duvoix A, Blasius R, Delhalle S, et al. (2005). Chemopreventive and therapeutic effects of curcumin. Cancer Lett 223:181–90
- Jose S, Prema, MT, Chacko AJ, et al. (2011). Colon specific chitosan microspheres for chronotherapy of chronic stable angina. Colloids Surf B Biointerfaces 83:277–83
- Kandhare AD, Raygude KS, Ghosh P, et al. (2012). Effect of hydroalcoholic extract of Hibiscus rosa sinensis Linn. leaves in experimental colitis in rats. Asian Pac J Trop Biomed 2:337–44
- Khatik R, Mishra R, Verma A, et al. (2013). Colon specific delivery of curcumin by exploiting Eudragit-decorated chitosan nanoparticles in vitro and in-vivo. J Nanopart Res 15:1893–907
- Lamprecht A, Yamamoto H, Takeuchi H, Kawashima Y. (2004). Design of pH-sensitive microspheres for the colonic delivery of the immunosuppressive drug tacrolimus. Eur J Pharm Biopharm 58:37–43
- Lao CD, Ruffin MT, Normolle D, et al. (2006). Dose escalation of a curcuminoid formulation. BMC CAM 6:10
- Li MG, Lu WL, Wang JC, et al. (2006). Preparation and characterization of insulin nanoparticles employing chitosan and poly(methylmethacrylate/methylmethacrylic acid) copolymer. J Nanosci Nanotechnol 6:2874–86
- Mehrabani D, Ziaei M, Hosseini SV, et al. (2011). The effect of Calendula Officinalis in therapy of acetic acid induced ulcerative colitis in dog as an animal model. Iran Red Crescent Med J 13:884–90
- Paharia A, Yadav AK, Rai G, et al. (2007). Eudragit coated pectin microspheres of 5-fluorouracil for colon targeting. AAPS Pharm Sci Tech 8:E87–93
- Perge L, Robitzer M, Guillemot C, et al. (2012). New solid lipid microparticles for controlled ibuprofen release: formulation and characterization study. Int J Pharm 422:59–67
- Philip AK, Dabas S, Pathak K. (2009). Optimized prodrug approach: a means for achieving enhanced anti-inflammatory potential in experimentally induced colitis. J Drug Target 17:235–41
- Rabiskova M, Bautzova T, Gajdziok J, et al. (2012). Coated chitosan pellets containing rutin intended for the treatment of inflammatory bowel disease: in vitro characteristics and in vivo evaluation. Int J Pharm 422:151–9
- Ruiz-Caro R, Veiga-Ochoa MD. (2009). Characterization and dissolution study of chitosan freeze-dried systems for drug controlled release. Molecules 14:4370–86
- Sareen R, Jain N, Dhar KL. (2013a). Development of colon specific microspheres of flurbiprofen for inflammatory bowel disease. Curr Drug Deliv 10:564–71
- Sareen R, Jain N, Pandit V. (2013b). Curcumin: a boon to colonic diseases. Curr Drug Target 14:1210–8
- Singh AN, Pathak K. (2011). Development and evaluation of dual controlled release microballoons containing riboflavin and citric acid: in-vitro and in-vivo evaluation. J Microencapsul 28:442–54
- Singh AK, Pathak K. (2013). Colon specific CODES based Piroxicam tablet for colon targeting: statistical optimization, in vivo roentgenography and stability assessment. Pharm Dev Technol. doi:10.3109/10837450.2013.860549 [Epub ahead of print]
- Singh BN. (2007). Modified release solid formulations for colonic delivery. Recent Pat Drug Deliv Formul 1:53–63
- Tahan G, Aytac E, Aytekin H, et al. (2011). Vitamin E has a dual effect of anti-inflammatory and antioxidant activities in acetic acid–induced ulcerative colitis in rats. Can J Surg 54:333–8
- Talaei F, Atyabi F, Azhdarzadeh M, et al. (2013). Overcoming therapeutic obstacles in inflammatory bowel diseases: a comprehensive review on novel drug delivery strategies. Eur J Pharm Sci 49:712–22
- Tsai SW, Yu DS, Tsao SW, Hsu FY. (2013). Hyaluronan-cisplatin conjugate nanoparticles embedded in Eudragit S100-coated pectin/alginate microbeads for colon drug delivery. Int J Nanomedicine 8:2399–407
- Wu X, Xu J, Huang X, Wen C. (2011). Self-microemulsifying drug delivery system improves curcumin dissolution and bioavailability. Drug Dev Ind Pharm 37:15–23
- Yadav PS, Kumar V, Singh UP, et al. (2013). Physicochemical characterization and in-vitro dissolution studies of solid dispersions of ketoprofen with PVP K30 and D-mannitol. Saudi Pharm J 21:77–84
- Zhao L, Zhu B, Jia Y, et al. (2013). Preparation of biocompatible carboxymethyl chitosan nanoparticles for delivery of antibiotic drug. BioMed Res Int 2013:236469. doi: 10.1155/2013/236469