Abstract
Purpose: A topical microemulsion (ME)-based hydrogel was developed to enhance permeation of an antifungal drug, sertaconazole (STZL) for effective eradication of cutaneous fungal infection.
Methods: Pseudo-ternary phase diagrams were used to determine the existence of MEs region. ME formulations were prepared with oleic acid, Tween 80, propylene glycol (PG) and water. Carbopol 940 (0.75% w/w) was used for preparation of hydrogel of STZL microemulsion (HSM) and characterized. The in vitro and in vivo evaluation of prepared HSM and commercial cream of STZL were compared.
Results: The viscosity, average droplet size and pH of HSM were 154.23 ± 0.54 to 162.52 ± 0.21 Pas, 42.3–91.7 nm and 6.9–7.2 , respectively. Permeation rate of STZL from optimized formulation (HSM-4), composed with oleic acid (8.75 % w/w), Tween 80 (33.35% w/w), PG (33.35% w/w) and water (24.55% w/w) was observed higher in compare with other HSMs and commercial cream. HSM-4 was stable, three times higher drug retention capacity in skin than commercial cream and did not caused any erythema or edema based on skin sensitivity study on rabbit. The average zone of inhibition of HSM-4 (23.54 ± 0.72 mm) was higher in compare with commercial cream (16.53 ± 0.63 mm) against Candida albicans.
Conclusion: The results of study showed that ME played a major role in permeation enhancing and skin retention effect of HSM and the concentration of STZL used for cutaneous fungal infection could be decreased by using ME based hydrogel preparation.
Introduction
Sertaconazole (STZL) nitrate is a potent imidazole derivative antifungal drug that maintains the antifungal activity against dermatophytoses, Tinea versicolor, cutaneous candidiasis, Seborrhoeic dermatitis and vaginal candidiasis by inhibiting the ergosterol synthesis. It is administered through topical drug delivery system like cream, gel, etc. (Carrillo-Muñoz et al., Citation2005). Although many drugs have been incorporated in microemulsion (ME) for topical delivery, STZL has not been evaluated yet. Various STZL commercial creams are available in the market and permeation ability of drug is a major challenge in recent year.
ME is commonly known as an water-in-oil (w/o) or oil-in-water (o/w) emulsion producing a transparent product having droplet size from 10 to 100 nm and does not have the tendency to coalesce (Lawrence & Rees, Citation2000; Kreilgaard, Citation2002). It is composed of oil phase, surfactant, co-surfactant and aqueous phase at appropriate ratio (Mohammed & Manoj, Citation2000). Physicochemical properties of ME include transparency, optical isotropy, low viscosity and thermodynamic stability (Baroli et al., Citation2000). It is promising for topical delivery of drugs as an efficient route of drug administration (Kreilgaard et al., Citation2000; Rhee et al., Citation2001; Baboota et al., Citation2007; Kamal et al., Citation2007; Yunpeng et al., Citation2014). Several mechanisms have been proposed to explain the advantages of ME for the topical and dermal delivery of drugs (Paolino et al., Citation2002; Zhao et al., Citation2006). First, the thermodynamics toward the skin is increased due to large amount of a drug incorporated in the formulation. Second, the increased thermodynamic activity of the drug may favor its partitioning into the skin. Third, the ingredients of ME may reduce the diffusional barrier of the stratum corneum and increase the permeation rate of drug via skin by acting as permeation enhancers. Also, the hydration effect of ME on the stratum corneum may influence the permeation ability of formulations (Delgado-Charro et al., Citation1997).
Carbopols are polymers of acrylic acid cross-linked with polyalkenyl ethers or divinyl glycol. They are essentially non-toxic and non-irritant materials with no evidence of their hypersensitivity in human subjects when used topically as hydrogel. Because of their hydrophilic nature, the cross-linked structures of carbopols make them potential candidates for the use as gel type formulation for topical use (Das et al., Citation2013). A hydrogel is a macromolecular polymer gel constructed of a network of crosslinked polymer chains. Hydrogels are formed from hydrophlic monomers by either chain or step growth, along with a functional crosslinker to promote network formation. A net-like structure along with void imperfections enhances the hydrogel's ability to absorb large amounts of water via hydrogen bonding (Carnali & Naser, Citation1992).
To enhance the cutaneous anti fungal activity of the drug, ME-based carbopol 940 hydrogels of STZL had been developed, evaluated and optimized in current work. Initially suitable oils among isopropyl myristate (IPM), di-isopropyl adaptate (DIA), isopropyl palmitate (IPP) and oleic acid; co-surfactants among propylene glycol (PG) and propanolol and surfactants among Cremophor-EL and tween 80 were selected through supersaturated solubility study of drug. Pseudo-ternary phase diagrams were used for the selection of concentration ranges of different components like oil, surfactant, co-surfactant and water for the development of MEs. The hydrogel of STZL (2% w/w) microemulsions (HSM) were prepared by incorporating 2.5% w/w STZL containing MEs in 0.75% w/w of carbopol 940 and characterized for physicochemical characteristics. The prepared formulations and commercial cream were evaluated for in vivo permeation and skin retention studies, skin sensitivity studies, stability studies and antifungal studies for comparison purpose.
Materials
STZL was obtained as a gift sample from Ajanta Pharmaceutical, Mumbai, India. Cremophor-EL (C-EL), tween-80, PG, IPM, DIA, IPP, Olive oil (OA) propanolol and carbopol 940 were procured from SD Fine Chemical Ltd., Mumbai, India.
Methods
Solubility study of drug
The solubility of STZL was carried out (Hilton et al., Citation1994) to select appropriate oil (OA, IPM, DIA and IPP), surfactant (Tween 80 and C-EL) and co-surfactant (PG and propanolol). An excess amount of STZL was added to 5 ml of oil or surfactant or co-surfactant and the resulting mixture was shaken reciprocally at 37 °C for 72 h followed by centrifugation for 10 min at 12 000 rpm. The supernatant was filtered through a membrane filter paper (0.45 µm) and the filtrate was analyzed in UV–Visible spectrophotometer (UV-1700, Shimadzu, Kyoto, Japan) at 260 nm with suitable dilution (Patel & Patel, Citation2012). The oil or surfactant or co-surfactant that showed high solubility of STZL was used in the preparation of MEs containing 2% w/w STZL.
Pseudo-ternary phase diagram
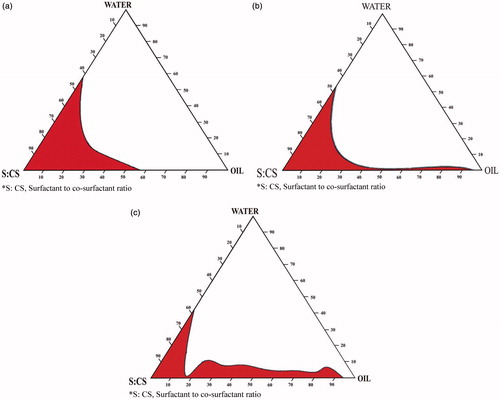
Pseudo-ternary phase diagram (Chen et al., Citation2006) were constructed to find the area of ME existence and to study the effect of different surfactant/co-surfactant weight ratios on the extent of stable ME region. The weight ratio of surfactant/co-surfactant varied as 1:2, 2:1 and 3:1. For each phase diagram a specific surfactant/co-surfactant weight ratio, the oily mixture containing oil, surfactant and co-surfactant were prepared with the weight ratio of oil to the mixture of surfactant and co-surfactant at 1:9, 1.5:8.5, 2:8, 2.5:7.5, 3:7, 3.5:6.5, 4:6, 4.5:5.5, 5:5, 5.5:4.5, 6:4, 6.5:3.5, 7:3, 7.5:2.5, 8:2, 8.5:1.5 and 9:1, respectively. Water was added drop wise to each oily mixture with continuous stirring at 37 °C until the mixture became clear at a certain point. The pseudo-ternary phase diagrams were prepared by considering three components (oil, the mixture of surfactant and co-surfactant, Water). The amount of components required for formulation of ME was chosen from phase diagram.
ME preparation
From the phase diagrams, STZL-loaded MEs were selected at different component ratios. ME systems were prepared by mixing oil with the mixture of surfactant and co-surfactant and, water was added precisely drop by drop into oily phases with magnetic stirring at 37 °C (). The systems were equilibrated with gently magnetic stirring for 30 min followed by dissolving of appropriate amount of STZL under ultrasonication (Zhu et al., Citation2008). The final concentration of STZL in ME formulation was 2.5% w/w.
Table 1. Composition of selected HSM formulations.
Hydrogel preparation
Carbopol 940 was selected as macromolecular polymer gel matrix based on previous reports (Zhu et al., Citation2009). Hydrogel of sertaconazole microemulsion (HSM) was prepared () using 0.75% w/w of carbopol 940 by swelling with a little water for 24 h. STZL-loaded ME (volume equivalent to 2% w/w of STZL in final HSM formulation) was slowly added in to high viscous solution of carbopol 940 under magnetic stirring. Triethylamine was added drop wise into it till a semisolid gel like consistency was obtained. The pH at gel consistency stage was within 6–8.
Characterization of ME and hydrogel
Electrical conductivity
Electrical conductivity of ME was measured using conductivity meter (DDS-11C, Shanghai Instrument, Shanghai, China). Based on electrical conductivity, the phase system of ME system were determined.
pH and refractive index
The pH of MEs was determined at 25 °C using the PH 110 digital acidometer (Lovibond, Wiltshire, UK) and refractive indices were measured with a thermostated Abbe refractometer (Shijiazhuang Optical Instrument Factory, Shijiazhuang, China).
Average droplet size and poly dispersity index
The average droplet size of both ME and HSM and, poly dispersity index of the ME were determined after suitable dilution in distilled water using photon correlation spectroscopy (Nano ZS90, Malvern Instruments, Worcestershire, UK). Measurements were performed at 25 °C using a He–Ne laser.
Rheology
The viscosity of ME and HSM were measured at 25 °C, using the NDJ-8S digital viscometer (Shanghai Precision and Scientific Instrument, Shanghai, China) with a No. 1 rotor set at 60 rpm.
Permeation study
Mice abdominal skin preparation
The animal study was conducted in accordance with the approval of the Animal Ethical Committee, Gayatri College of Pharmacy, Sambalpur, Odisha, India. Male mice weighing 20–24 g were sacrificed using anesthetic ether. The hair of test animals was carefully trimmed with electrical clippers and the full thickness skin was removed from the abdominal region. The epidermis was prepared surgically by heat separation technique (Chen et al., Citation2007), which involved soaking the entire abdominal skin in water at 60 °C for 45 s, followed by careful removal of the epidermis. The epidermis was washed with water, stored at 4 °C overnight and then used for in vivo permeability studies.
In vivo permeation studies
Franz diffusion cell with an effective diffusion area of 2.8 cm2 was used for ex vivo permeation studies (Kreilgaard et al., Citation2002; Valenta & Schultz, Citation2004) of HSM and commercial cream “Onabet”. The skin samples were mounted carefully on diffusion cells and donor compartment consisting of test HSM. The receiver compartment was consisted of 30 ml ethanolic water to ensure sink condition and its temperature was maintained at 37 ± 0.5 °C with magnetic stirring at 600 rpm through out the experiment. For each experiment, 1 ml sample of the receiver medium was withdrawn at predetermined time and replaced immediately with an equal volume of fresh receiver medium (Peltola et al., Citation2003). The samples were analyzed in UV–Visible spectrophotometer (UV-1700, Shimadzu) at 260 nm with suitable dilution (Patel et al., Citation2012). The cumulative amount of STZL permeated through mice skin was plotted as a function of time.
Calculation of in vivo data
Cumulative amount of drug (Qn, µg cm−2) in all preparations in the receptor chamber was plotted as a function of time (t, h). The cumulative amount (% w/w) of STZL permeated through excised mice skins was determined based on the following equation (Zhu et al., Citation2008):
(1)
where Cn stands for the drug concentration of the receptor medium at each sampling time, Ci for the drug concentration of the ith sample, and V0 and Vi stand for the volumes of the receiver solution and the sample, respectively, S for the effective diffusion area.
Difference and similarity factors
The in vivo drug permeation profile of the HSM (test) was compared with the drug permeation profile of commercial Onabet cream (reference) determining the “difference factor”, f1 and “similarity factor”, f2 (Geoffroy et al., Citation1998; Hamed & Sakr, Citation2001). The difference factor (f1) measures the percentage of error between the two curves over all time points and was calculated by using Equation (Equation2(2) ).
(2)
where n is the number of sampling points, Rj and Tj are the percentage of dissolved reference and test products at each time point j, respectively.
The similarity factor (f2) is a logarithmic transformation of the sum of squared error of differences between the test Tj and the reference products Rj over all time points. It was calculated as Equation (Equation3(3) ).
(3)
where wj is an optional weight factor and other terms are as defined earlier.
Determination of t50 and t90
The time required for 50% of drug permeation (t50) and 90% of drug permeation (t90) was calculated by fitting data to the Korsmeyer and Peppas equation (Korsmeyer et al., Citation1983):
(4)
where Mt/M∞ represents the fraction of drug permeate at time t, k is the release rate constant, and n is the diffusion coefficient. The entire curve-fitting analysis was performed using Excel (Microsoft) software.
Skin retention study
Protocol for skin retention study was approved by the Institutional Animal Ethics Committee. Skin was obtained from the abdomen of male mice weighing 20–24 g. The full-thickness skin was excised after hair was removed with a depilatory. Subcutaneous fat and other extraneous tissues were trimmed; the skin was washed with physiological saline followed by phosphate buffered saline (pH 7.4) and then visually inspected for integrity to ensure the absence of holes or other imperfections. The excised mice skin were stored at −20 °C and used within 1 week of harvest. The skin was placed with stratum corneum facing upward (inside of the tube) and dermal side downward (to face the medium). The position-fixed skin was made water tight by a rubber band. Assembly was adjusted as previously mentioned under the in vivo diffusion study. After 24 h, the effective diffusion area of the skin was separated, washed several times with distilled water, to remove formulation excess and then cut into small pieces. The segments obtained were vortexed with ethanol and then left for soaking for 24 h to ensure effective extraction of the retained drug from the skin. The resulting mixture was then filtered using 0.45 μm syringe filter and 1 ml from the filtrate was diluted with receptor fluid then filtered and STZL was quantified using UV spectrophotometry at λmax 260 nm (Patel & Patel, Citation2012).
Skin sensitivity study
This study was performed on rabbits (weighing 2.0–2.5 kg) to evaluate the irritant potential of the developed formulation after topical application (Draize et al., Citation1944). The hairs on the back of rabbit were removed 24 h prior to the administration of formulations (Osborne et al., Citation1991), whereas the control group was treated with normal saline. HSM-4 and the saline solution containing 2% w/w STZL was applied to the treatment group twice a day for 5 d consecutively (n = 3) (Richardson et al., Citation1988). The animals were observed for any signs of itching or change in skin such as erythema, papule, flakiness and dryness for a period of 5 d (Jain et al., Citation1996). After withdrawal, observation for single or multiple administrations was continued for 3 d (Dreher et al., Citation1996). The irritation scores of the test area were obtained by judging the extent of erythema and edema according to the criteria (Paolino et al., Citation2002). Erythema and edema were graded as follows: 0 for no visible reaction, 1 for just present reaction, 2 for slight reaction, 3 for moderate reaction and 4 for severe reaction. Eventually, the total scores for irritation test in each condition were calculated using the following Equation (Equation5(5) ) (Osborne et al., Citation1991; Butani et al., Citation2014).
(5)
Stability study
The optimized HSM was filled in lacquered aluminum collapsible tubes and stored at three different temperatures 5 ± 2 °C, 25 ± 2 °C and 40 ± 2 °C for a period of 6 months. Samples were withdrawn after specified intervals and evaluated for drug content, pH, transparency, clarity, non-grittiness and color change. The centrifuge test was also carried out to assess the physical stability of formulations by centrifuging HSM at 13 000 rpm for 30 min.
Antifungal study
The in vitro antifungal studies of optimized stable HSM formulation was carried out by the cup plate method using Candida albicans. Sterilized Sabouraud’s agar nutrient medium (25 ml) was poured into sterilized Petri plates (diameter 15 cm) under laminar air flow and allowed to solidify. The 0.4 ml aqueous suspension of C. albicans was spread uniformly on solidified Sabouraud’s agar nutrient medium. The cups were cut and formulations were filled into different cups using sterilized syringes, under laminar air flow. Plates were covered with lids and incubated in BOD at 32 °C for 40 h. The zones of inhibition were measured after 40 h (Alam et al., Citation2007).
Comparison of optimized formulation with the commercial cream
In vivo permeation behavior, skin retention characteristics and antifungal potential of optimized HSM formulation (STZL content, 2% w/w) were compared with the commercial cream (STZL content, 2% w/w) as a control one.
Results and discussion
Solubility study of drug
The solubility of STZL in various media was analyzed in order to screen components for ME. Previous reports indicated that the superior dermal flux appeared mainly due to large solubilizing capacity of the MEs, which led to larger concentration gradient toward the skin (Lawrence & Rees, Citation2000). Among four oils, the solubility of STZL was highest in oleic acid (35 mg/ml), followed by IPM (29 mg/ml), DIA (18 mg/ml) and IPP (16.5 mg/ml). It has reported that oleic acid is a powerful permeation enhancer for dermal delivery since it could increase fluidity of lipid portion of the stratum corneum (Kogan & Garti, Citation2006; Baboota et al., Citation2007). So oleic acid was chosen as oil for the preparation of the MEs containing STZL. In two used surfactants, STZL had a higher solubility in Tween 80 (47 mg/ml) than Cremophor-EL (36.5 mg/ml). Among two co-surfactants the solubility of STZL in PG (28.4 mg/ml) was more than propanolol (17.2 mg/ml). PG has a good ability in forming MEs with oleic acid and Tween 80 and its aqueous solution has a good solubility of STZL which can form a concentration gradient. So oleic acid, Tween 80 and PG were subsequently used as the oil phase, surfactant and co-surfactant for the formulation of MEs containing STZL in this study.
Pseudo-ternary phase diagram
The studied systems composed of safe constituents including oleic acid, PG, Tween 80 and water. The construction of phase diagrams makes it easy to find out the concentration range of components for the existence range of MEs. The pseudo-ternary phase diagrams with various weight ratios of Tween 80 to PG are described in . The translucent ME region is presented in phase diagrams. No distinct conversion from w/o to o/w MEs was observed. The gel area shows the transparent and high viscosity region. The rest of the region on the phase diagram represents the turbid and conventional emulsions based on visual observation. No liquid crystalline structure was observed using cross polarizer. The area of ME isotropic region changed slightly in size with the increasing ratio of surfactant to cosurfactant. A similar result was obtained from an ethyl laurate-based ME system with Tween 80 as surfactant, PG and ethanol as cosurfactant (Li et al., Citation2002).
Preparation and characterization of formulations
The results of this investigation indicate development of successful ME formulations of STZL with optimum characteristics. All ME formulations were clear and transparent which were incorporated into hydrogel. The conductivity of different MEs was found in range between 137.0 ± 2.3 µs cm−1 and 194.5 µs cm−1. This result shows the MEs were o/w type. The drug content in ME was within the range between 97.86 ± 0.54% and 99.74 ± 0.35%. The refractive index of all ME was ranged between 1.35 and 1.40 signifies that prepared ME were clear and transparent. Polydispersity index is a measure of particle homogeneity and it varies from 0.139 to 0.363. It signifies particle in ME had narrow size distribution. When the MEs were mixed with carbopol 940 which had adequately swelled in water, the appearance of carbopol 940 viscous solution became ivory white, which was in accordance with the results obtained by Chen et al. The possible reason is that the dehydration of some ingredients such as surfactant and co-surfactant in ME makes carbopol 940 dissociated from hydrated state. The prepared HSMs were subjected for drug content, viscosity, pH, droplet size measurement studies and results are depicted in . The drug content in HSM was within the variation of 100 ± 2%. The viscosity of HSMs (154.23 ± 0.5 to 162.52 ± 0.2 Pas at 25 °C) was more than the viscosity of ME (89.0 ± 0.4 to 356.7 ± 0.6 mPa s at 25 °C) which is suitable for topical application. The pH value of HSM was in between 6.4 and 6.9. The pH near to 7 indicates HSM could result less stimulation to skin than ME. The average droplet size of ME in gels was ranged from 42.3 to 91.7 nm, respectively. It indicated that the gel network of carbopol 940 could not markedly influence the diameter of ME droplets.
Table 2. Characterization of HSM.
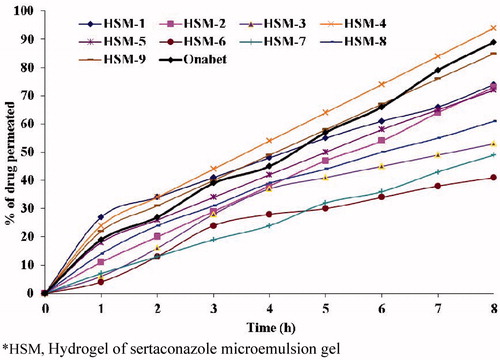
In vivo permeation study
The in vivo permeation profiles of HSM and commercial cream, Onabet through excised abdominal skins of mice are shown in . The time required to release 50% (t50) and 90% (t90) of drug was calculated and results are depicted in The HSM having less t50 and t90 values indicate faster permeation rate. It is observed from , t50 (3.4) and t90 (8.4) of HSM-4 are less than other formulations including Onabet cream (t50, 6.5 h and t90, 12.1 h). It indicates the permeation rate of STZL in HSM-4 is higher than the other formulations and Onabet cream. Hence, it was considered as best formulation and selected for antifungal study. The ingredients such as OA and PG in ME act as permeation enhancers could significantly reduce the barrier of stratum corneum and increase the diffusion coefficient of drug in skin, in addition to the contributions produced by ME as the nano carrier for drug, such as a very large surface area for drug transfer to skin due to the very small droplet size and high drug concentration within the upper layers of the skin which results in a higher concentration gradient as the driving force for transdermal drug delivery. However, f1 and f2 were determined to know the formulations best fitted with permeation profile of commercial cream. For f1 and f2, in vivo permeation profiles of Onabet were considered as reference and prepared HSM was considered as test. The f1 value less than 15 and f2 value more than 50 signifies permeation profile of test is good agreement wit reference. In this study (), the permeation profiles of HSM-3 (f1, 11.7; f2, 66.9) and HSM-8 (f1, 13.5; f2, 63.5), was good agreement with the permeation profile of Onabet.
Table 3. In vitro drug permeation study.
Skin retention study
The skin retention of STZL was 32.68, 62.42 and 103.26 μg for plain drug solution, commercial cream and HSM-4, respectively. HSM-4 had 3 and 1.5 times higher drug deposition capacity than commercial cream and plain drug solutions, respectively. This might be due to faster release observed for the drug from HSM-4 compared to commercial cream and plain drug solution thereby more drugs penetrates into the skin by which more amount of drug retains in the skin. If more amount of drug retains in the skin, fungus present in deep tissues were treated more effectively. Hence it can be inferred from skin retention study that the optimized formulation, HSM-4S was more efficient for topical fungal diseases.
Skin sensitivity study
The intensity criterion of skin irritation followed the protocol, that scores of <0.5 meant no irritation, 0.5–3 for slight irritation, >6 showed severe irritation and others showed moderate irritation (Butani et al., Citation2014). The irritation studies showed very little visible irritation after application of optimized HSM-4 and aqueous saline solution containing 2% w/w STZL for 5 d on the rabbit skin. The average response scores of skin irritation found for HSM-4 are 0.06 ± 0.000 (48 h) and 0.11 ± 0.003 (72 h); and for saline solution with 2% w/w STZL are 0.74 ± 0.032 and 1.28 ± 0.027. The saline solution of STZL induced significant erythema, edema and irritation. This is because of the irritation property of STZL. In contrast HSM-4 showed very minute irritation may be due to encapsulation of STZL by ME in HSM.
Stability study
Optimized HSM exhibited transparency, clarity and no drug precipitation or color change when it was subjected to stability study at 5 ± 3 °C, 25 ± 2 °C and 40 ± 2 °C for 6 months. The organoleptic features like gel viscosity, gel strength, physical appearance were also observed and no significant change was found in these characters. The centrifuge tests showed that all formulations had good physical stability. The o/w structure of HSM and suitable pH ranging from 6.0 to 7.0 provided a suitable circumstance for avoiding the hydrolysis of drug (Zhao et al., Citation2006).
Anti-fungal study
The anti-fungal efficacy of the developed HSM-4 was compared with commercial formulations (Onabet). Developed HSM-4 was found to be more effective anti-fungal activity in compare to commercial cream. The average zone of inhibition of HSM-4 against C. albicans was 23.54 ± 0.72 mm compared to 16.53 ± 0.63 mm of Onabet (), indicating significantly higher efficacy of HSM-4 gel (p < 0.001). From the results of in vitro antifungal activity it is clear that the developed HSM-4 was more effective than the tested commercial formulation.
Table 4. Comparison of zone of inhibition of optimized HSM gel with commercial cream.
Conclusion
In current study, the application of ME systems in gel form for topical delivery of STZL was investigated and pseudo ternary phase diagram was used to optimize the formulations. The results suggested that the ME played a role in permeation enhancing and skin retention effect of hydrogel (0.75% w/w of carbopol 940) of STZL microemulsion (HSM). Compared with commercial cream, the skin permeation ability of STZL was significantly increased by HSM-4. The optimized formulation, HSM-4 containing drug loaded ME composed with oleic acid (8.75% w/w), Tween 80 (33.35 % w/w), PG (33.35% w/w) and water (24.55% w/w), was stable after storing at 5 ± 3 °C, 25 ± 2 °C and 40 ± 2 °C for 6 months. The results of in vitro antifungal activity infer that the developed HSM-4 was more effective than the tested commercial formulation. It is promising that the concentration of STZL used to treat cutaneous fungal infection could be decreased due to the high permeation and anti-fungal ability of STZL in HSM-4.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Alam MA, Ahmad FJ, Khan ZI, et al. (2007). Development and evaluation of acid buffering bioadhesive vaginal tablet for mixed vaginal infections. AAPS PharmSciTech 8:Article 109
- Baboota S, Al-Azaki A, Kohl K, et al. (2007). Development and evaluation of a microemulsion formulation for transdermal delivery of terbinafine PDA. J Pharm Sci Technol 61:276–85
- Baroli B, Lopez-Quintela MA, Delgado-Charro MB, et al. (2000). Microemulsions for topical delivery of 8-methoxsalen. J Control Release 69:209–18
- Butani D, Yewale C, Misra A, Amphotericin B topical microemulsion. (2014). Formulation, characterization and evaluation. Coll Surfaces B Biointerfaces. 116:351–8
- Carnali JO, Naser MS. (1992). The use of dilute solution viscometry to characterize the network properties of carbopol microgels. Colloids Polym Sci 270:183–93
- Carrillo-Muñoz AJ, Giusiano G, Ezkurra PA, et al. (2005). Updated review of a topical antifungal agent. Expert Rev Anticanc 3:333–42
- Chen HB, Chang XL, Du DR, et al. (2006). Microemulsion based hydrogel formulation of ibuprofen for topical delivery. Int J Pharm 315:52–8
- Chen H, Mou D, Du D, et al. (2007). Hydrogel-thickened microemulsion for topical administration of drug molecule at an extremely low concentration. Int J Pharm 341:78–84
- Das B, Nayak AK, Nanda U. (2013). Topical gels of lidocaine HCl using cashew gum and Carbopol 940: preparation and in vitro skin permeation. Int J Biol Macromol 62:514–17
- Delgado-Charro MB, Iglesias-Vilas G, Blanco-Ménde J, et al. (1997). Delivery of a hydrophilic solute through the skin from novel microemulsion systems. Eur J Pharm Biopharm 43:37–42
- Draize J, Woodard G, Calvery H. (1944). Methods for the study of irritation and toxicity of substances topically applied to skin and mucous membranes. J Pharmacol Exp Ther 82:377–90
- Dreher F, Walde P, Luisi P, Elsner P. (1996). Human skin irritation studies of a lecithin microemulsion gel and of lecithin liposomes. Skin Pharmacol 9:124–9
- Geoffroy JM, Fredrickson JK, Shelton JT. (1998). A mixture experiment approach for controlling the dissolution rate of a sustained-release tablet. Drug Dev Ind Pharm 24:799–806
- Hamed E, Sakr A. (2001). Application of multiple response optimization technique to extended release formulation design. J Control Release 73:329–38
- Hilton J, Woollen BH, Scott RC, et al. (1994). Vehicles effect on in-vitro percutaneous absorption through rat and human skin. Pharm Res 11:396–1400
- Jain GK, Sharma A, Agrawal S. (1996). Transdermal controlled administration of verapamil—enhancement of skin permeability. Int J Pharm 130:169–77
- Kamal MA, Iimura N, Nabekura T, Kitagawa S. (2007). Enhanced skin permeation of diclofenac by ion-pair formation and further enhancement by microemulsion. Chem Pharm Bull 55:368–71
- Kogan A, Garti N. (2006). Microemulsions as transdermal drug delivery vehicles. Adv Colloid Interface Sci 123–126:369–85
- Korsmeyer RW, Doelker GEP, Peppas NA. (1983). Mechanisms of potassium chloride from compressed, hydrophilic, polymeric matrices: effect of entrapped air. J Pharm Sci 72:1189–91
- Kreilgaard M. (2002). Influence of microemulsions on cutaneous drug delivery. Adv Drug Deliv Rev 54:77–98
- Kreilgaard M, Pedersen EJ, Jaroszewski JW. (2000). NMR characterisation and transdermal drug delivery potential of microemulsion systems. J Control Release 69:421–33
- Lawrence MJ, Rees GD. (2000). Microemulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev 45:89–121
- Li L, Nandi I, Kim KH. (2002). Development of an ethyl laurate based microemulsion for rapid-onset intranasal delivery of diazepam. Int J Pharm 237:77–85
- Mohammed C, Manoj V. (2000). Aerosol-OT microemulsions as transdermal carriers of tatracaine hydrochloride. Drug Dev Ind Pharm 26:507–12
- Osborne DW, Ward AJ, O'Neill KJ. (1991). Microemulsions as topical drug delivery vehicles: in-vitro transdermal studies of a model hydrophilic drug. J Pharm Pharmacol 43:450–4
- Paolino D, Ventura CA, Nisti S, et al. (2002). Lecithin microemulsion for the topical administration of ketoprofen: percutaneous adsorption through human skin and in vivo human skin tolerability. Int J Pharm 244:21–31
- Patel A, Patel J. (2012). Design development and in vitro evaluation of sertaconazole mucoadhesive vaginal tablet. Der Pharma Lett 4:418–27
- Peltola S, Saarinen-Savolainen P, Kiesvaara J, et al. (2003). Microemulsions for topical delivery of estradiol. Int J Pharm 254:99–107
- Rhee YS, Choi JG, Park ES, Chi SC. (2001). Transdermal delivery of ketoprofen using microemulsions. Int J Pharm 228:161–70
- Richardson SE, Bannatyne RM, Summerbell RC, et al. (1988). Disseminated fusarial infection in the immunocompromised host. Rev Infect Dis 10:1171–81
- Valenta C, Schultz K. (2004). Influence of carrageenan on the rheology and skin permeation of microemulsion formulations. J Control Release 95:257–65
- Yunpeng F, Lin M, Weimin Z, et al. (2014). Microemulsion can improve the immune-enhancing activity of propolis flavonoid on immunosuppression and immune response. Int J Biol Macromol 63:126–32
- Zhao X, Liu JP, Zhang X, Li Y. (2006). Enhancement of transdermal delivery of theophylline using microemulsion vehicle. Int J Pharm 327:58–64
- Zhu W, Yu A, Wang W, et al. (2008). Formulation design of microemulsion for dermal delivery of penciclovir. Int J Pharm 360:184–90
- Zhu W, Guo C, Yu A, et al. (2009). Microemulsion-based hydrogel formulation of penciclovir for topical delivery. Int J Pharm 378:152–8