Abstract
Baclofen is a centrally acting skeletal muscle relaxant with a short elimination half-life, which results in frequent daily dosing and subsequent poor patient compliance. The narrow absorption window of baclofen in the upper gastrointestinal tract limits its formulation as extended release dosage forms. In this study, baclofen extended release superporous hydrogel (SPH) systems, including conventional SPH, SPH composite and SPH hybrid (SPHH), were prepared aiming to increase the residence of baclofen at its absorption window. The applicability of different polymers, namely, gellan gum, guar gum, polyvinyl alcohol and gelatin, was investigated in preparation of SPHH systems. The prepared SPH systems were evaluated regarding weight and volume swelling ratio, porosity, mechanical properties, incorporation efficiency, degree of erosion and drug release. In vivo assessment was performed in dogs to evaluate gastric residence time by X-ray studies. In addition, the oral bioavailability of baclofen relative to commercially available Lioresal® immediate release tablets was also investigated. The novel baclofen gellan SPHH cross linked with calcium chloride was characterized by optimum mechanical properties, acceptable swelling properties as well as extended drug release. It also exhibited a prolonged plasma profile when compared to twice daily administered Lioresal®.
Introduction
Several approaches are currently used to achieve increased residence time for the dosage form in the stomach with a controlled release pattern. These approaches include floating systems, high density systems, expanding systems, swelling systems, bioadhesive systems and magnetic systems (Deshpande et al., Citation1996; Whitehead et al., Citation1996; Hwang et al., Citation1998; Mojaverian et al., Citation1988; Streubel et al., Citation2006). Hydrogels were discovered by Wichterle & Lim in 1960 (Wichterle & Lim, Citation1960). Since then, considerable progress was made in the synthesis and the applications of hydrogels, especially in the area of controlled drug delivery technology (Omidian et al., Citation2005b).
Chen et al. prepared superporous hydrogel systems (SPHs) with fast swelling kinetics for the first time (Chen et al., Citation1999). SPH is a three-dimensional network of a hydrophilic polymer that absorbs a large amount of water in a very short period of time due to the presence of interconnected microscopic pores (Chen & Park, Citation1999). These features allow dried SPHs when in contact with water to swell fast to a very large size, developing drug extended gastric retention devices, in order to achieve oral-controlled drug delivery systems (Chen et al., Citation2000; Dorkoosh et al., Citation2002a,Citationb; Polnok et al., Citation2004). However, a fully swollen conventional SPH is mechanically too weak to overcome the gastric contractions (Chen et al., Citation1999).
SPH composites (SPHC) and hybrids (SPHH) were developed to maintain high mechanical strength even after fast swelling and to improve gastric residence (Gemeinhart et al., Citation2000). Park et al. (Citation2001) first introduced SPHCs by modifying conventional SPHs through the addition of strengtheners like cross-linked sodium carboxymethylcellulose (Ac-Di-Sol) or methylcellulose polymer (Chen & Park, Citation2000b; Park et al., Citation2001).
Aiming to enhance the mechanical properties, elastic third generation SPHH was prepared (Omidian et al., Citation2005a; Omidian et al., Citation2006). In SPHH, a second polymer network is incorporated in the SPH frame to form interpenetrating polymer networks (IPN). This is done by including a hybrid agent that can be cross-linked, by adding suitable post cross-linker, after the SPH is formed. Different water-soluble hydrocolloids, including sodium alginate, sodium carboxymethylcellulose and chitosan, have been used as hybrid agents. To induce ionotropic gelation of these hydrocolloids, calcium, iron and phosphates have been used, respectively, as post crosslinkers (Omidian & Rocca, Citation2006).
Baclofen is a centrally acting skeletal muscle relaxant. It is rapidly and extensively absorbed following oral administration, with bioavailability of 70%–85%. It is primarily eliminated by renal excretion in unchanged form (renal clearance: 10–17 L/h) (He et al., Citation2014). Its recommended dose is 15–60 mg daily, maximum of 100 mg daily in divided doses. Baclofen suffers from short plasma half life, about 2–4 h, which results in frequent daily administration and poor patient compliance. It is only commercially available as immediate release tablets such as Lioresal® (Galichet, Citation2005). Baclofen has an absorption window in the small intestine. Hence, it is difficult to be formulated in extended release dosage form because upon arrival to the colon, or even before, absorption will be low or nonexistent (Kriel et al., Citation1997). As previously reported, gastroretentive dosage forms are efficient in developing extended release products for drugs that are characterized by absorption window in the proximal gut like baclofen (Davis, Citation2005).
In this study, different polymers, namely, gellan gum, guar gum, polyvinyl alcohol and gelatin were investigated in the preparation of SPHH systems, where post crosslinking was induced using calcium chloride, glutaraldehyde (glut), borate and glut as post crossilinkers, respectively (Kennedy et al., Citation1984; Alhaique et al., Citation1996; Gliko-Kabir et al., Citation1998; Martucci et al., Citation2006; Kale et al., Citation2011). Furthermore, the feasibility of developing an extended release gastroretentive dosage form by incorporating baclofen, as a model drug, in the prepared SPH systems was investigated. Finally, X-ray examination and in vivo pharmacokinetic study of baclofen from the selected extended release SPH system were carried out in dogs to confirm gastric residence of the hydrogel system up to six hours and to evaluate drug bioavailability in comparison with the commercially available Lioresal® tablet.
Materials and methods
Materials
Baclofen (Batch no. BF/0709008) was procured from Misr Company (Giza, Egypt). Lioresal® tablets (Batch no. T004) were procured from Novartis Pharmaceuticals (Cairo, Egypt). Acrylamide (AM), acrylic acid (AA), N,N methylenebisacrylamide (Bis), ammonium persulfate (APS) and N,N,N,N tetramethylethylenediamine (TEMED) were procured from MP Biomedical (Santa Ana, CA). Pluronic (PF127), alginic acid sodium salt, chitosan (medium molecular weight 200–800 cP), gelatin (Ph Eur grade), gellan gum, glut, methylcellulose (medium viscosity methocel, 27.5–32% methoxyl basis), guar gum, acetonitrile, methanol and hydrochloric acid were procured from Sigma Aldrich Chemical Company (St Louis, MO). Carboxymethylcellulose sodium salt, poly vinyl alcohol (MW: 57 000–66 000), borate and tripolyphosphate were procured from Alfa Aesar (Karlsruhe, Germany). Sodium bicarbonate, acetic acid (ac. a), calcium chloride, absolute ethanol and ferric chloride (FeCl3) were procured from Prolabo (Briare, France). Potassium dihydrogen phosphate was procured from EMerck (Darmstadt, Germany). Barium sulfate was procured from Elnasr Chemical (Giza, Egypt). All materials were used as received, and all solutions were prepared using double-distilled water.
Preparation of baclofen SPH systems
The composition of different SPH systems are listed in . SPHs were synthesized by synchronization between two processes: polymerization and foaming processes (gas blowing technique) (Chen et al., Citation1999; Omidian et al., Citation2006). AM solution was poured into a glass test tube (11.0 mm internal diameter and 100 mm height). Bis solution was added and then the glass tube was shaken using vortex shaker. To this combined solution, 25 mg of baclofen and PF127 were added. The mixture was shaken and adjusted to pH 5.1 using AA. To initiate the polymerization (gelling) at room temperature, APS and TEMED were added as an initiator and a catalyst, respectively. The NaHCO3/acid system was used as a special trigger system for foam synthesis. Sodium bicarbonate (30 mg) was added to the reaction mixture 45 s after the addition of APS with shaking for 10 s. The addition of sodium bicarbonate was followed by foaming and gelation, which occurred over a period of 50–75 s. The foam stayed stable at its maximum height. Then the hydrogel was removed from the test tube after 10 min and dried out to a constant weight in an oven at 50 °C overnight.
Table 1. Composition of baclofen hydrogel systems (SPHs).
Baclofen SPHC systems were prepared using similar components and procedure as used in the preparation of SPHs, but an aqueous solution of the strengthener (10% methocel) was added before the initiation of the polymerization using APS/TEMED. The formed SPH was dried to a constant weight at room temperature for five days.
Similarly, baclofen SPHH systems were prepared using similar components and procedure, but different types of hybrid agents were used, also the pH was adjusted to 5.1 using ac. a. After removal of the hydrogel from the test tube, it was immersed immediately for 30 min in an aqueous solution of post crosslinker (depending on the type of strengthener). Then, it was dried to constant weight in oven at 50 °C overnight.
Morphological examination
About 200 mg of the prepared SPH, SPHC and SPHH systems in cylindrical shapes were photographed after being fully swollen in 0.01 N HCl at ambient temperature. The inner porous structure of the SPH, SPHC and SPHH systems was examined using scanning electron microscope (SEM) (Jeol, Jxa-840A, Tokyo, Japan). The dried hydrogels were cut and fixed on a brass stub using double-sided tape and then gold coated in vacuum by a sputter coater before imaging by the SEM.
Porosity measurements
The porosity of the prepared SPH, SPHC and SPHH systems was measured using the solvent replacement method. The dried hydrogel was immersed in absolute ethanol for 4 h and weighed after blotting excess ethanol on the surface. The porosity % was calculated based on the following equation (Yin et al., Citation2007):
where M1 and M2 are the mass of the hydrogel before and after being immersed in absolute ethanol, respectively, ρ is the density of ethanol and V is the initial volume of the hydrogel before being immersed in absolute ethanol and was determined by the multiplication of the area of base (πr2, where r is the radius of the circular base) by the height. The entire process was performed in triplicate.
Determination of apparent density
The apparent density (d) was calculated by dividing the weight of dried sample (Wd) over the volume of dried sample Vd (Chen et al., Citation1999). The entire process was performed in triplicate.
Swelling studies
The hydrogel samples were weighed and measured (diameter and length) and then immersed into the swelling medium (0.01 N HCl) at room temperature. At predetermined time points, the sample was taken out of the swelling medium and excess HCl was removed from the surface using filter paper. The weight, diameter and length of swollen samples were measured. The weight swelling ratio (Qw) is determined as (Chen & Park, 2000a):
where Ws is the weight of swollen SPH sample and Wd is the weight of dried SPH sample. The volume swelling ratio (Qv) is determined as (Qiu & Park, Citation2003):
where Vs is the volume of swollen SPH sample and Vd is the volume of dried SPH sample. The time for SPH sample to reach the equilibrium swollen state could be defined as the swelling time. The curves of weight and volume swelling ratios were plotted to compare the swelling kinetics of hydrogel samples. The entire process was repeated to get three values, and the average was calculated.
Tensile strength testing
Mechanical properties of swollen SPH systems were examined using the Instron series IX automated materials testing system (Buckinghamshire, UK) (Qiu & Park, Citation2003). The hydrogel samples were cut into certain lengths of cylindrical shape with a radius of 1 cm and a height of 1 cm, then they were allowed to swell in 0.01 N HCl up to equilibrium. The swollen hydrogel samples were mounted on the testing system. Both maximum loaded force (N) and the displacement (i.e. elongation in mm) at breaking point were determined. All the tests were carried out under test speed of 20.0 mm/s. The entire process was performed in triplicate.
Erosion determination
SPHs were cut into uniform cylindrical samples then were placed in an USP dissolution vessel containing 1000 ml of 0.01 N HCl (pH = 2) at 37 ± 0.5 °C, and the apparatus was stirred at 100 rpm using paddle. At predetermined time points, 0, 0.5, 1, 2, 3, 4 and 6 h, the samples were taken out and dried in an oven at 40 °C until reaching a constant weight. The degree of erosion E% (expressed as percentage erosion of the polymer content) (Durig & Fassihi, Citation2002; Avachat & Kotwal, Citation2007) was determined using the following equation:
where Wi and Wf are the masses of the initial and the same dried and partially eroded sample, respectively. The process was performed in triplicate.
Estimation of incorporation efficiency
An accurately weighed amount of the dried SPH system expected to contain 25 mg of baclofen was added to a beaker containing 250 ml of 0.01 N HCl and stirred for 24 h. Sample of 5 ml was withdrawn and assayed for baclofen content by determining the absorbance at λmax = 220. The entire process was performed in triplicate. The incorporation efficiency was calculated using the following equation
In vitro release of baclofen from SPH systems
In vitro release studies of baclofen from the prepared baclofen gastroretentive SPH systems (containing 25 mg baclofen) were performed in a 1000 ml dissolution medium of 0.01 N HCl at pH 2 for 6 h, kept at 37 ± 0.5 °C and stirred at 100 rpm, using USP dissolution apparatus, Apparatus II. For comparison, drug release was performed from the commercially available Lioresal® tablets (containing 25 mg baclofen). A 5-ml sample was withdrawn and filtered through a 0.45 µm filter and was replaced with fresh dissolution medium at 0, 0.25, 0.5, 1, 1.5, 2, 2.5, 3, 4, 5 and 6 h. The amount of baclofen released was determined spectrophotometrically at λmax 220 nm.
X-ray examination
The study was conducted to confirm gastric residence of the hydrogel system up to six hours in dogs under fed condition. The study was approved by the Cairo University Ethics Committee. On the day of the study, the animals were fasted for 10 h but had free access to water. Then, each dog was given a standard meal (450 g of canned food) before the oral administration of the treatment without water. The gastric retention of the optimum extended release SPH system was examined in dogs using X-ray machine (General Electric Corporation, Fairfield, CT). In each baclofen SPH system (cylindrical shape with diameter 1 cm and height 0.5 cm), a total of three BaSO4 pellet markers were incorporated (Chen et al., Citation2000). Two systems were administered to the dog to ensure the results, and the X-ray pictures were taken at time intervals of 0, 3 and 6 h.
In vivo pharmacokinetic evaluation
The objective of this study is to compare the pharmacokinetics of baclofen in dogs after the oral administration of Lioresal® commercially available immediate release tablets containing 25 mg baclofen (reference product) and the selected extended release gastroretentive SPH system containing 50 mg baclofen (test product) under fed condition. The study was approved by the Cairo University Ethics Committee.
The study was conducted in randomized cross over design. The dogs (n = 6) were divided randomly into two groups. In period one, one group administered the test product and the other administered the reference product. After one week washout period, the reverse product was administered. The two treatments were administered as follows: two tablets Lioresal® were administered, one tablet every 8 h (q8h), whereas, the test product was only administered in a single dose. On the day of the study, the animals were fasted for 10 h but had free access to water. Then, each dog was given a standard meal (450 g of canned food) before the oral administration of the treatment without water.
Blood samples (4.0 ml) were collected from the dogs’ forearm cubital vein using a hypodermic syringe via an indwelling venous cannula over a period of 24 h according to the following time intervals: 0 (predose), 1, 2, 3, 4, 6, 8, 9, 10, 11, 12 and 24 h after drug administration. The plasma was immediately obtained by centrifuging the blood samples at 3000 rpm for 5 min., then transferred to labeled tubes and stored −20 °C, before baclofen analysis by HPLC. The quantitative determination of baclofen was performed using a validated reversed-phase high-performance liquid chromatographic method reported by Rustum (R2 = 0.9915 and cover the range 50–1600 ng/ml) (Rustum, Citation1989).
Pharmacokinetic parameters for baclofen, following oral administration of the treatments, were determined from the plasma concentration time data by means of a model-independent method using Kinetica® software version 5 (Thermo Fischer Scientific, Waltham, MA). The maximum drug concentration (Cmax, µng/ml) and the time to reach Cmax (Tmax, h) were obtained from the individual plasma concentration–time curves. The area under the curve AUC0–24 (µg h/ml) was determined as the area under the plasma concentration–time curve up to the last measured sampling time and calculated by the linear trapezoidal rule. The area under the curve from zero to infinity AUC0–∞ (µg h/ml) was calculated according to AUC0–∞ = AUC0–24 + Ct/K where Ct is the last measured concentration at the time t. All the results were expressed as mean ± standard deviation. The pharmacokinetic parameters Cmax, AUC0–24 and AUC0–∞ were compared between treatments statistically using ANOVA with a level of significance of 0.05, and a p value smaller than 0.05 was considered significant. The percent relative bioavailability (F) of baclofen from the tested preparation in comparison to the reference preparation was calculated with respect to AUC0–24 and AUC0–∞ (Rouge et al., Citation1998).
Results and discussion
Morphological examination
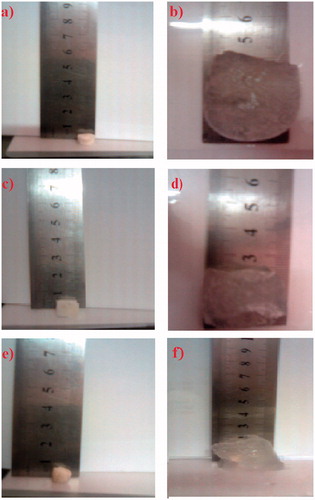
depict the morphological appearance of baclofen SPH system (formula 1S) in the dried and wet (swollen) states, respectively. It could be observed that the dried state of 1S system appear to be white and rigid. System 1S exhibited the largest increase in volume among the tested systems, and the system appeared transparent. As shown in , the dried state of formula 2SC was whiter than formula 1S. Furthermore, the swollen state was more opaque and showed smaller increase in volume after swelling when compared to formula 1S (). Such a difference between SPH (1S) and SPHC (2SC) systems could be attributed to the presence of methocel in system 2SC. The morphological appearance of SPHH system (9SH) in the dried and swollen states is shown in , respectively. It could be observed that the dried state of 9SH system appeared to be yellowish white, and it showed the least swelling volume compared to other systems.
Figure 1. Photographs of initial dried state (a, c and f) and fully swollen states after immersion in 0.01 N HCl at ambient temperature (b, d and f) of SPH (formula 1S), SPHC (formula 2SC) and SPHH (formula 9SH) systems, respectively.
The inner structures of SPH systems were examined under SEM at magnification (40×). shows the detailed morphology of SPH system (1S). The inner surface of 1S system was highly porous and showed the largest pore size relative to the other systems. As clearly shown in , the SPHC system (2SC) showed a porous surface with a pore size smaller than the pore size of SPH system (1S). Many of the capillary channels were partially closed. Finally, SPHH system (9SH) possessed porous surface with the smallest pore size compared to other systems ().
Porosity measurements
Porosity percent is a key characteristic, controlling the swelling rate behavior of SPH systems. Generally, high value of the porosity percent leads to fast swelling manner of SPH, SPHC and SPHH systems. It is obvious from that the porosity percent of SPH formula (1SH) was the highest (74.676 ± 1.52%) among all systems followed by SPHC formula 2SC (68.515 ± 1.12%). SPHH showed the smallest porosity ranging from 50.474 ± 2.36% to 63.046 ± 3.00%. This was in accordance to the SEM photos as previously shown (). The addition of the strengthener in the synthesis of SPHC or SPHH systems did not result in the loss of the high porosity manner. Despite that the introduction of strengthener enhanced the viscosity of the reaction solution, which could result in retention of more bubbles in the polymer and save the gel porosity, yet the presence of too much strengthener would lead to the inflation and blowing out of many of the formed bubbles; and thus fewer pores are maintained in the polymer, which accounts for the decreased porosity of SPHC or SPHH (Yin et al., Citation2007).
Table 2. Porosity, apparent density, tensile strength testing and incorporation efficiency of baclofen SPH, SPHC and SPHH systems.
Determination of apparent density
Measured density is related to the porosity of the prepared systems. As shown in , the apparent densities of dried SPH system was the lowest (0.416 ± 0.15 g/cm3), since SPH systems are very porous as observed by SEM (), followed by that of dried SPHC system (0.756 ± 0.2115 g/cm3). Dried SPHH systems possessed the highest apparent densities (ranged from 0.830 to 0.995 g/cm3). These results are in accordance with the differences in the number and the size of the pores between the different prepared systems.
Swelling studies
The results of weight and volume swelling ratios after immersion in the swelling medium are shown in and . It is worthy to note that, in spite the difficulty in determination of the volume of the swollen system, the results of volume swelling ratio are very critical in determining the gastroretentive properties of SPH by preventing it from passing through the pyloric sphincter. Our target was to formulate a SPH system that is characterized by fast swelling (20 min or less) to prevent premature empting from the stomach. Furthermore, the system must swell to a suitable size and maintain its swelling for an appropriate period of time (Chen et al., Citation2000).
Figure 3. Weight swelling ratio (Qw) of SPH system formulae 1S (a), SPHC system formula 2SC (a) and SPHH systems (b).
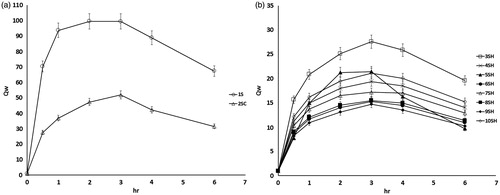
Figure 4. Volume swelling ratio (Qv) of SPH system formulae 1S (a), SPHC system formula 2SC (a) and SPHH systems (b).
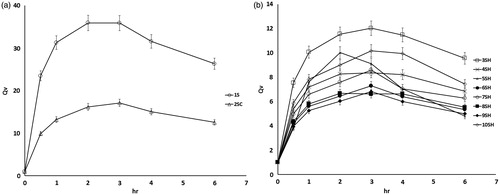
Generally, it is observed that the swelling of all systems gradually increased until reaching equilibrium and then gradually decreased. SPH system (formula 1S) possessed the highest swelling profile with a strong absorbing capacity. The weight and volume swelling ratios were 70 and 23.5 times, respectively, after 0.5 h and reached their maximum values of 100 and 36 times, respectively, at 2 h. This result is related to the highly porous structure of the system, which allowed the rapid diffusion of large amount of swelling medium.
The weight and volume swelling ratios of SPHC system (formula 2SC) was lower than that of SPH system (formula 1S). The weight and volume swelling ratios were 28 and 10 times, respectively, after 0.5 h and reached their maximum values of 52 and 17 times, respectively, at 3 h. This could be attributed to porosity reduction and density increase of formula 2SC after the addition of methocel in the system.
The presence of interpenetrating network greatly reduced the weight and volume swelling ratios of the SPHH systems by decreasing its porosity. The weight swelling ratio ranged from 8 to 16 times after 0.5 h and exhibited its maximum values at 3 h and ranged from 15 to 28 times. Volume swelling ratio ranged from 4 to 8 times after 0.5 h and reached their maximum values after 3 h and ranged from 7 to 12 times except for formulae 5SH, 4SH and 8SH, which showed their maximum values at 2 h and were 10, 8 and 7 times, respectively. Although SPHH systems possess lower volume swelling ratio, these ratios could potentially satisfy the needs required for the gastric retention property because their lengths in swollen state were greater than 15 mm as shown in (Davis, Citation2005).
Tensile strength testing
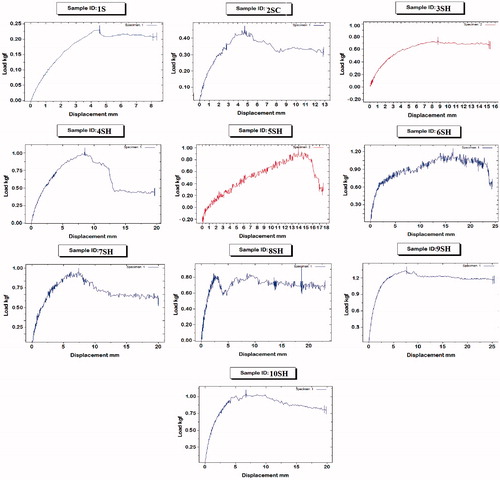
Not only fast and large swelling is important in developing gastroretentive SPH systems but also the mechanical properties of the system are of great importance. Our target was to formulate a SPH system that maintains high mechanical strength even after fast swelling to large size so as to overcome pressure resulting from the contractions of the stomach (Chen et al., Citation2000). The results of tensile strength testing are displayed in and illustrated graphically in .
It is clear that the tensile strength of SPH formula 1S was the lowest among all systems. It showed a maximum force of only 2.5 N and displacement of 8 mm at the breaking point. The tensile strength of SPHC system (formula 2SC) was higher than that of SPH (formula 1S). It exhibited a maximum force of 4 N and displacement of 12.8 mm at breaking point. The incorporation of methocel in the preparation of SPHC system (formula 2SC) increased the overall cross-linking density of the SPHC by physical entanglement of the polymer chains with methocel fibers (Park et al., Citation2001). This structure is expected to share the mechanical load between methocel fibers and the polymer structure resulting in increased tensile strength.
In an attempt to achieve further improvement in mechanical strength while keeping the fast swelling kinetics, SPHH systems were prepared and several pairs of strengthener and post crosslinkers were examined as shown in . These systems showed maximum force ranging from 8 to 12 N and displacement ranging from 15.5 to 25.133 mm at the breaking point. The improved mechanical strength of the SPHH systems could be ascribed to the introduction of the interpenetrating network structure. The entanglement of acrylate chains with strengthener fibers improved the structural integrity of the hydrogel, which enhanced its ability to withstand pressure (Qiu & Park, Citation2003). The difference between the various systems could be attributed to the different mechanical properties of the interpenetrating network resulted from each pair of strengthener and post crosslinker used. Formula 9SH system, prepared with gellan gum as strengthener and calcium chloride as post crosslinker, showed the highest tensile strength properties. It exhibited maximum force of 12 N and a displacement of 25.133 mm at the breaking point.
Erosion determination
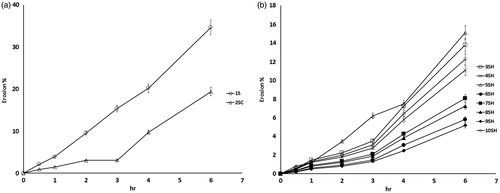
A swellable matrix will start to erode when hydration is severe (highly swollen) because the interchain intermolecular forces will no longer be able to resist any external forces. Once the hydrogel erodes, it breaks up into smaller pieces, exposing more surface to the fresh swelling medium and hence more drug will be released (Avachat & Kotwal, Citation2007). The time-dependent erosion behavior of all prepared formulae is shown in . It is clear that erosion of all the systems increased progressively with time and was dependent on the composition of the studied systems.
SPH system (formula 1S) showed the fastest and largest degree of erosion. The erosion reached 9.5% after 2 h and increased linearly with time up to 34.7% after 6 h. This result was expected taking into consideration the highly porous structure and low tensile strength of the system, which accelerated liquid uptake and facilitated erosion process.
The SPHC system (formula 2SC) exhibited slower and smaller percentage of erosion when compared to SPH system (formula 1S). Initially, the erosion was slow and reached 3% after 3 h, then there was sudden increase in the rate of erosion and reached 19.4% after 6 h.
Generally, the SPHH systems showed the slowest and lowest percentage of erosion compared with SPH and SPHC systems. The relatively low porosity together with denser interpenetrating network structure greatly reduced the erosion behavior. System 9SH exhibited the lowest percentage of erosion among the other SPHH systems. The percentage of erosion increased gradually till it reached 5.178% after 6 h. This result correlates with the previously discussed results.
Estimation of incorporation efficiency
The incorporation efficiency of SPHC formula 2SC (84.01%) was substantially higher than SPH system in formula 1S (74.76%). SPHH systems possessed higher incorporation efficiency percentage (ranged from 89.82% to 94.21%). This could be attributed to the higher permeability behavior of the SPH and SPHC systems when compared to the SPHH systems in terms of greater number and size of pores, which might facilitate the diffusion of a part of the entrapped drug into the surrounding medium during the preparation process.
In vitro release of baclofen from SPH systems
The results of the release of baclofen from the different prepared SPH systems are illustrated graphically in . It is clear that the commercially available Lioresal tablets showed fast release of baclofen, where the time of 80% dissolution (T80%) was about 0.5 h. The SPH system (formula 1SC) showed the fastest release of baclofen (T80% = 1.5 h) among other SPH systems. It is worthy to note that the development of extended release gastroretentive dosage form of baclofen is highly challenging taking into consideration its high solubility in acid medium (25 mg/ml in 0.1 M HCL) (Gande & Rao, Citation2011).
Figure 7. Dissolution profiles of SPH system formulae 1S (a), SPHC system formula 2SC (a) and SPHH systems (b).
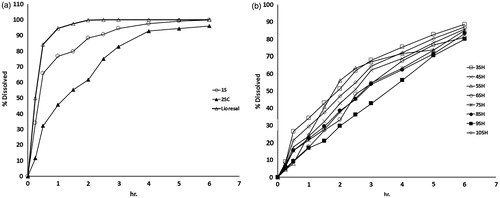
Baclofen SPHC systems (formula 2SC) possessed a slower release rate (T80% = about 3 h) than SPH system (formula 1S) and the commercially available Lioresal® tablets. The incorporation of methocel as strengthener improved the mechanical properties and reduced the erosion percentage of baclofen SPHC systems. Furthermore, the less porous structure of SPHC system (formula 2SC) might have resulted in lower penetration of the dissolution medium. Hence, lower drug diffusion and more delay in drug release were observed.
As anticipated from the previous results, baclofen SPHH systems showed the most extended release of baclofen, where T80% was about 6 h. These results were attributed to the relative low porosity, the denser IPN structure and the high tensile strength of SPHH systems. The alginate SPHH system (formula 3SH) exhibited comparably the fastest baclofen release rate among the SPHH systems. On the other hand, gellan gum SPHH (formula 9SH) showed the slowest baclofen release rate. Moreover, baclofen release from gellan gum SPHH (formula 9SH) followed zero-order kinetics (data are not shown). Typically, zero-order release kinetics is desirable for extended release dosage forms (Hardy et al., Citation2007).
X-ray examination
As previously discussed, gellan gum SPHH (formula 9SH) exhibited the most extended release profile of baclofen, showing the most optimum tensile strength properties as well as acceptable swelling results. Accordingly, it was selected for the X-ray examination and in vivo pharmacokinetic evaluation.
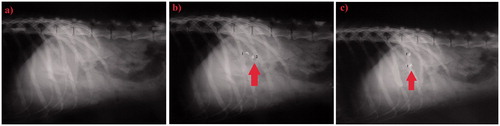
shows the control stomach with no X-ray markers at 0 h, while were taken after 3 and 6 h, respectively, following the oral administration of two 9SH systems to the dog. It is clear that the amount of X-ray opaque material in these pellets was sufficient to ensure visibility by X-ray. The 9SH system remained intact where all the three BaSO4 pellet markers maintained their positions in the stomach of the dog (indicated by arrows on ). Therefore; it could be concluded that baclofen 9SH system could be considered as a successful gastroretentive dosage form throughout the studied period of six hours.
In vivo pharmacokinetic evaluation
Plasma concentrations of baclofen versus time profile following oral administration of Lioresal® tablets and gellan SPH system (9SH) in dogs are illustrated in . The plasma concentrations time curve of Lioresal® tablet was characterized by the occurrence of two peaks, corresponding to the twice daily administration of 25 mg Lioresal® tablet.
Figure 9. Mean plasma concentration-time curve of baclofen after q8h oral administration of Lioresal® tablets (25 mg baclofen) and q.d. oral administration of formula 9SH gellan gum SPHH system (50 mg baclofen) to six dogs under fed condition.
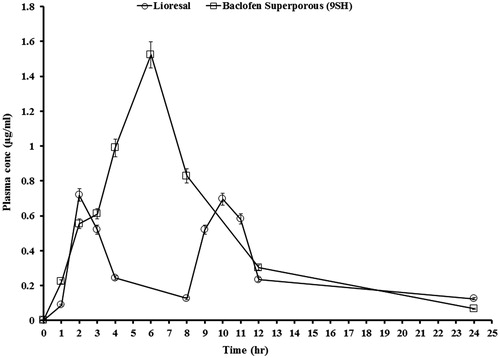
The mean pharmacokinetic characteristics are summarized in . The values of the Cmax of baclofen following the administration of two Lioresal® tablets (each contain 25 mg baclofen) were 0.72 and 0.70 µg/ml for the first and second peaks, respectively. Cmax of baclofen following administration of single dose gellan SPH (9SH) system (containing 50 mg baclofen) was 1.52 µg/ml. Tmax values were 2 and 6 h, following the administration of two Lioresal® tablets and single dose gellan SPH (9SH) system, respectively. The AUC0–24 increased from 6.18 (two doses of Lioresal® tablets) to 10.64 µg h/ml after administration of single 50 mg oral dose of baclofen gellan SPH (formula 9SH). Similarly, AUC0–∞ increased from 7.38 (two doses of Lioresal® tablets) to 11.08 µg h/ml after administration of single 50 mg oral dose of baclofen gellan SPH (formula 9SH).
Table 3. Mean pharmacokinetic parameters ± SD of baclofen after oral administration of 25 mg in Lioresal tablets or 50 mg in SPHH system (9SH) to six dogs under fed conditions.
Statistical analysis of pharmacokinetic parameters showed that there were significant differences (p < 0.05) between the values of Tmax, AUC0–24 and AUC0–∞ of gellan SPH preparation (9SH) when compared to two doses of the commercially available immediate-release Lioresal® tablets. The mean percent relative bioavailability (F) of baclofen from the prepared SPH preparation (9SH) with respect to AUC0–24 and AUC0–∞ were 172.2% and 150.07%, respectively.
Finally, it could be concluded that the gastroretentive extended release of baclofen from gellan gum SPHH system (formula 9SH) for a period of 6 h resulted in retardation in drug absorption and higher bioavailability as evident by the significant delayed Tmax and higher AUC0–24 and AUC0–∞.
Conclusions
A potential baclofen gastroretentive system intended for once daily oral administration was successfully developed. The fast swelling rate, optimum tensile strength, gastroretentive properties and extended drug release from gellan gum SPHH system (9SH) resulted in a retardation of drug absorption as well as increased bioavailability with a prolonged plasma profile following single oral dose administration compared to twice daily administration of the commercially available Lioresal® immediate release tablet.
Declaration of interest
The authors report no declarations of interest.
References
- Alhaique F, Santucci E, Carafa M, et al. (1996). Gellan in sustained release formulations: preparation of gel capsules and release studies. Biomaterials 17:1981–6
- Avachat A, Kotwal V. (2007). Design and evaluation of matrix-based controlled release tablets of diclofenac sodium and chondroitin sulphate. AAPS Pharm Sci Tech 8:51–6
- Chen J, Blevins WE, Park H, Park K. (2000). Gastric retention properties of superporous hydrogel composites. J Control Release 64:39–51
- Chen J, Park H, Park K. (1999). Synthesis of superporous hydrogels: hydrogels with fast swelling and superabsorbent properties. J Biomed Mater Res A 44:53–62
- Chen J, Park K. (2000a). Synthesis of fast-swelling, superporous sucrose hydrogels. Carbohydr Polymer 41:259–68
- Chen J, Park K. (2000b). Synthesis and characterization of superporous hydrogel composites. J Control Rel 65:73–82
- Chen JUN, Park K. (1999). Superporous hydrogels: fast responsive hydrogel systems. J Macromol Sci A 36:917–30
- Davis SS. (2005). Formulation strategies for absorption windows. Drug Discov Today 10:249–57
- Deshpande AA, Rhodes CT, Shah NH, Malick AW. (1996). Controlled rlease drug delivery system for prolonged gastric residence: an overview. Drug Dev Ind Pharm 22:531–9
- Dorkoosh FA, Borchard G, Rafiee-Tehrani M, et al. (2002a). Evaluation of superporous hydrogel (SPH) and SPH composite in porcine intestine ex-vivo: assessment of drug transport, morphology effect, and mechanical fixation to intestinal wall. Eur J Pharm Biopharm 53:161–6
- Dorkoosh FA, Verhoef JC, Ambagts MHC, et al. (2002b). Peroral delivery systems based on superporous hydrogel polymers: release characteristics for the peptide drugs buserelin, octreotide and insulin. Eur J Pharm Sci 15:433–9
- Durig T, Fassihi R. (2002). Guar-based monolithic matrix systems: effect of ionizable and non-ionizable substances and excipients on gel dynamics and release kinetics. J Control Rel 80:45–56
- Galichet LY. (2005). Clarke's analysis of drugs and poisons. : Pharmaceutical Press
- Gande S, Rao Y. (2011). Sustained-release effervescent floating matrix tablets of baclofen: development, optimization and in vitro-in vivo evaluation in healthy human volunteers. Daru 19:202–9
- Gemeinhart RA, Chen J, Park H, Park K. (2000). pH-sensitivity of fast responsive superporous hydrogels. J Biomater Sci Polym 11:1371–80
- Gliko-Kabir I, Yagen B, Penhasi A, Rubinstein A. (1998). Low swelling, crosslinked guar and its potential use as colon-specific drug carrier. Pharm Res 15:1019–25
- Hardy IJ, Windberg-Baarup A, Neri C, et al. (2007). Modulation of drug release kinetics from hydroxypropyl methyl cellulose matrix tablets using polyvinyl pyrrolidone. Int J Pharm 337:246–53
- He Y, Brunstrom-Hernandez JE, Thio LL, et al. (2014). Population pharmacokinetics of oral baclofen in pediatric patients with cerebral palsy. J Pediatr. [Epub ahead of print]
- Hwang SJ, Park H, Park K. (1998). Gastric retentive drug-delivery systems. Crit Rev Ther Drug Carrier Syst 15:243–84
- Kale SN, Mona J, Dhobale S, et al. (2011). Intramolecular and intermolecular crosslinked poly(vinyl alcohol)–borate complexes for the sustained release of fertilizers and enzymes. J Appl Polymer Sci 121:2450–7
- Kennedy JF, Kalogerakis B, Cabral JMS. (1984). Surface immobilization and entrapping of enzymes on glutaraldehyde crosslinked gelatin particles. Enzyme Microb Technol 6:127–31
- Kriel RL, Krach LE, Hoff DS, et al. (1997). Failure of absorption of baclofen after rectal administration. Pediatr Neurol 16:351–352
- Martucci JF, Ruseckaite RA, Vázquez A. (2006). Creep of glutaraldehyde-crosslinked gelatin films. Mater Sci Eng A 435–436:681–6
- Mojaverian P, Vlasses PH, Kellner PE, Rocci M. (1988). Effects of gender, posture and age on gastric residence time of an indigestible solid: pharmaceutical considerations. Pharm Res 5:639–44
- Omidian H, Qiu Y, Yang S, et al. (2005a). Hydrogels having enhanced elasticity and mechanical strength properties. US Patent 6960617
- Omidian H, Rocca JG. (2006). Formation of strong superporous hydrogels. US Patent 7056957
- Omidian H, Rocca JG, Park K. (2005b). Advances in superporous hydrogels. J Control Rel 102:3–12
- Omidian H, Rocca JG, Park K. (2006). Elastic superporous hydrogel hybrid of polyacrylamide and sodium alginate. Macromol Biosci 6:703–10
- Park K, Chen J, Park H. (2001). Hydrogel composites and superporous hydrogel composites having fast swelling, high mechanical strength, and superabsorbent properties. US Patent 6271278
- Polnok A, Verhoef JC, Borchard G, et al. (2004). In vitro evaluation of intestinal absorption of desmopressin using drug-delivery systems based on superporous hydrogels. Int J Pharm 269:303–10
- Qiu Y, Park K. (2003). Superporous IPN hydrogels having enhanced mechanical properties. AAPS Pharm Sci Tech 4:406–12
- Rouge N, Allemann E, Gex-Fabry M, et al. (1998). Comparative pharmacokinetic study of a floating multiple-unit capsule, a high-densitymultiple-unit capsule and an immediate-release tablet containing 25 mg atenolol. Pharmaceutica Acta Helvetiae 73:81–7
- Rustum AM. (1989). Simple and rapid reversed-phase high-performance liquid chromatographic determination of baclofen in human plasma with ultraviolet detection. J Chromatogr 487:107–15
- Streubel A, Siepmann J, Bodmeier R. (2006). Gastroretentive drug delivery systems. Expert Opinion Drug Deliv 3:217–33
- Whitehead L, Fell JT, Collett JH. (1996). Development of a gastroretentive dosage form. Eur J Pharm Sci 4:S182
- Wichterle O, Lim D. (1960). Hydrophilic gels for biological use. Nature 185:117–8
- Yin L, Fei L, Tang C, Yin C. (2007). Synthesis, characterization, mechanical properties and biocompatibility of interpenetrating polymer network – superporous hydrogel containing sodium alginate. Polymer Int 56:1563–71