Abstract
Context: Artemether and lumefantrine combination therapy is well-accepted for uncomplicated malaria treatment. However, the current available formulation has several pharmacokinetic mismatches such as drug degradation in gastrointestinal tract, erratic absorption, etc. Hence, need of the hour is the injectable formulation, which can overcome the pharmacokinetic mismatch associated with current available formulation in the market.
Objective: To fabricate artemether and lumefantrine co-loaded injectable nanostructured lipid carriers (NLCs) formulation.
Materials and methods: Artemether and lumefantrine co-loaded NLCs were fabricated using homogenization followed by ultra-sonication method. Fabricated NLCs were evalauated for their physicochemical characteristics, and suitability of the formulation for malaria treatment was evaluated using in vivo animal model (Plasmodium berghei-infected mice).
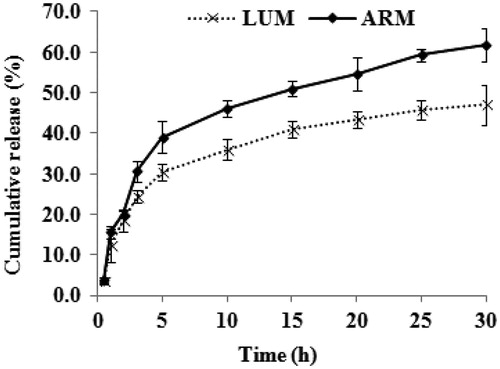
Results, discussion and conclusion: Artemether and lumefantrine co-loaded NLCs had a hydrodynamic diameter of ∼145 nm with the surface charge of −66 mV. Due to the lipophilic nature of both antimalarial drugs, both single drugs-loaded and co-loaded NLCs have shown high encapsulation efficiency, which is 84% for artemether and 79% for lumefantrine. In vitro drug release study has shown a biphasic drug release pattern, which has shown 63% artemether release and 45% of lumefantrine release over a time period of 30 h. Plasmodium berghei-infected mice treated with artemether and lumefantrine co-loaded NLCs showed better antimalarial activity with respect to parasitemia progression and survivability period.
Introduction
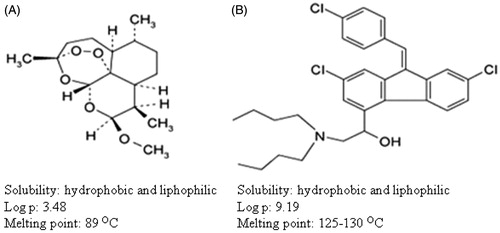
Malaria, once said to be burden to developing countries, is now wide spread in the developed and developing countries also. The major hurdle in fighting malaria is the development of rapid drug resistance, wide spread presence and limited participation from private sector due to the lack of economic benefits. In addition, bringing new drug into the market takes a long time and requires lot of investment (Aditya et al., Citation2012). To overcome this problem, proper utilization of currently existing drugs and enhancing the therapeutic efficiency of these drugs are of prime importance. In this stair, combination therapy is preferred over monotherapy for malaria treatment; and among various combination therapies, artemisinin-based combination therapy is the most preferred one (Nandakumar et al., Citation2006). Artemisinin-based combination therapy mainly involves the partnering of two antimalarial drugs with distinct site of action or mode of action. In principle, artemisinin or its derivative acts first and rapidly reduces the parasite burden, and the partner drug, clears the remaining parasites in blood or from the parasite reservoir and avoid the recrudescence. Among the artemisinin combination therapy, artemether and lumefantrine () combination is the first-line treatment for uncomplicated malaria due to its non toxicity, cost effectiveness and high success rate (Egunsola & Oshikoya, Citation2013). This combination therapy consists of artemether and lumefantrine at 1:6 ratios. In this combination, artemether first acts on the malaria parasites and reduces the parasite burden instantly, later lumefantrine acts on the remaining parasites or the parasites, which comes from the hibernation, which are mostly merozoites. Currently, artemether and lumefantrine combination are available as tablets for oral administration. Even though this combination has high success rate, the major disadvantage in the oral dosage form is the (a) requirement to take this drugs along with fat supplemented food to avoid low and/or erratic absorption, (b) requirement of drug administration twice a day and (c) degradation of drugs in acidic condition, etc. (Yeung et al., Citation2004). These problems propose the need for the development of an injectable formulation of artemether and lumefantrine, which is not available at present. However, high hydrophobicity and the pharmacokinetic mismatch of this combination (wide difference in their plasma half life) make the formulation of conventional injectable unsuitable for effective management of the disease particularly to overcome the problem of controlled release, bioavailability, drug degradation, etc. These situations warrant the application of novel drug delivery approaches, which have shown success in the past in overcoming the pharmacokinetic mismatches such as controlled release, bioavailability, stability, etc. to other drug molecules, which are in use to treat similar parasitic infections in general and malaria in particular (Date et al., Citation2007; Santos-Magalhães & Mosqueira, Citation2010; Aditya et al., Citation2013b). Thus, the aim of this research is to fabricate injectable (intraperitoneal; i.p.) co-loaded (artemether and lumafentrine) nano lipid drug delivery system to get the following benefits: (a) route of administration: fabrication of injectable formulation will avoid degradation of drugs in gastrointestinal tract, dependency of bioavailability on the fat intake, low patient compliance and inconsistent assimilation etc. (Aditya et al., Citation2010), (b) drug delivery systems: among the various drug delivery technologies, lipid nanoparticles were preferred over the other carrier materials in the current formulation due to lipophilicity of core materials (artemether and lumefantrine) and successes in the past in using these lipids as carrier materials in developing injectable delivery systems (Joshi & Muller, Citation2009). In addition, second generation of lipid nanocarriers, i.e. nanostructured lipid carriers (NLCs) were chosen due to their advantages such as enhanced drug loading, cost effectiveness, non-toxicity over the other types of lipid carriers such as liposomes, solid lipid nanocarriers, (c) co-loaded nanoparticles: co-loaded nanoparticles were preferred over the single drug-loaded nanoparticles since, co-loading has proven to increase the therapeutic efficiency of drugs by increasing protection rate, reducing the drug dosage, etc., which was observed in recent studies by our group and others (Aditya et al., Citation2012; Memvanga et al., Citation2013).
To the best of our knowledge, this is the first report that shows the therapeutic benefits of artemether and lumefantrine co-loaded NLCs. The fabricated NLCs were characterized for their physicochemical properties such as particle size, zeta potential, encapsulation, drug loading, morphology which are the indicators of formulation stability and in vitro and in vivo performance. The suitability of fabricated system was studied in vivo using Swiss Albino mice, which were infected with animal malaria parasite model parasite strain Plasmodium berghei.
Materials and methods
Chemicals, reagents and instrumentation
The solid lipid glycerol monostearate (GMS) was obtained from CDH laboratory reagents (New Delhi, India). Soybean oil was provided by Kamani Oil Industries Ltd. (Mumbai, India). Artemether and lumefantrine were procured as a gift sample from IPCA Laboratories Ltd. (Mumbai, India). Field stains A (buffered solution of azure dye) and B (buffered solution of eosin) and dialysis bags (MW: 110 kDa) were procured from HIMEDIA Laboratories Pvt. Ltd., (Mumbai, India).
Preparation of NLCs of artemether and lumefantrine
For fabrication of NLCs containing lipophilic antimalarial drugs (artemether and lumefantrine), homogenization followed by ultra-sonication method was employed as described early with slight modification (Aditya et al., Citation2010). Lipid phase, which constituted of GMS as solid lipid and/or soybean oil as liquid lipid, was heated up to 68 ± 3 °C, which is ∼10 °C above the melting point of the solid lipid. To this lipid phase, aqueous phase, which is also heated up to 68 ± 3 °C composing of double-distilled water and the hydrophilic surfactant Pluronic F68 was added under homogenization, which was carried out using high speed homogenizer (HG-15 D, DAIHAN Scientific, Co., Ltd., Seoul, Korea) for three minutes at 5000 rpm. This warm emulsion was further treated with sonicated using probe-type sonicator (S-400-010, Misonix, Farmingdale, NY) for three minutes. NLCs were obtained by allowing the hot nanoemulsion to cool to room temperature. Artemether and/or lumefantrine-loaded NLCs were produced by the same process with the addition of drugs to the lipid phase. To obtain formulation with minimum size and maximum encapsulation, ratio of solid lipid to liquid lipid was varied, as listed in . Five percent of trehalose was used as cryoprotectant to lyophilize the NLCs after fabrication. Lyophilization was carried out for 24 h at −30 °C and a vacuum of 0.40 mbar, followed by a secondary drying phase of one day at −20 °C.
Table 1. Composition of various formulations.
Physicochemical characterization
Morphology
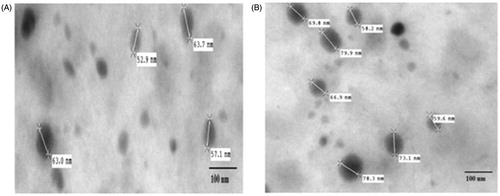
The morphology of the lyophilized NLCs was assessed by using high-resolution transmission electron microscopy (TEM; Hitachi H-7500, Amsterdam, the Netherlands). A drop of particle dispersion which was diluted suitably was spread onto a 200-mesh copper grid coating, and the excess droplets were removed using filter paper and dried at room temperature for 5–10 min. Later, copper grid with NLCs was negatively stained using 2% (w/v) phosphotungstic acid. The grid was dried at room temperature and observed under TEM.
Particle size and zeta potential analysis
Particle size and zeta potential of the formulations was determined after suitable dilution in water by photon correlation spectroscopy (PCS) using particle size analyzer (Delsa Nano C, Beckman Coulter Inc., Tokyo, Japan) at fixed angle of 90° at 25 °C. All measurements were performed in triplicate.
Entrapment efficiency
Entrapment efficiency was determined by mini column centrifugation method. In brief, Sephadex® G 50 solution (10% w/v) was prepared in water and allowed to swell for 24 h. To prepare mini column, swollen Sephadex was added carefully to 1 ml syringe; 100 µl of artemether and/or lumefantrine-loaded NLCs were slowly added on prepared column and centrifuged at 500 rpm for three minutes. Here, un-entrapped drug molecules were bound to the swollen Sephadex beads while drug entrapped NLCs gets eluted out. Amount of drug loaded in NLCs were diluted with methanolic HCl and heated at 80 °C to rupture the NLCs and centrifuged for three minutes at 3000 rpm. Thereafter, the amount of entrapped drug in the supernatant liquid was detected using an ultraviolet-visible spectrophotometer (UV 1700 Pharm Spec, Schimadzu Corporation, Kyoto, Japan) by measuring absorbance at 260 nm for artemether and at 286.2 nm for lumefantrine against the blank as described earlier (Parashar et al., Citation2013). The following equation was used to calculate entrapment efficiency (EE).
where Ainitial drug represents the initially added amount of antimalarial drug and Aentrapped drug represents the amount of antimalarial drug entrapped in the NLCs.
In vitro release of artemether and lumefantrine
In vitro release studies were performed using 110 kDa dialysis membrane. Dialysis bag containing 5 ml of artemether and/or lumefantrine-loaded NLCs was immersed in 20 ml phosphate-buffered saline (pH 7.4, maintained at 37 ± 1 °C and rotation of ∼75 rpm). At pre-determined time intervals, 3 ml of dissolution medium was removed and same volume of fresh medium was replaced. The withdrawn samples were assayed for drug content by measuring absorbance at 260 nm for artemether and at 286.2 nm for lumefantrine against the blank using UV spectrophotometer (UV 1700 Pharm Spec, Shimadzu Corporation, Japan) using the equation.
where drug is either artemether or lumefantrine.
In vivo antimalarial activity testing in P. berghei infected mice
The animal studies were carried out after getting approval from Institutional Animal Ethics Committee under registration no. 816/PO/a/04/CPCSEA. Only male Swiss Albino mice were selected to disallow hormonal influence or any other gender related physiological factors that may obliterate the pharmacological action of the drug. The animals were fed with standard mice cubes and water ad libitum. Six Swiss Albino Mice (25–30 g) were housed per cage in the animal house of I.S.F. College of Pharmacy. Initially, P. berghei-infected mice was procured from National Institute of Malaria research, Delhi, India. When the parasitemia was >60%, blood was collected from infected mice by heart puncturing, and these parasites were injected in the new healthy mice through i.p. route (100 µl) as described earlier (Aditya et al., Citation2010). Mice were divided into six animal groups with six animals each (n = 6), and then all the formulations were given according to , and the observations were recorded for four weeks. Parasitemia level on the day of treatment (three days after the infection) was 2–4%. To evaluate parasite progression, blood was drawn from the tail vein of mice, and blood smears were prepared by staining with field stain A and B and observed under light microscope. Mortality was observed till the end of the study.
Table 2. Distribution of animals for in vivo antimalarial activity testing (n = 6).
Results and discussion
Morphology, particle size and zeta potential analysis
Both SLN and NLCs were lyophilized as lyophilization offers physical and chemical stability against Ostwald ripening and hydrolysis (Mehnert & Mader, Citation2001). White spongy NLCs were formed in case of blank and artemether-loaded NLCs, whereas it was yellow in case of lumefantrine and artemether and lumefantrine co-loaded NLCs. Lyophilized NLCs were easily dispersible in distilled water after hand shaking for ∼1 min, which has shown the suitability of trehalose as an effective cryoprotectant at used concentration (5%). This is in accordance with earlier studies that have shown similar effect of trehalose in maintaining the size and monodispersity of NLCs after lyophilization, which otherwise results in aggregation (Madden et al., Citation1985; Aditya et al., Citation2012). The size of the SLN was 125 ± 3.2 nm, whereas size decreased slightly in NLCs where part of the solid lipid was replaced with liquid lipid (). NLCs with 55% solid lipid and 45% liquid lipid resulted in the formation of monodispersed nanoparticles of 109 ± 1.39 nm particle size. Addition of artemether or/and lumefantrine to the formulation resulted in increase in size of nanoparticles from ∼109 to ∼145 nm. Even though this size increase is significantly different in terms of statistics, it is insignificant as far as biological systems is concerned. Moreover, size and PDI of the formulations remained within the acceptable range for administration of drugs through various injectable routes such as i.p., intravenous, etc., which is generally <200 nm (Nayak et al., Citation2010; Aditya et al., Citation2012).
Table 3. Determination of entrapment efficiency in various formulations.
Furthermore, to study the morphology and to confirm the size, TEM was used (). TEM images showed round-shaped NLCs with the size of 60–80 nm in both drug-loaded and placebo formulations. The diameter of particle observed by TEM is smaller than that from particle size analyzer. The reason may be the formation of hydration layer around the NLCs dispersed in water to measure size in case of PCS method, while the NLCs measured in TEM were taken after drying them on the copper grid, which are void of these hydration layer.
Zeta potential is an important indicator for the stability of the nanoparticles. In this regard, zeta potential value increased from −43 mV in SLN to −62 mV in NLC (). This drift may be due to layering of liquid lipid on the surface of the solid lipid, which were not accommodated within the core of the NLCs or due to the formation of small emulsions or oil droplets. This corroborates with earlier studies, which have shown similar trend of increasing zeta potential along with increase in liquid lipid concentration (Nayak et al., Citation2010). In all the formulations, zeta potential was high enough to stabilize the nanoparticles against aggregation (Teng et al., Citation2013). Addition of either artemether or lumefantrine has no effect on the zeta potential of the nanoparticles.
Determination of encapsulation efficiency
A vital concern regarding the use of nano carriers in drug delivery is their capacity to accommodate and deliver the sufficient therapeutic dosage. Due to lipophilicity of both artemether and lumefantrine, higher encapsulation efficiency was observed () (Amin et al., Citation2013). Encapsulation efficiency was 84.10% when artemether was loaded alone and it was 79.10% when lumefantrine was loaded alone. In co-loaded NLCs, encapsulation efficiency was 82% and 79% for artemether and lumefantrine, respectively. This is an important observation where co-loading has practically no effect on the encapsulation efficiency of each of the drugs. This is in agreement with earlier result, which has shown similar results when curcumin and genistein were co-loaded in NLCs (Aditya et al., Citation2013a).
In vitro release study of artemether and lumefantrine
The cumulative percentages of artemether and lumefantrine released from NLCs were investigated in vitro over a period of 30 h using dialysis bag method using artemether and lumefantrine co-loaded NLCs. After placing the artemether and lumefantrine co-loaded NLCs into the releasing medium, within the first 2 h burst release was observed, which resulted in the release of ∼20% of both drug molecules, which later turned into a sustained release pattern (). At the completion of the release study for 30 h, ∼62% of artemether and ∼47% of lumefantrine was released. It is very difficult to pin point the exact reason behind the burst release. But the presence of drug molecules on the wall of the NLCs, the drug molecules that were solubilized in surfactant or those that are entrapped in emulsion formed from the excessive liquid oil might have contributed to this. Later, sustained release indicates the presence of the drug within the core of the NLCs, which takes time to diffuse out from the internal core region to outer releasing medium. In addition, artemether released quickly compared to lumefantrine. This may be due to (a) high hydrophobicity of lumefantrine (log p = 9.19) compared to artemether (log p = 3.48), which shows the preference of lumefantrine to stay within the hydrophobic core of NLCs rather than releasing into the hydrophilic media (Amin et al., Citation2013), (b) the smaller molecular structure of artemether compared to lumefantrine, which might have diffused easily from the nanostructure core into the releasing media faster than its counterpart. In artemether and lumefantrine combination therapy, artemether first reduces the parasite burden and later lumefantrine helps to clear the parasite and avoid the recrudescence, which perfectly matches with the observed release pattern in our formulation. Since only 45% of lumefantrine was released from the NLCs after 30 h, it strongly indicates that fast release of artemether will help to reduce the parasite burden initially, and lumefantrine will be available for the system for prolonged time period to clear the remaining parasite. Thus, this delivery system is especially suitable for delivery of artemether and lumefantrine combination.
In vivo antimalarial activity testing in P. berghei-infected mice
Percent parasitemia and survival time was used to assess the therapeutic potency of the fabricated NLCs. In co-loaded NLCs, the ratio of artemether to lumefantrine was 1:6 (4:24 mg/kg body weight), which is the standard ratio used in clinical trials to treat malaria patients. Drug was given (single dose) to the infected mice on the third day of the infection when the parasitemia was ∼3%.
As anticipated, blank NLC-treated mice along with control group mice (untreated) showed continuous increase in parasitemia and died without showing any resistance between 7 and 10 d after parasitemia reach >60% (). Even though decrease in parasitemia was observed in artemether alone and lumefantrine alone treated group, both the monotherapy treatment option failed to completely cure the mice from malaria infection. In artemether-loaded NLC-treated group, the parasitemia was ∼31.9 ± 13% on day 19, and all mice died before 28 d. In lumefantrine-loaded NLC-treated groups, on the 19th d, parasitemia was 22 ± 15% leading to death of five mice in a group within 28 d, and only single mice survived up to 28 d. Similar type of enhanced antimalarial activity was observed in earlier studies after encapsulating either artemether or lumefantrine in various carrier systems like NLCs, emulsions, liposome’s (Joshi et al., Citation2008; Santos-Magalhães & Mosqueira, Citation2010; Sosnik & Amiji, Citation2010). In contrary, mice treated with the artemether and lumefantrine combination either in NLCs or simple oil suspension, treated animals have shown significant decrease in parasitemia on day 19, and ≥50% mice survived beyond 28 d. The increased antiparasitic activity may be due to the presence of two drugs, which have shown similar increased therapeutic efficiency when used in combination in earlier clinical trials (van Vugt et al., Citation2000). In addition, between these two groups, artemether and lumefantrine co-loaded NLC-treated group has shown ≥90% decrease in parasitemia (4 ± 3%) in comparison to artemether and lumefantrine oil suspension-treated animals, which have shown the parasitemia of ∼13 ± 4% on day 19 due to the availability of artemether for prolonged period, which helps to reduce the parasitemia followed by availability of the lumefantrine, which will clear the remaining parasites and artemether resistant parasites if any. Similarly, earlier studies have shown the increased therapeutic efficacy of other antimalarial drugs such as chloroquine when they were given through i.p. route using lipid-based delivery systems. This effect may be due to controlled release of drugs from the drug depots making it available to the system for the prolonged period of time (Peeters et al., Citation1989a, Citationb). Recently, artemether and lumefantrine co-loaded lipid delivery system (emulsion) has been developed, which has shown the enhanced antimalarial activity in terms of parasitemia progression and infected animal survivability when given through oral route (Patil et al., Citation2013). Similarly, in this study, artemether and lumefantrine co-loaded NLCs are most effective in treating the infected animals, which resulted in the lowest parasitemia percentage and prolonged survival period of more than four weeks when given through i.p. route.
Table 4. Survivability and parasitemia in Swiss Albino mice infected with P. berghei.
Conclusion
Nano structured lipid carriers co-loaded with artemether and lumefantrine was designed successfully, and physicochemical characterization was carried out to evaluate the suitability of fabricated nano carrier system as injectable formulation. The artemether and lumefantrine co-loaded NLCs treatment resulted in greater clearance of parasites from the infected mice and has shown clear benefit in comparison to individual drug-loaded NLCs in terms of survivability and parasitemia progression. Even though this study has given a preliminary information about the suitability of co-loaded NLCs for malaria treatment, further studies regarding the suitability of the formulation to use for intravenous injection, toxicity, pharmacokinetics, effectiveness in treating parasite strains that infects humans (Plasmodium falciparum and Plasmodium vivax) and drug resistance parasites needs to be studied using suitable in vivo methods and clinical trials.
Acknowledgements
Authors would like to acknowledge Kamani Oil Industries, Mumbai, for their kind gift of soybean oil and IPCA Laboratories for their kind gift of artemether and lumefantrine.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Aditya NP, Chimote G, Gunalan K, et al. (2012). Curcuminoids-loaded liposomes in combination with arteether protects against Plasmodium berghei infection in mice. Exp Parasitol 131:292–9
- Aditya NP, Patankar S, Madhusudhan B, et al. (2010). Arthemeter-loaded lipid nanoparticles produced by modified thin-film hydration: pharmacokinetics, toxicological and in vivo anti-malarial activity. Eur J Pharm Sci 40:448–55
- Aditya NP, Shim M, Lee I, et al. (2013a). Curcumin and genistein coloaded nanostructured lipid carriers: in vitro digestion and antiprostate cancer activity. J Agric Food Chem 61:1878–83
- Aditya NP, Vathsala PG, Vieira V, et al. (2013b). Advances in nanomedicines for malaria treatment. Adv Colloid Interface Sci 201–202:1–17
- Amin NC, Fabre H, Blanchin MD, et al. (2013). Determination of artemether and lumefantrine in anti-malarial fixed-dose combination tablets by microemulsion electrokinetic chromatography with short-end injection procedure. Malaria J 12:202
- Date AA, Joshi MD, Patravale VB. (2007). Parasitic diseases: liposomes and polymeric nanoparticles versus lipid nanoparticles. Adv Drug Deliv Rev 59:505–21
- Egunsola O, Oshikoya KA. (2013). Comparative safety of artemether-lumefantrine and other artemisinin-based combinations in children: a systematic review. Malaria J 12:385
- Joshi M, Pathak S, Sharma S, Patravale V. (2008). Design and in vivo pharmacodynamic evaluation of nanostructured lipid carriers for parenteral delivery of artemether: nanoject. Int J Pharm 364:119–26
- Joshi MD, Muller RH. (2009). Lipid nanoparticles for parenteral delivery of actives. Eur J Pharm Biopharm 71:161–72
- Madden TD, Bally MB, Hope MJ, et al. (1985). Protection of large unilamellar vesicles by trehalose during dehydration – retention of vesicle contents. Biochim Biophys Acta 817:67–74
- Mehnert W, Mader K. (2001). Solid lipid nanoparticles – production, characterization and applications. Adv Drug Deliv Rev 47:165–96
- Memvanga PB, Coco R, Préat V. (2013). An oral malaria therapy: curcumin-loaded lipid-based drug delivery systems combined with β-arteether. J Control Rel 172:904–13
- Nandakumar DN, Nagaraj VA, Vathsala PG, et al. (2006). Curcumin-artemisinin combination therapy for malaria. Antimicrob Agents Chemother 50:1859–60
- Nayak AP, Tiyaboonchai W, Patankar S, et al. (2010). Curcuminoids-loaded lipid nanoparticles: novel approach towards malaria treatment. Colloids Surf B Biointerfaces 81:263–73
- Parashar D, Kumar A, Aditya NP, Rayasa RM. (2013). Simultaneous estimation of artemether and lumefantrine in pharmaceutical dosage forms using derivative spectrophotometry. Asian J Res Chem 6:226–31
- Patil S, Suryavanshi S, Pathak S, et al. (2013). Evaluation of novel lipid based formulation of β-artemether and lumefantrine in murine malaria model. Int J Pharm 455:229–34
- Peeters PAM, Deleest K, Eling WMC, Crommelin DJA. (1989a). Chloroquine blood-levels after administration of the liposome-encapsulated drug in relation to therapy of murine malaria. Pharm Res 6:787–93
- Peeters, PAM, Huiskamp CWEM, Eling WMC, Crommelin DJA. (1989b). Chloroquine containing liposomes in the chemotherapy of murine malaria. Parasitology 98:381–6
- Santos-Magalhães NS, Mosqueira VCF. (2010). Nanotechnology applied to the treatment of malaria. Adv Drug Deliv Rev 62:560–75
- Sosnik A, Amiji M. (2010). Nanotechnology solutions for infectious diseases in developing nations. Adv Drug Deliv Rev 62:375–7
- Teng Z, Li Y, Luo Y, et al. (2013). Cationic Β-lactoglobulin nanoparticles as a bioavailability enhancer: protein characterization and particle formation. Biomacromolecules 14:2848–56
- Van Vugt M, Looareesuwan S, Wilairatana P, et al. (2000). Artemether-lumefantrine for the treatment of multidrug-resistant falciparum malaria. Trans R Soc Trop Med Hyg 94:545–8
- Yeung S, Pongtavornpinyo W, Hastings IM, et al. (2004). Antimalarial drug resistance, artemisinin-based combination therapy, and the contribution of modeling to elucidating policy choices. Am J Trop Med Hyg 71:179–86