Abstract
The objective of this study was to develop and evaluate olanzapine (OZP) -loaded microemulsions (OZPME) for intranasal delivery in the treatment of schizophrenia. The OZPME was formulated by the spontaneous microemulsification method and characterized for physicochemical parameters. Pharmacodynamic assessments (apomorphine – induced compulsive behavior and spontaneous locomotor activity) were performed using mice. All formulations were radiolabeled with technetium-99 (99mTc), and biodistribution of drug in the brain was investigated using Swiss albino rats. Brain scintigraphy imaging in rabbits was performed to determine the uptake of the OZP into the brain. OZPME were found clear and stable with average globule size of 23.87 ± 1.07 nm. In pharmacodynamic assessments, significant (p < 0.05) difference in parameters estimated were found between the treated and control groups. 99mTc-labeled OZP solution (OZPS)/OZPME/OZP mucoadhesive microemulsion (OZPMME) were found to be stable and suitable for in vivo studies. Brain/blood ratio at all sampling points up to 8 h following intranasal administration of OZPMME compared to intravenous OZPME was found to be five to six times higher signifying larger extent of distribution of the OZP in brain. Drug targeting efficiency and direct drug transport were found to be highest for intranasal OZPMME, compared to intravenous OZPME. Furthermore, rabbit brain scintigraphy also demonstrated higher intranasal uptake of the OZP into the brain. This investigation demonstrates a prompt and larger extent of transport of OZP into the brain through intranasal OZPMME, which may prove beneficial for treatment of schizophrenia.
Introduction
Schizophrenia is one of many brain diseases that may include delusions, loss of personality, confusion, agitation, social withdrawal, psychosis, bizarre behavior, high risk of suicide and other life-threatening behaviors. Key issue in the schizophrenia management is adherence with the treatment. The reasons for non adherence to medication include lack of efficacy, intolerability and patient decision (Canuso et al., Citation2008). The need for treatment options tailored to improved delivery of the existing treatment class has been emphasized by the recent Clinical Antipsychotic Trials of Intervention Effectiveness study, until new agents with novel mechanisms of action become available (Nasrallah et al., Citation2005; Zacher & Grady, Citation2007; Canuso et al., Citation2008; Patel et al., Citation2013a–c).
Oral route of drug administration is most convenient and well accepted. However, in many instances, oral administration is not suitable when drug undergoes significant degradation in the gastrointestinal tract or is metabolized to a high degree via the first-pass effect in the liver. Furthermore, patients who are not able to swallow cannot be medicated by oral route, which limits its utility in particular population of patients. Hence, an alternative route of administration should be preferable. Reportedly, nasal mucosal membrane offers a practical non invasive route of administration for therapeutic effect of many drugs (Li et al., Citation2002; Vyas et al., 2006a; Jogani et al., Citation2008; Kaur & Kim, Citation2008; Patel et al., Citation2013b; Elshafeey et al., Citation2009). Nasal route has advantages of rapid absorption of drug, higher bioavailability allowing lower doses, fast onset of therapeutic action, avoidance of liver or gastrointestinal metabolism, avoidance of irritation of the gastrointestinal membrane, reduced risk of overdose, non-invasive administration, ease of convenience and self-medication and improved patient compliance (Li et al., Citation2002; Vyas et al., 2006b; Jogani et al., Citation2008; Kaur & Kim, Citation2008; Elshafeey et al., Citation2009; Patel et al., Citation2013b). Researchers are making many attempts to find efficient and new way to prevent extensive first-pass metabolism and target the drug to the receptor site in brain for treating brain disorders (Chow et al., Citation1999; Li et al., Citation2002; Zhang et al., Citation2004; Vyas et al., Citation2005; Vyas et al., Citation2006a,Citationb; Jogani et al., Citation2008; Kaur & Kim, Citation2008; Kumar et al., Citation2008a,Citationb; Porecha et al., Citation2009; Sharma et al., Citation2009; Patel, Citation2013b).
Olanzapine (OZP), chemically 2-methyl-4-(4-methyl-1-piperazinyl)-10H-thieno [2, 3-b] benzodiazepine is a novel antipsychotic agent used in the treatment of schizophrenia (Hiriyanna et al., Citation2008a). OZP is available as tablet dosage form, which shows extensive first-pass metabolism before reaching the systemic circulation (Kumar et al., 2008). To overcome the bioavailability problems of tablet dosage form, OZP is available as disintegrating wafers and intramuscular (IM) injection. The approved indications for OLZ IM injection are to rapidly control agitation and disturbed behaviors in patients with schizophrenia or manic episodes when oral therapy is inappropriate. However, IM administration of OZP is associated with several adverse events (Kumar et al., 2008a; Patel et al., Citation2010, Citation2013a). These adverse events, beside the need of a therapeutic prompt action, make OLZ a possible candidate for the development of a transnasal formulation. Furthermore, the site of action for OZP is the brain, thus an alternate route of administration, an intranasal delivery, is advantageous, which not only improves the bioavailability but also helps in achieving the desired concentration of OZP at the targeted site of action, consequently reduces the adverse events at non targeting sites (Kumar et al., 2008a).
In contrast, the limitation for intranasal drug delivery is the fact that most drugs diffuse poorly and slowly through the nasal mucosa and thus the desired levels of the therapeutic agent cannot be achieved. Furthermore, the nasal cavity accommodate limited volume of formulation, approximately 150 µL per nostril, and excess volume will drain out into the pharynx and be swallowed (Patel et al., Citation2013a,Citationb; Shinde et al., Citation2011).
This study deals with above-mentioned problems and explored the suitable drug delivery vehicle, microemulsion (ME), which is able to dissolve the OZP in required concentration, yet which is not irritating to the nasal mucosa. The intranasal administration of drug can be increased by co-administering a permeation enhancer (Patel et al., Citation2013b). ME have been thoroughly studied as a drug carrier due to their special features, such as ease of preparation due to spontaneous formation, thermodynamic stability, high solublization capacity for the hydrophilic and lipophilic drugs, transparent and elegant appearance, enhanced penetration through the biological membranes, increased bioavailability and less inter- and intra-individual variability in drug pharmacokinetics (Li et al., Citation2002; Zhang et al., Citation2004; Kaur & Kim, Citation2008; Elshafeey et al., Citation2009; Shinde et al., Citation2011).
An additional constraint concerning nasal administration is the natural defense mechanism; nasal mucociliary clearance. Therefore, it is essential to localize the formulation on a mucosal layer of nasal cavity to enhance drug absorption and prevent rapid nasal clearance. Addition of a well-designed mucoadhesive agent to ME can prevent rapid nasal clearance of formulation (Patel et al., Citation2013b).
Thus, this study describes development, characterization and pharmacodynamic–pharmacokinetic evaluation of OZP-loaded MME designed using generally regarded as safe-listed ingredients and polycarbophil AA-1 as a mucoadhesive component.
Materials and methods
Drug and reagents
OZP pure powder was obtained as gratis sample from Torrent Pharmaceutical Ltd. (Ahmedabad, India) with 99.9% purity. Caprylocaproyl polyoxylglycerides, diethylene glycol monoethyl ether, (Gattefosse, Saint-Priest, France) was procured as gratis samples from Gattefosse Asia Ltd. (Mumbai, India). Polyoxyl 40 hydrogenated castor oil was procured as gratis sample form BASF (Mumbai, India). Polycarbophil (AA-1, pharmagrade, molecular weight approximately 3.5 million) was procured as gratis sample from Lubrizol Advanced Material India Pvt. Ltd. (Mumbai, India). cis-9-octadecenoic acid, potassium dihydrogen phosphate, methanol and propylene glycol were purchased from SDfine Chemicals (Ahmedabad, India). Ethanol was purchased from Baroda Chemical Ind. Ltd. (Dabhoi, India). Double distilled water was used throughout the study. All other chemicals and solvents were of analytical reagent grade and used as received without further purification.
Preparation and characterization of formulations
The ME for OZP was formulated by the spontaneous microemulsification method (Patel et al., Citation2013a). The calculated amount of drug (8 mg/mL of OZP) was added to the oily phase (oleic cis-9-octadecenoic acid) of ME and magnetically stirred until dissolved followed by addition of emulsifier mixture (Emix) [caprylocaproyl polyoxylglycerides and Polyoxyl 40 hydrogenated castor oil (nonionic emulsifiers) (1:1) to diethylene glycol monoethyl ether (coemulsifier)] in a fixed proportion (3:1) to produce clear mixture. Then, a defined proportion of water was added to produce crystal clear ME of OZP (OZPME). The mucoadhesive ME of OZP (OZPMME) was prepared by initially preparing ME of the drug using minimum volume of external phase and then adding the required volume of polymer solution (1%, wt/vol) so that the final concentration of polymer in the MME was 0.5% (wt/wt). After the addition of polymer solution, the MME was allowed to homogenize for 10 min. The OZP solution (OZPS) meant for comparative evaluation of MME-based systems was prepared by dissolving OZP (80 mg) in 10 mL of propylene glycol resulting in a solution of 8 mg/mL (Vyas et al., Citation2005; Vyas et al., 2006b; Kumar et al., 2008b; Porecha et al., Citation2009; Sharma et al., Citation2009; Patel, Citation2013a).
OZP content in the formulations was estimated using a high-performance thin layer chromatography (TLC) (Patel et al., Citation2010). The average droplet size and polydispersity index (PDI) of ME was determined using photon correlation spectroscopy with in-built Zetasizer (Nano ZS, Malvern Instruments, Malvern, UK) at 633 nm. Helium–neon gas laser having intensity of 4 mW was the light source. The droplet size was calculated using Stokes–Einstein relationship by Zetasizer Software. Electrophoretic mobility (µm/s) was measured using small volume disposable zeta cell and converted to zeta potential by in-built software using Helmholtz–Smoluchowski equation (Vyas et al., Citation2005; Vyas et al., 2006b; Kumar et al., 2008b; Porecha et al., Citation2009; Sharma et al., Citation2009; Patel, Citation2013a). The pH value of ME was determined using digital pH meter (HI 98107, Hanna Instruments, Mumbai, India), standardized using pH 4 and 7 buffers before use (Patel et al., Citation2013a). The viscosity of ME was measured using a Brookfield Viscometer LVDV – IIIU (Brookfield Engineering Labs, Stoughton, MA) with spindle SC 18 at 100 rpm using interval of 30 s. All aspects of testing were controlled using Rheocalc Software (Patel et al., Citation2013a).
Stability studies
The formulations, OZPME and OZPMME, were subjected to stability studies for a period of six months at room temperature and refrigerated conditions (4 °C). After six months of storage, the formulations were characterized for physical stability (creaming, phase separation or flocculation), accelerated centrifugation cycle (3000 × g for 15 min), drug content, particle size and zeta potential determinations (Vyas et al., Citation2005, 2006a; Kumar et al., 2008a; Porecha et al., Citation2009; Sharma et al., Citation2009; Patel, Citation2013a).
Pharmacodynamic study
The protocol for animal experimentations was approved by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) and the Institutional Animal Ethics Committee (IAEC) of A. R. College of Pharmacy & G. H. Patel Institute of Pharmacy, Vallabh Vidyanagar, Gujarat.
Apomorphine – induced compulsive behavior
Apomorphine – induced compulsive behavior was evaluated in six groups of six mice in each of either sex with average weight of 25 g. One group received distilled water as control while other groups received OZPME (IV), OZPS (IN), OZPME (IN) and OZPMME (IN), remaining group received haloperidol (1 mg/kg body weight (BW)) (Kulkarni & Jisheph, Citation1998) as positive control. The intranasal formulations were administered in mice using micropipette (5 μl/nostril) at the dose of 0.08 mg OZP (equivalent to 3.20 mg/kg of BW). After 1 h of treatment, apomorphine hydrochloride (2 mg/kg of BW) was administered intraperitoneally to all animals. The mice were individually placed in glass containers of 250 mL capacity and the sign of stereotyped behavior, which include sniffing, rearing, licking and gnawing, were observed at 0, 15, 30, 45 and 60 min after apomorphine administration. The intensity of stereotypy was recorded by the following scoring system (Shibuya & Nishimori, Citation1982; Kulkarni & Jisheph, Citation1998, Patel et al., Citation2013b):
Discontinuous sniffing and constant exploratory activity.
Continuous sniffing, periodic exploratory activity and small head movements.
Continuous sniffing, small body and head movements, discontinuous gnawing, biting and licking the container wall and brief spurts of locomotor activity.
Continuous gnawing, biting and licking the cage wall and no ambulation except for occasional backward movement.
Spontaneous motor activity
Mice of either sex with an average weight of 25 g were taken and deprived of food and water for 24 h before the test. To avoid any influence of the circadian rhythm, the experimentation was performed only between 8.00 and 12.00 am. Six mice were taken per time point. The intranasal formulations (OZPS, OZPME and OZPMME) were administered in mice using micropipette (5 μl/nostril) at the dose of 0.08 mg OZP (equivalent to 3.20 mg/kg of BW) followed by intraperitoneal administration of L-dopa (13 mg/kg of BW) and carbidopa (3.25 mg/kg of BW) after 30 min. The locomotor activity was measured for 10 min by placing the animals in digital photoactometer (Inco, Mumbai, India). For the formulation OZPME, given by intravenous route, the locomotor activity was measured after 2 min for a period of 10 min (Patel et al., Citation2013b).
Pharmacokinetic study
The protocol for animal experimentation was approved by the Committee for the Purpose of CPCSEA and the IAEC of Veterinary Nuclear Medicine Centre, Bombay Veterinary College, Mumbai, India.
Preparation of radiolabeled formulations
Drug in OZPS and the formulations, OZPME and OZPMME, were radiolabeled using technetium-99 (99mTc) by direct labeling method (Eckelman, Citation1995; Babbar et al., Citation2000; Li et al., Citation2002; Zhang et al., Citation2004; Vyas et al., 2006b; Kaur & Kim, Citation2008; Patel et al., Citation2013b). To 1.0 mL of OZP formulation, 200 μL of stannous chloride dehydrate (2 mg/mL in 10% acetic acid) was added, the pH was adjusted to 6.0–6.5 using 50 mM sodium bicarbonate solution. To the resultant mixture (filtered through 0.22 μ nylon 66 membrane), required volume of sterile 99mTc-pertechnetate (5 mCi) was added over a period of 60 s with continuous mixing such that the resultant solution had a radioactivity of 5 mCi/mL and was incubated at 30 °C ± 5 °C for 30 min with continuous nitrogen purging. The final volume was made up to 2.5 mL using 0.9% (w/v) sterile sodium chloride solution. The resultant formulations obtained had 100 μCi/20 μL activity.
The radiochemical purity (Eckelman, Citation1995; Babbar et al., Citation2000; Patel et al., Citation2013b) of 99mTc-labeled OZPS (99mTc-OZPS), 99mTc-labeled OZPME (99mTc-OZPME) and 99mTc-labeled OZPMME (99mTc-OZPMME) was determined by ascending instant TLC using silica gel-coated fiberglass sheets and acetone as the mobile phase. The effects of incubation time, pH and stannous chloride concentration on radiolabeling efficiency were studied to achieve optimum conditions. The radiolabeled formulations were challenged to assess bonding strength at different molar concentrations (25–100 mM) of diethylene triamine penta acetic acid (Eckelman, Citation1995; Babbar et al., Citation2000; Patel et al., Citation2013b). The optimized radiolabeled-drug formulations were evaluated for in vitro stability in 0.90% (w/v) sodium chloride (normal saline) and in plasma (Eckelman, Citation1995; Babbar et al., Citation2000; Patel et al., Citation2013b). The optimized, stable radiolabeled drug formulations of OZP were used for biodistribution study in rats.
Bio-distribution studies
Wistar rats (male, aged 4–5 months), weighing between 200 and 250 g were selected for the study. Four rats for each formulation per time point were used in the study. The radiolabeled complex of 99mTc-OZPME (100 μCi/20 μL) containing 0.056–0.064 mg OZP (equivalent to 0.29–0.32 mg/kg BW) was injected through tail vein of Wistar rats. Similarly, radiolabeled complex of 99mTc-OZPS/OZPME/OZPMME (100 μCi/20 μL) containing 0.056–0.064 mg OZP (equivalent to 0.29–0.32 mg/kg BW) was administered (10 μL) in each nostril. Prior to nasal administration of the formulations, the rats were anaesthetized using 50 mg/kg ketamine IM injection, and the formulations were instilled into the nostrils with the help of micropipette (10–100 μL) attached with low density polyethylene tube having 0.1 mm internal diameter at the delivery site. The rats were held from the back in slanted position during nasal administration of the formulations. The rats were sacrificed at predetermined time intervals, and blood was collected using cardiac puncture. Subsequently, different tissues/organs including brain and spinal cord were dissected, washed twice using normal saline solution and made free from adhering tissue/fluid and weighed. The radioactivity present in each tissue/organ was measured using shielded well-type gamma scintillation counter. The radiopharmaceutical uptake per gram in each tissue/organ was calculated as a fraction of administered dose (Li et al., Citation2002; Zhang et al., Citation2004; Vyas et al., Citation2005; Vyas et al., 2006a; Jogani et al., Citation2008; Kaur & Kim, Citation2008; Kumar et al., 2008a; Porecha et al., Citation2009; Sharma et al., Citation2009; Patel, Citation2013a).
To assess the brain targeting efficiency, two indexes drug targeting efficiency (DTE, %) and nose-to-brain direct transport percentage (DTP, %) were adopted as mentioned below. DTE (%): DTE (%) represents time average partitioning ratio, which has been derived from Equation (1). In order to define nose–brain direct transport clearly, “the brain drug DTP (%)’’; which has been derived from Equation (2).
(1)
(2)
where, AUC is area under the curve; Bx is brain AUC fraction contributed by systemic circulation through the BBB following intranasal administration; Bi.v is AUC0→480 (brain) following intravenous administration; Pi.v. is AUC0→480 (blood) following intravenous administration; Bi.n. is AUC0→480 (brain) following intranasal administration; and Pi.n. is AUC0→480 (blood) following intranasal administration.
Literature review revealed that the drug uptake into the brain from the nasal mucosa mainly occurs via three different pathways (Li et al., Citation2002; Zhang et al., Citation2004; Vyas et al., Citation2005, 2006a; Jogani et al., Citation2008; Kaur & Kim, Citation2008; Kumar et al., 2008a; Porecha et al., Citation2009; Sharma et al., Citation2009; Patel, Citation2013a). One is the systemic pathway by which some of the drug is absorbed into the systemic circulation and subsequently reaches the brain by crossing BBB. The others are the olfactory pathway and the trigeminal neural pathway by which partly the drug travels directly from the nasal cavity to CSF and brain tissue. We can conclude that the amount of drug reaches in the brain tissue after nasal administration is attributed to these three pathways. Thus, we can assume that the brain AUC fraction contributed by systemic circulation through BBB (represented by Bx), divided by plasma AUC from nasal route is equal to that of IV route (see Equation (1)). Therefore, DTP (%) represents the percentage of drug directly transported to the brain via the olfactory pathway and the trigeminal neural pathway. DTP (%) and DTE (%) were calculated using tissue/organ distribution data following intranasal and intravenous administrations.
Gamma scintigraphy imaging
The New Zealand rabbits (2.00–2.50 kg) were selected for the study. The radiolabeled complex of 99mTc-OZPS (100 mCi/100 μL) containing 0.32–0.40 mg OZP (equivalent to 0.16–0.21 mg/kg BW) was IV injected through the ear vein of the rabbit. Similarly, the radiolabeled complex of 99mTc-OZPS/99mTc-OZPME/99mTc-OZPMME (100 mCi/100 μL) containing 0.0.32–0.40 mg OZP (equivalent to 0.16–0.21 mg/kg BW) was administered through intranasal route (50 μL in each nostril). The rabbits were held from the back in slanted position during nasal administration of formulations. The rabbits were anesthetized using 1 mL ketamine hydrochloride IM injection (50 mg/mL) and placed on the imaging platform. Imaging was performed using Single Photon Emission Computerized Tomography (LC 75-005, Diacam, Siemens AG; Erlanger, Germany) gamma (Li et al., Citation2002; Zhang et al., Citation2004; Vyas et al., Citation2005, 2006a; Jogani et al., Citation2008; Kaur & Kim, Citation2008; Kumar et al., 2008a; Porecha et al., Citation2009; Sharma et al., Citation2009; Patel, Citation2013a).
Statistical analysis
All data are reported as mean ± SEM and the difference between the groups were tested using Student's t-test at the level of p < 0.05. More than two groups were compared using ANOVA, with p < 0.05 considered statistically significant.
Results and discussion
Formulation development and characterization
ME formulations containing cis-9-octadecenoic acid as an oil phase were prepared at caprylocaproyl polyoxylglycerides and polyoxyl 40 hydrogenated castor oil: diethylene glycol monoethyl ether (1:1) fixed Emix ratios of 3:1 (). The ME containing 4% wt/wt cis-9-octadecenoic acid, 32% wt/wt Emix and 64% wt/wt distilled water showed highest solubilizing capacity for OZP. Polycarbophil (0.5% wt/wt) was used as a mucoadhesive polymer and incorporated in ME formulation to obtained OZP-loaded MME. All formulations were prepared and characterized for the various physicochemical parameters (). The OZP content (%) of OZPS, OZPME and OZPMME was found to be 99.78 ± 1.22, 101.03 ± 0.77 and 102.18 ± 1.56, respectively of the theoretical value (8 mg/mL). The narrow globule size range of 23.87 ± 1.07 nm and 31.66 ± 1.14 nm and PDI of 0.121 ± 0.016 and 0.251 ± 0.032 for OZPME and OZPMME, respectively, indicated that the ME approached a monodispersed stable system and could deliver the drug effectively owing to larger surface area. The presence of zeta potential to the tune of −35.14 ± 2.12 and −42.15 ± 3.08 mV on the globules of OZPME and OZPMME, respectively, conferred physical stability to the system. OZPME showed net negative charge, and addition of mucoadhesive agent further contributed negativity to the system. This may be attributed to the fact that the increase in surfactant level resulted in a decrease in surface tension and surface free energy of the formed micelles. Therefore, net negative charge (anionic) of the ME increased (Li et al., Citation2002; Zhang et al., Citation2004; Vyas et al., 2006a; Jogani et al., Citation2008; Kaur & Kim, Citation2008; Elshafeey et al., Citation2009; Shinde et al., Citation2011; Patel et al., Citation2013a,Citation2013b). The MEs were expected to have good physical stability (phase separation) as zeta potential is less than −30 to −40 mV (Vyas et al., 2006b; Jogani et al., Citation2008; Patel et al., Citation2013b). Moreover, addition of mucoadhesive polymer (polycarbophil) may further stabilize the system since it increased negative charge of the system (Li et al., Citation2002; Zhang et al., Citation2004; Vyas et al., Citation2005; Vyas et al., 2006a; Jogani et al., Citation2008; Kaur & Kim, Citation2008; Kumar et al., 2008b; Porecha et al., Citation2009; Sharma et al., Citation2009; Patel, Citation2013a). The pH of all the OZPS, OZPME and OZPMME ranged from 5.65 to 5.95, approximating the normal pH range of nasal fluids (Patel et al., Citation2013b), which is one of the formulation considerations that may help reducing the irritation produced upon instillation. It was observed that the viscosity of the ME formulations generally was very low. This was expected because one of the characteristics of ME formulations is of lower viscosity (Patel et al., Citation2013a). Low viscosity values of OZPME (75 ± 5.00 cp) and OZPMME (93 ± 6.21 cp) ensure easy handling, packing and hassle-free nasal administration of formulations.
Table 1. Composition of olanzapine microemulsion (OZPME) and olanzapine mucoadhesive microemulsion (OZPMME).
Table 2. Characterization parameters of optimized olanzapine microemulsions (OZPME) and Olanzapine mucoadhesive microemulsions (OZPMME) (n = 3).
In stability studies, the ME exhibited no precipitation of drug, creaming, phase separation and flocculation on visual observation and was found to be stable after centrifugation (3000 × g for 15 min) both at room temperature and at 2–8 °C. The results of stability studies showed that there are negligible changes in the parameters such as drug content, % transmittance, globule size and zeta potential of OZPME and OZPMME after six months of storage at room temperature and refrigerated condition, thus substantiating the stability of formulations for six months (data not shown).
Pharmacodynamic evaluation
Compulsive behavior is defined as purposeless activity exhibited by the animal. This purposeless activity is supposed to be identical to the behavioral disorder seen in schizophrenia (psychotic) patients who also show repetitive purposeless activity. This behavioral abnormality in schizophrenia is due to the excessive neuronal activity of dopamine in the limbic system. Apomorphine, a dopamine receptor agonist, through its dopaminergic activity induces compulsive stereotyped behavior in mice. The stereotyped behavior includes rearing (repetitive standing), continuous sniffing (touching the nose to the wall of the container) and licking the wall of container (Patel et al., Citation2013b).
As shown in , all animals administered OZP formulations by either routes exhibited significant difference (p < 0.05) in inhibition of apomorphine-induced stereotype behavior. Absence of apomorphine-induced stereotype behavior was observed in mice pretreated with haloperidol. Increased passage of OZP via paracellular or transcellular mechanism to the brain via the olfactory region of the nasal cavity was demonstrated by lower value of score for stereotype behavior for OZPME when compared with OZPS. Furthermore, OZPMME showed lower score for stereotype behavior () when compared with OZPME, both administered intranasally, indicating the superiority of intranasal mucoadhesive microemulsion over OZPME and clearly demonstrates the role of polycarbophil (mucoadhesive agent) that prevent nasociliary clearance thereby prolonging the residence time, and is also suggested to play a role in the enhancement of penetration of the formulation across the cells (Braso et al., Citation2003). OZPMME formulation administered by intranasal routes showed a lower value of score for stereotype behavior than OZPME administered intravenously, suggesting better brain uptake of the drug by intranasal route as MME when compared with other OZP formulations.
Table 3. Pharmacodynamic study (apomorphine – induced compulsive behaviour) of olanzapine (OZP) formulation in normal mice (n = 6).
In central nervous system (CNS), the neurotransmitter dopamine mediates a wide array of physiological functions including regulation of locomotor activity. It is generally believed that locomotor activation results from brain activation, which is manifested as a result of excitation of central neurons and an increase in cerebral metabolism. While different neurochemical mechanisms are involved in brain activation, dopamine appears to play an essential role; pharmacological blockade of dopamine transmission inhibits spontaneous locomotor activity (Le & Simon, Citation1991; Chow et al., Citation1999; Salamone et al., Citation2005; Patel et al., Citation2013b).
The animals of the control group (not administered OZP formulation) showed maximum locomotor activity due to the D2 receptor activation by L-dopa and carbidopa that were used to develop the behavior model of schizophrenia in mice by improving dopamine concentration in the mesolimbic system where the drug(s) acts on 5HT2 and D2 receptor (Le & Simon, Citation1991; Chow et al., Citation1999; Salamone et al., Citation2005; Patel et al., Citation2013b). Whereas the groups that were administered OZP formulations (OZPS, OZPME and OZPMME) exhibited significant reduction (p < 0.05) in the locomotor activity (). The reduction in locomotor activity by intranasal administration of OZPS, OZPME and OZPMME was better when compared with OZPME administered intravenously, suggesting superiority of nose-to-brain delivery of OZP over intravenous route of administration. The results demonstrate superior delivery of OZP to the brain via intranasal route from the formulation containing mucoadhesive agent polycarbophil (0.50% by weight). The mucoadhesive agent used in the formulation shall not only prevent the nasociliary clearance but is also suggested to play a role in the enhancement of penetration of the formulation across the cells. These pharmacodynamic reports are consistent with the earlier findings reported in literature (Patel et al., Citation2013b).
Table 4. Pharmacodynamic study (spontaneous motor activity) of olanzapine (OZP) formulation in normal mice (n = 6).
Pharmacokinetic evaluation
OZPS, OZPME and OZPMME formulations were effectively radiolabeled with 99mTc and optimized for maximum labeling efficiency and stability. Radiochemical purity achieved was 96.80%, 97.10% and 97.70% for OZPS, OZPME and OZPMME, respectively, when evaluated for reduced/hydrolyzed 99mTc and free 99mTc. The optimal SnCl2 2H2O concentration was found to be 200 μL (2 mg/mL) at pH 6.0–7.0 with an incubation time of 30 min. 99mTc-OZPS/OZPME/OZPMME were found to be stable in normal saline solution and plasma up to 24 h (degradation < 5% w/w). Bonding strength of 99mTc-OZPS/OZPME/OZPMME was also investigated by the DTPA challenging test, and the percent trans-chelation of the labeled complex was 3.26% w/w at 25 mM DTPA concentration, while at 100 mM, it increased to 4.52% w/w. The results suggested high bonding strength and stability of 99mTc-OZPS/OZPME/OZPMME. Thus these formulations were found suitable for biodistribution studies of the drug in rats.
Biodistribution studies (Chow et al., Citation1999; Li et al., Citation2002; Zhang et al., Citation2004; Vyas et al., Citation2005; Vyas et al., Citation2006a,Citationb; Jogani et al., Citation2008; Kaur & Kim, Citation2008; Kumar et al., Citation2008a,Citationb; Porecha et al., Citation2009; Sharma et al., Citation2009; Patel, Citation2013b) of 99mTc-OZP formulations following IV administration (OZPME) and intranasal (OZPS, OZPME and OZPMME) administration on Wistar rats were performed and the radioactivity was estimated at predetermined time intervals up to 8 h. The brain/blood ratio of the drug at all time points for different formulations were also calculated and recorded in . The pharmacokinetic parameters were calculated from and reported in .
Figure 1. (A) OZP concentration in rat blood at different time intervals following 99mTc-OZPME (IV), 99mTc-OZPME (IN), 99mTc-OZPMME (IN) and 99mTc-OZPS (IN) administrations. (B) OZP concentration in rat brain at different time intervals following 99mTc-OZPME (IV), 99mTc-OZPME (IN), 99mTc-OZPMME (IN) and 99mTc-OZPS (IN) administrations.
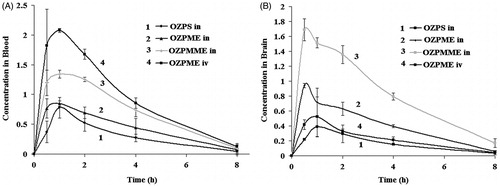
Table 5. Compartmental distribution of 99mTc-OZPME (IV), 99mTc-OZPME (IN), 99mTc-OZPMME (IN) and 99mTc-OZPS (IN) at different time intervals in normal Wistar rats.a.
Table 6. Pharmacokinetics of 99mTc-OZPME (IV), 99mTc-OZPME (IN), 99mTc-OZPMME (IN) and 99mTc-OZPS (IN) at different time intervals in normal Wistar rats.a.
Drug concentrations in brain following intranasal administrations of OZPME and OZPMME were found to be significantly higher at all sampling time points compared with IV administration of OZPME. The brain/blood ratio at 0.5 h for OZPME (IN) and OZPMME (IN) was found to be five- to sixfold higher as compared with OZPME (IV). This finding may be attributed to direct nose-to-brain transport. Reports in the literature revealed that following intranasal administration, preferential nose-to-brain transport bypassing the BBB occurred due to the unique connection between the nose and the CNS (Vyas et al., 2006a; Jogani et al., Citation2008; Patel et al., Citation2013b). The substantially higher uptake in the brain with intranasal administration suggests a larger extent of selective transport of OZP from nose-to-brain. Many researchers (Li et al., Citation2002; Zhang et al., Citation2004; Vyas et al., Citation2005, 2006a; Jogani et al., Citation2008; Kaur & Kim, Citation2008; Kumar et al., Citation2008a,Citationb; Porecha et al., Citation2009; Sharma et al., Citation2009; Patel, Citation2013b) have reported a unique connection between the nose and the brain and intranasal delivery of drugs to the brain by passing the blood–brain barrier. The T1/2 of 1.54–1.77 h (blood), 1.82–1.97 h (brain) and Kel 0.38–0.45 (blood), 0.36–0.38 (brain) were observed irrespective of the routes of administration and the type of the formulations ().
When OZPME (IV) was compared to OZPME (IN) and OZPS (IN), significantly lower Cmax and AUC were observed. The mucociliary clearance under normal circumstances rapidly clears the instilled formulation. However, when mucoadhesive agent was incorporated in the formulation (OZPMME), significant improvement in Cmax and AUC was observed. Comparable AUC to OZPME (IV) was achieved with OZPMME (IN). This demonstrates the value of the mucoadhesive agent in prolonging the contact time of the formulation with the nasal mucosa. Significantly higher AUC and Cmax for OZPME (IN) nasal compared to OZPS (IN) are attributed to microemulsion formulation (Li et al., Citation2002; Zhang et al., Citation2004; Vyas et al., Citation2005, 2006; Jogani et al., Citation2008; Kaur & Kim, Citation2008; Kumar et al., Citation2008a,Citationb; Porecha et al., Citation2009; Sharma et al., Citation2009; Patel, Citation2013b).
The DTE (%) and brain drug DTP (%) were also calculated for nasally administered formulations and are listed in . The OZPMME showed the highest DTE (%) and DTP (%) values among all the three formulations followed by OZPME and then OZPS (). The 2.13-fold higher DTE (%) and 1.38-fold higher DTP (%) for OZPMME compared to OZPS show the benefit of the mucoadhesive microemulsion formulation. The higher DTE (%) and DTP (%) suggest that OZPMME has better brain targeting efficiency mainly because of substantial direct nose-to-brain transport. These findings are in congruence with the observations reported in Literature (Li et al., Citation2002; Zhang et al., Citation2004; Vyas et al., Citation2005, 2006a; Jogani et al., Citation2008; Kaur & Kim, Citation2008; Kumar et al., Citation2008a,Citation2008b; Porecha et al., Citation2009; Sharma et al., Citation2009; Patel, Citation2013b) that microemulsion increases nose-to-brain uptake of the drugs.
Table 7. Brain targeting efficiency and direct nose-to-brain transport following intranasal administration of 99mTc-OZPME, 99mTc-OZPMME and 99mTc-OZPS.a.
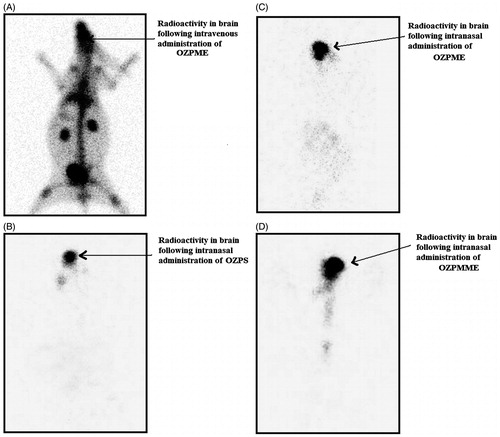
To visualize brain uptake of 99mTc-OZP formulation, following IN and IV administrations, gamma scintigraphy camera was used to derive comprehensive biodistribution information. The gamma scintigraphy images of rabbit 0.50 h post IV injection and IN administrations are shown in . Gamma scintigraphy images showed accumulation of significantly higher radioactivity in the rabbit brain after IN administration of OZP compared with IV administration. Among IN formulations, OZPMME shows higher radioactivity compared with OZPME and OZPS. The scintigraphy images were consistent with the results listed in , and high uptake of OZPMME into the brain was observed.
Figure 2. Gamma scintigraphy of anteroposterior (AP) view of rabbit following intravenous administration of 99mTc-OZPME (A), intranasal administration of 99mTc-OZPS (B), 99mTc-OZPME (C) and 99mTc-OZPMME (D). Rabbits were administered 100 µCi radioactivity by intravenous and intranasal administration.
Conclusion
In this investigation, the efficiency of ME as carrier for nasal delivery of OZP was studied. All formulations were successfully prepared, characterized and found suitable for intranasal administration. The pharmacodynamic studies revealed that OZPMME showed lower score for stereotype behavior when compared with OZPME, both administered intranasally, indicating the superiority of intranasal OZPMME over OZPME. The reduction in locomotor activity by intranasal administration of OZPME and OZPMME was better when compared with OZPME administered intravenously, suggesting superiority of nose-to-brain delivery of OZP over intravenous route of administration. The results demonstrate superior delivery of drug to the brain via intranasal route from the formulation containing mucoadhesive agent polycarbophil. The pharmacokinetic studies reveal that the intranasal administration of OZPMME provide higher brain drug concentrations compared to the intravenous administration. As a consequence of this, decrease in the dose and frequency of administration for drugs is possible to achieve the desired therapeutic activity. Furthermore, intranasal administration of the drugs will avoid unwanted peripheral tissue distribution of the drugs and hence the associated peripheral side effects. These together will improve the therapeutic efficacy of the drugs.
Given the results of this investigation, the in vitro and in vivo studies demonstrated the potential of developed MME for intranasal delivery of OZP and confirms the existence of a transport pathway for a drug (OZP) to the brain directly from the nasal cavity. However, clinical benefits to the risk ratio of the formulation developed in this investigation will decide its appropriateness in the clinical practice for the treatment of schizophrenia.
Acknowledgements
All India Council for Technical Education (AICTE), New Delhi, is gratefully acknowledged for financial support (Grant no. 8023/BOR/RID/RPS-147). Authors are thankful to Torrent Pharmaceutical Ltd. for providing the gift sample of pure powder of drugs, Gattefosse, Colorcon (Asia) Pvt. Ltd. (Mumbai, India), BASF, Noveon (Cleveland, USA) for readily providing gratis samples of excipients, to Veterinary Nuclear Medicine Centre, Bombay Veterinary College, Mumbai, and Institute of Nuclear Medicine and Allied Science (INMAS), New Delhi, for permitting to carry out experimental work, and the authors also thank Sophisticated Instrumentation Center for Applied Research and Testing (SICART), Vallabh Vidyanagar, for providing facilities and research assistant.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Babbar AK, Singh AK, Goel HC, et al. (2000). Evaluation of 99mTc-labeled photosan-3, a hematoporphyrin derivative, as a potential radiopharmaceutical for tumor scintigraphy. Nucl Med Biol 27:419–26
- Braso A, Princep M, Schmid M, et al. (2003). Developmental pharmacology: behavior assay in antipsychotics. Methods Find Expt Clin Pharmacol 25:1–2
- Canuso CM, Youssef EA, Bossie CA, et al. (2008). Paliperidone extended-release tablets in schizophrenia patients previously treated with risperidone. Int Clin Psychopharm 23:209–15
- Chow HS, Chen Z, Matsuura GT. (1999). Direct transport of cocaine from the nasal cavity to brain following intranasal cocaine administration in rats. J Pharm Sci 88:754–8
- Eckelman WC. (1995). Radiolabeling with technetium-99m to study highcapacity and low-capacity biochemical systems. Eur J Nucl Med 22:249–63
- Elshafeey AH, Bendas ER, Mohamed OH. (2009). Intranasal microemulsion of sildenafil citrate: in vitro evaluation and in vivo pharmacokinetic study in rabbits. AAPS PharmSciTech 10:361–4
- Hiriyanna SG, Basavaiah K, Goud PSK, et al. (2008). Identification and characterization of olanzapine degradation products under oxidative stress conditions. Acta Chromatogr 20:81–93
- Jogani VV, Shah PJ, Mishra P, et al. (2008). Intranasal mucoadhesive microemulsion of tacrine to improve brain targeting. Alzheimer Dis Assoc Disord 22:116–24
- Kaur P, Kim K. (2008). Pharmacokinetics and brain uptake of diazepam after intravenous and intranasal administration in rats and rabbits. Int J Pharm 364:27–35
- Kulkarni SK, Jisheph P. (1998). Psychopharmacological profile of sitone granules, A herbal drug preparation. Ind drug 35:536–44
- Kumar M, Misra A, Mishra AK, et al. (2008a). Mucoadhesive nanoemulsion-based intranasal drug delivery system of olanzapine for brain targeting. J Brain Targ 16:806–14
- Kumar M, Misra A, Babbar AK, et al. (2008b). Intranasal nanoemulsion based brain targeting drug delivery system of risperidone. Int J Pharm 358:285–91
- Le M, Simon H. (1991). Mesocorticolimbic dopaminergic network functional and regulatory roles. Physiol Rev 71:155–234
- Li L, Nandi I, Kim KH. (2002). Development of an ethyl laurate-based microemulsion for rapid-onset intranasal delivery of diazepam. Int J Pharm 237:77–85
- Nasrallah HA, Targum SD, Tandon R, et al. (2005). Defining and measuring clinical effectiveness in the treatment of schizophrenia. Psychiatr Serv 56:273–82
- Patel RB, Patel MR, Bhatt KK, Patel BG. (2013a). Formaulation and evaluation of microemulsion based drug delivery system for intranasal administration of olanzapine. Int J Bimed Pharm Sci 7:20–7
- Patel RB, Patel MR, Bhatt KK, et al. (2013b). Risperidone microemulsion for transnasal delivery: pharmacodynamic and pharmacokinetic evaluation. Pharm Nanotech 1:44–53
- Patel RB, Patel MR, Bhatt KK, Patel BG. (2013c). Paliperidone loaded mucoadhesive microemulsion in treatment of schizophrenia: formulation consideration. J Pharm Inn 8:195–204
- Patel RB, Patel MR, Bhatt KK, Patel BG. (2010). Development and validation of an HPTLC method for determination of olanzapine in formulations. J AOAC Int 93:811–9
- Porecha S, Shah T, Jogani V, et al. (2009). Microemulsion based intranasal delivery system for treatment of insomnia. Drug Del 16:128–34
- Salamone JD, Correa M, Mingote SM, Weber SM. (2005). Beyond the reward hypothesis: alternative functions of nucleus accumbens dopamine. Curr Opin Pharmacol 5:34–41
- Sharma G, Mishra AK, Mishra P, Misra A. (2009). Intranasal cabergoline: pharmacokinetic and pharmacodynamic studies. AAPS PharmSciTech 10:1321–30
- Shibuya TT, Nishimori MH. (1982). Behavioural pharmacological studies in the monkey with DD-3480. Int J Clin Pharmaco Ther Toxicol 20:251–4
- Shinde RL, Jindal AB, Devarajan PV. (2011). Micromeulsions and nanoemulsions for targeted drug delivery to the brain. Current Nanosci 7:119–33
- Vyas TK, Babbar AK, Sharma RK, Misra A. (2005). Intranasal mucoadhesive microemulsion of zolmitriptan: preliminary studies on brain targeting. J Drug Target 13:317–24
- Vyas TK, Babbar AK, Sharma RK, et al. (2006a). Intranasal mucoadhesive microemulsions of clonazepam: preliminary studies on brain targeting. J Pharm Sci 95:570–80
- Vyas TK, Babbar AK, Sharma RK, et al. (2006b). Preliminary brain targeting studies of intranasal mucoadhesive microemulsions of sumatriptan. AAPS PharmSci Tech 7:E49–57
- Zacher JL, Grady SE. (2007). Paliperidone extended-release tablets (Invega). Psychopharma Rev 42:51–8
- Zhang Q, Jiang X, Jiang W, et al. (2004). Preparation of nimodipine-loaded microemulsion for intranasal delivery and evaluation on the targeting efficiency to the brain. Int J Pharm 275:85–96
