Abstract
Sildenafil citrate, a drug used to treat erectile dysfunction, is available in tablet form but has three major problems. First, the drug displays poor aqueous solubility, which delays its onset of action. Second, the drug undergoes extensive first-pass metabolism, resulting in a low (40%) bioavailability. Third, the gastrointestinal effects of sildenafil citrate include dyspepsia and a burning sensation. The objective of this study was to prepare sildenafil citrate using a fast orodissolvable film (ODF) containing the drug in a solid dispersion (SD) to mitigate the abovementioned problems. The solubility of sildenafil citrate in β-cyclodextrin derivatives was estimated, and SDs were prepared and characterized. To develop an ODF that disintegrates rapidly and releases the maximum amount of sildenafil citrate, a 33 Box–Behnken experimental design was used to estimate the effects of different concentrations of film forming polymer (X1), the film modifier (X2), and the plasticizer (X3) on the responses, i.e. the disintegration time (Y1) and the amount of drug released (Y2). Pharmacokinetic studies with the optimized (ODF) were conducted on human volunteers. SD prepared using hydroxybutyl-β-cyclodextrin enhanced the solubility of sildenafil citrate by more than eightfold. The Y1 for the optimized ODF was 89 seconds, and the Y2 was 86%; this formula also exhibited a rapid onset of action, and its bioavailability was enhanced by 2.25-fold compared with that of the marketed tablet. The ODF is a promising formulation for sildenafil citrate that results in higher solubility, a rapid onset of action, and enhanced systemic bioavailability.
Introduction
Sildenafil citrate is the most common drug used for the treatment of erectile dysfunction. This compound is nearly insoluble in water and undergoes extensive first-pass metabolism, which leads to its low oral bioavailability (40%) and an onset of action that is attained within 60 minutes Al-Ghazawi et al. Citation2010. The drug lasts for four hours, although a lower response is observed at this time point compared with that at two hours Walker et al. Citation1999. The major adverse effects of this drug include gastrointestinal effects, such as dyspepsia, burning sensation, and interactions with food; in particular, fatty foods hinder its absorption Nichols et al. Citation2002.
Cyclodextrins have recently been recognized as useful excipients and form a group of cyclic oligosaccharides containing a truncated cone or torus structure. These structures can accommodate several types of lipophilic species within their cavities, enhancing the solubility of the lipophilic molecules due to the hydrophilic exterior surface and nonpolar interior cavity Fernandes et al. Citation2002. Typical natural β-cyclodextrins contain seven units of glucose monomers Chen and Jiang Citation2011. Recently, several grades of synthetic β-cyclodextrins, such as hydroxypropyl-β-cyclodextrin and hydroxybutyl-β-cyclodextrin, have become available.
Several techniques have been used in research and manufacturing to improve oral bioavailability. The utilization of solid dispersion technology through the formation of an inclusion complex between the drug and some soluble carriers is the most common technique and results in improvements in the solubility and enhancements in the dissolution rate Dahima et al. Citation2010. Therefore, this technology should improve the bioavailability of drugs, such as sildenafil citrate.
Orodissolvable films (ODFs) are a relatively new oral dosing form; these postage stamp-sized strips of thin polymeric films are formulated to disintegrate or dissolve almost instantaneously when placed on the tongue Cilurzo et al. Citation2010, El-Setouhy and El-Malak Citation2010. A drug in an ODF is absorbed through the oral mucosa and thus enters the systemic circulation without undergoing first-pass hepatic metabolism Malke et al. Citation2010. This formulation is administered easily and provides fast pharmacological action, thereby improving patient compliance. Furthermore, ODFs can be formulated using solid dispersions, thereby increasing the solubility of the drug.
The Box–Behnken experimental design is one of the response surface models used to hit the target, reduce variability in the experiment, maximize/minimize a response that increases the production yield or decreases the amount of waste, and represent opportunities for extensive financial gain Karnachi and Khan Citation1996. As this model has an orthogonal design, the factor levels are evenly spaced and coded for low, medium, and high settings: −1, 0, and +1, respectively. Therefore, the process is optimized to obtain the levels of the independent variables that provide the optimum response values Rajput et al. Citation2011.
To mitigate the abovementioned problems associated with the commercially marketed product, this work aimed to prepare sildenafil citrate citrate as a fast ODF containing the drug in a solid dispersion (SD) with β-cyclodextrin derivatives through the utilization of Box–Behnken experimental design.
Experimental methods
Materials
Sildenafil citrate was generously donated by EIPICO (Cairo, Egypt). Propylene glycol was purchased from Fluka AG, Buchs SG (Switzerland). Hydroxypropyl cellulose (HPC) and guar gum were purchased from Aqualone, London (UK); cyclodextrin (β-CD, hydroxypropyl-β-CD, and hydroxybutyl-β-CD) was donated by Nihon Shokuhin Kako Co., Ltd. Tokyo (Japan). All other chemicals were of analytical grade.
Methodology
Phase solubility studies
The phase solubility was analyzed according to Highuchi and Connors Citation1965. Excess sildenafil citrate was dispersed into a 20 mL aqueous solution of a hydrophilic carrier (β-cyclodextrin, hydroxypropyl-β-cyclodextrin, and hydroxybutyl-β-cyclodextrin) at various concentrations (1–10 mM/L). The mixture was then stirred for 24 h at 37 °C ± 0.5 °C. After equilibrating, the samples were filtered through a 0.22 μm nylon disc filter, and the absorbance was read after suitable dilution at 291 nm (Shimadzu 1700 UV-Visible spectrophotometer). The apparent stability constant (Ks) was calculated according to the hypothesis of a 1:1 stoichiometric ratio of complexes using the following equation:
where So is the equilibrium solubility of sildenafil citrate in water.
Preparation of the solid dispersion
After the phase solubility analysis, hydroxybutyl-β-cyclodextrin was chosen for the solid dispersion with sildenafil citrate. The SDs were prepared using two different techniques: kneading and coevaporation. For the kneading method, the required amounts of hydroxybutyl-β-cyclodextrin and distilled water were mixed in a mortar until a homogeneous paste was obtained. Sildenafil citrate was then added portion-wise while triturating with small amounts of methanol and an appropriate amount of water. Subsequently, the pastes were dried at 40 °C. The obtained SD was crushed and passed through an 80-mesh screen before being stored in a dry place until use Veiga et al. Citation1996. For coevaporation, sildenafil citrate and hydroxybutyl-β-cyclodextrin were mixed at a 1:1 molar ratio, and methanol was added under constant stirring until dissolution. The solvent was then evaporated, and the resultant solid dispersion was crushed and passed through an 80-mesh screen sieve before being stored in a dry place until use Patel and Patel Citation2007.
Solubility analysis of the prepared solid dispersion
Excess SD was dispersed in 5 mL of distilled water to obtain a supersaturated solution. These dispersions were shaken for 24 h at 25 °C until equilibrated. Two milliliters of the supersaturated solution was filtered through a 0.22 μm nylon filter, and 1 ml of the filtrate was diluted with methanol. The absorbance was measured at 291 nm. Solubility studies were also performed with pure sildenafil citrate.
Box–Behnken experimental design
Preliminary studies revealed the formulation variables that significantly affect the characteristics of sildenafil citrate (ODF). These factors include the concentration of hydroxypropyl cellulose (HPC) used as the film-formation polymer (X1), the concentration of guar gum (GG) used as the film modifier (X2), and the concentration of propylene glycol (PG) used as the plasticizer (X3). The level for each formulation variable was selected based on the aforementioned studies and is reported in . The responses (Y1 and Y2) and their constraints are presented in . The Box–Behnken model is shown in the following equation:
where b0 through b9 are the regression coefficients, and E is the experimental error. The experimental design was optimized to calculate the levels of the different independent variables that provide the optimal dependent responses based on the regression equation generated using System Statistical software (SAS).
Table 1. Variables and their levels in the Box–Behnken design.
Table 2. Experimental runs and observed values of responses for BBD.
Film preparation
The films were prepared using a solvent casting method Aliaa and Arwa Citation2011; hydroxypropyl cellulose was used as the film-formation polymer (X1) at different concentrations according to a Box–Behnken experimental design, whereas guar gum was used as the film modifier (X2). Both the compounds were dispersed in 20 mL of distilled water with continuous stirring for 30 min and maintained in a refrigerator for 24 h to remove all of the air bubbles. Accurately weighed quantities of the sildenafil citrate solid dispersion, propylene glycol, which was used as the plasticizer (X3), and 1% aspartame, which was used as a sweetener, were dissolved separately in 2.5 mL of 50% ethanol solution in another beaker. After completely hydrating the polymer with water, the drug solution was added and mixed thoroughly in the polymer solution; the volume was increased to 25 mL with distilled water. The final solutions were poured onto a glass Petri dish and allowed to dry before being carefully removed and cut into strips. Each strip contained 50 mg of sildenafil citrate, measured 2 × 2.5 cm, and was stored in an air-tight container.
Determination of physicochemical parameters
The average weight each of 10 samples of each formulation was determined. The thickness of each of sample was measured using micrometer screw gauge at five locations, and the mean thicknesses were calculated. The folding endurance was determined by repeatedly folding one film at the same place till it broke or folded up to 300 times which is considered satisfactory to reveal good film properties. The number of times the film could be folded at the same place without breaking gives the value of the folding endurance.
In vitro disintegration of films
The in vitro disintegration time of the film strips was determined using the method described by Misra and Amin Citation2009. The film strip was placed in a glass Petri dish (6.5 cm in diameter) containing 25 mL of distilled water at 37 °C with swirling every 5 s. The disintegration time was recorded as the time at which the film began to break or disintegrate.
Release studies of sildenafil citrate from films
The release studies were conducted in a paddle dissolution apparatus. Each film strip was fixed to a glass slab at the bottom of the dissolution vessel before the dissolution test to prevent the film strips from floating. The dissolution medium was 250 mL of PBS at pH 6.8 and was maintained at 37 ± 0.5 °C and stirred at 50 rpm. Three-milliliter samples were withdrawn at 1, 5, 10, 15, 20, 25, 30, 45, and 60 min; the volume was replenished with fresh buffer at 37 ± 0.5 °C. The sildenafil citrate concentration in the filtered samples was analyzed using UV-Vis spectroscopy at 291 nm.
In vivo studies on human volunteers
To compare the pharmacokinetics of sildenafil citrate in the optimized orodispersible film (treatment A) with the conventional Viagra® tablet (Pfizer, Cairo, Egypt; treatment B), a single dose of sildenafil citrate (50 mg) was given to the volunteers using a double blind, randomized, cross-over design. Fasting subjects were administered a single 50-mg oral dose of sildenafil citrate orodispersible film or the reference product during each period of the study.
Subject population
Twelve healthy Egyptian male volunteers aged between 30 and 45 years (median weight = 72 ± 5.5 kg and median height = 177 ± 6.7 cm) were chosen. The health status of the volunteers was confirmed using a complete medical history and laboratory analyses performed at the beginning of the study. No medication was allowed during the study.
Sample collection
The study was conducted according to the Helsinki agreement protocol and the requirements of the ethical committee of the Faculty of Medicine at Beni Suef University in Egypt. The drug was administered during each period of the study with a one-week washout period. Blood samples (5 mL) were collected at the following time intervals: 0.25, 0.5, 0.75, 1, 2, 4, 6, 8, 10, 12, 18, and 24 h. The blood samples were centrifuged, and the plasma was collected and stored at −20 °C until assayed.
Chromatographic conditions
The pharmacokinetic parameters of sildenafil citrate were determined using a method based on that developed by Sheu et al. Citation2003 involving the extraction of sildenafil citrate with dichloromethane. The DCM was then allowed to evaporate under nitrogen, and the residue was dissolved in 0.2 mL of the mobile phase (acetonitrile and potassium dihydrogen phosphate [30 mM] 56:44, v/v). The column was an RPC18.
Pharmacokinetic analysis
The pharmacokinetic parameters were estimated using WinNonlin® (version 1.5, Scientific consulting, Inc., Cary, NC). Cmax (ng/mL) and Tmax (h) were the observed maximum drug concentration and the time needed to reach the maximum concentration, respectively. AUC(0–24) (ng·h/mL) was calculated using the relative bioavailability (AUC test/AUC standard × 100).
Statistical analysis
The statistical analyses were performed using a t-test for paired data with SPSS® 7.5 for Windows. The level of statistical significance was p ≤ 0.05.
Results
Phase-solubility study and solubility analysis of the prepared solid dispersion
The phase-solubility analysis evaluated the affinity between the cyclodextrin and drug molecules in water. shows the phase-solubility curve of sildenafil citrate in the presence of CDs; an increase in the concentration of the CDs resulted in increases in the solubility of sildenafil citrate. The solubility of sildenafil citrate was increased nine-fold with 50 mM/L hydroxybutyl-β-cyclodextrin, sevenfold with 50 mM/L β-cyclodextrin, and fivefold with 50 mM/L hydroxypropyl-β-cyclodextrin.
Figure 1. The phase solubility diagrams of sildenafil citrate with different concentrations of the three β-cyclodextrin carriers.
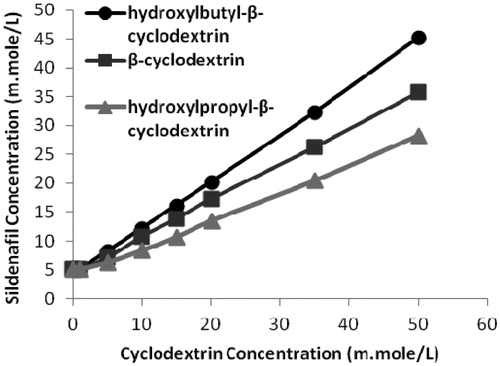
The phase-solubility plots were type A for all three CDs, indicating that a 1:1 (sildenafil citrate:CD) inclusion complex was formed. The Ks values were 954 M−1 for hydroxybutyl-β-CD, 357 M−1 for β-CD, and 205 M−1 for hydroxypropyl-β-CD, indicating that a more suitable and stable complex was formed with hydroxybutyl-β-CD compared with the other two polymers.
The saturation solubility studies revealed that the aqueous solubility of pure sildenafil citrate was 3.38 ± 0.23 mg/mL and that this solubility increased with its dispersion in hydroxybutyl-β-CD to 9.54 ± 0.42 mg/mL in a SD prepared using the kneading method and to 24.33 ± 0.61 mg/ml in a SD prepared using coevaporation.
Box–Behnken experimental design
For the 33 Box–Behnken experimental design, 15 different formulae were prepared as shown in .
Estimation of the quantitative effects of the factors
Student’s t-test was used to estimate the quantitative effects of the factors. The effects of X1, X2, and X3 on Y1 and Y2 determined through ANOVA are presented in .
Table 3. Factor effects and associated p values for Y1 and Y2 responses.
The analysis of the variance of Y1 revealed that four effects had p values less than 0.05, whereas the analysis of the variance of Y2 found six effects with p values less than 0.05. As shown in , the concentration of the film-forming polymer has a significant synergistic effect on the disintegration time and a significant antagonistic effect on the concentration of sildenafil citrate released from the film. However, the plasticizer concentration has a significant antagonistic effect on Y1 and a significant synergistic effect on Y2. Additionally, Y1 and Y2 were significantly affected by the synergistic effect of the quadratic term X1², whereas Y2 was significantly affected by the quadratic term X3². The interaction effects X1X3 and X2X3 have significant synergistic effect on the disintegration time and the percentage of sildenafil citrate released, respectively. In contrast, the interaction effect X1X2 has a significant antagonistic effect on the percentage of sildenafil citrate released. In addition, the concentration of the film modifier (guar gum) and its quadratic term (X2²) exhibited no significant effect on either the disintegration time or the concentration of sildenafil citrate released.
Table 4. Regression Equations of the fitted models.
Response surface and contour plots
The relationship between the factors and responses can be understood by plotting the response surface and contour for the estimated effects ().
Figure 2. (A) Response surface plot (3D) showing the effect of X1 and X3 on Y1. (B) contour plot showing relationship between various levels of two variables to attain fixed values of Y1. (C) Response surface plot (3D) showing the effect of X1, X2, and X3 on Y2. (D) Contour plot showing relationship between various levels of two variables to attain fixed values of Y2.
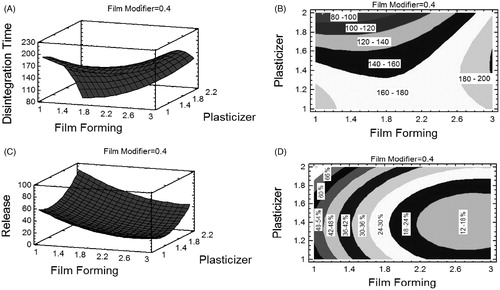
Based on the results shown in , at a fixed percentage of X2 (0.4%), an increase in X1 to 3% and a decrease in X3 to 1% decreases the disintegration time to 165 s. The use of a low level of X1 (1%) and the increase in X3 up to 2% decreases the Y1 to 100 s. The contour plot () suggests the exact percentages of X1 and X3 that minimize the disintegration time for a fixed X2 (0.4%). The use of a low level of X1 and a high level of X3 can produce a film that disintegrates within 80 to 100 s.
The effects of X1 and X3 on the cumulative percentage release (Y2) are displayed in a response surface plot (). A at fixed level of X2 (0.4%), an increase in X1 to 3% and a decrease in X3 to 1% decreases Y2 to 18.18%. The use of a low level of X1 (1%) and increasing X3 to 2% increases Y2 to 79.22%. Additionally, the contour plot () suggests the exact percentages of X1 and X3 that produces a film that releases the maximum amount of drug within 30 min. The use of a low level of X1 with a high X3 can release the maximum amount of drug, as in the case of F1.
Optimized formulation
reveals the calculated optimal combination of factor levels that attain the target by fulfilling the requirements for each response. To verify the validity of the design, the responses of the optimized formulations were evaluated () to ensure the desired disintegration time and drug release profile of the sildenafil citrate ODF. All the fabricated film formulations prepared were smooth, almost transparent with good flexibility. It was observed that result of uniformity of weight; thickness and drug content were satisfactory with respect to variation of these parameters between films of same formulation.
Table 5. Optimum desirability with observed values of the responses for the optimized sildenafil citrate ODFs formulation.
In vivo absorption studies
The mean plasma concentration-time curves of the prepared optimized ODF and Viagra tablet are illustrated in . The pharmacokinetic parameters were evaluated using WinNonLin® Professional software. The total drug absorbed over a period of 24 h is shown in ; this measurement was determined using the differences in Tmax between the two treatments. The AUCs were statistically significant (p < 0.05). Moreover, the relative bioavailability of the optimized sildenafil citrate ODF was 225% compared with Viagra® tablets as the reference standard.
Figure 3. Plasma concentration of Sildenafil citrate following the administration of the reference (Viagra® tablets), and optimized ODF. Data represent the mean values of n = 6 ± S.D.
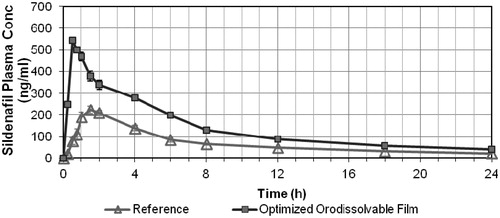
Table 6. Pharmacokinetic parameters ± S.D of sildenafil following the administration of a single oral dose (50 mg) of the market formulation (Viagra® tablets), and the selected optimized ODF formulation.
Discussion
The phase-solubility analyses evaluated the affinity between the cyclodextrin and sildenafil citrate molecules in water. The solubility of sildenafil citrate increased as the CD concentrations increased because CDs have a nonpolar cavity interior that attract lipophilic drugs, such as sildenafil citrate, due to either dipolar or induced dipolar interactions, as well as Van der Waals hydrophobic interactions Fernandes et al. Citation2002. Additionally, the surfactant-like properties of CD carriers arise from the hydrophilicity of the exterior surface, which reduce the interfacial tension between the water and sildenafil citrate molecules Fernandes et al. Citation2002.
The stability constants (Ks) for the sildenafil citrate complexes indicate that the drug’s binding potential with hydroxybutyl-β-CD is higher than that with β-CD or hydroxypropyl-β-CD. This difference is likely due to the presence of alkyl chains on hydroxybutyl-β-CD, which increased the hydrophobicity around the cavity Omar et al. Citation2007. The stability constant for hydroxypropyl-β-CD was low because this polymer is more soluble in water and less hydrophobic than β-CD; therefore, it does not form stable complexes with the hydrophobic sildenafil citrate molecules.
The preparation of sildenafil citrate SD through coevaporation was more efficient than the kneading method; the solubility of sildenafil citrate increased approximately eightfold compared with that obtained in pure state due to its higher affinity toward inclusion in the hydrophobic CD cavity. This process was aided by heating and stirring during coevaporation, which provided sufficient energy for sildenafil citrate to interact in the correct orientation Liu and Zhu Citation2006. In contrast, the kneading method produced true inclusion complexes inefficiently due to the hindered sildenafil citrate-CD interactions in a semisolid medium; the solubility increased only threefold relative to that of the pure state.
A three-level, three-factor Box–Behnken experimental design was utilized to evaluate the effects of the concentrations of hydroxypropyl cellulose, which was used as the film-forming polymer (X1), guar gum, which was used as the film modifier (X2), and propylene glycol, which was used as the plasticizer (X3), in order to minimize the disintegration time (Y1) and maximize the amount of drug released (Y2). Polymers impart the necessary mechanical properties to the oral film and influence the release of the active ingredient into the oral cavity. This design is suitable for exploring the quadratic response surfaces, constructing second-order polynomial models, and optimizing the process using a small number of experimental runs. The analysis of the variance in the disintegration time and the statistical significance of each effect indicated that the concentration of the film-forming material has a significant synergistic effect on the disintegration time and a significant antagonistic effect on the amount of drug released; the p values were 0.025 and 0.0001, respectively. These results may be attributed to the improvement in the swelling of the polymer upon contact with the dissolution medium, the hardening of the release path of the drug from the film, and the increase in the time required for disintegration obtained by increasing the concentration of the film-forming material Kianfar et al. Citation2012. However, the concentration of the plasticizer has a strong antagonistic effect on the disintegration time and a strong synergistic effect on the amount of drug released; the p values were 0.009 and 0.004, respectively. The plasticizer level affected the mechanical properties of the film by lowering the glass transition temperature (Tg) and influencing the disintegration and dissolution characteristics Repka and McGinity Citation2000. Furthermore, the plasticizer molecules inserted themselves between the polymer strands, thereby breaking the polymer–polymer interactions and increasing the molecular mobility of the polymer strands. Accordingly, increases in the concentration of the plasticizer increased resulted in decreases in the film stiffness and increases in the film ductility.
The pharmacokinetic study revealed that the administration of sildenafil citrate as a fast ODF can modify its pharmacokinetic profile, increasing its bioavailability by more than 2.25-fold relative to that of the marketed oral tablet. Compared with the marketed tablet, the Tmax for the ODF containing a sildenafil citrate SD was significantly lower (p < 0.05) because sildenafil citrate is a lipophilic drug with poor aqueous solubility. The preparation of this drug as a SD enhanced its solubility and tissue permeability, thereby shortening its onset of action. The onset of action for the tablet begins after 1 h because the plasma level reaches 215 ng/mL at this point; this concentration was attained after 10 min using the ODF, as shown in .
These results demonstrate that the ODF containing sildenafil citrate SD was easily absorbed, which indicates that the ODF circumvented the delayed onset observed with the tablet. Additionally, the pharmacokinetics of drugs delivered via ODF formulations are dictated by the properties of the ODF rather than the physicochemical properties of the drug molecules. When an ODF is administered orally, it is instantly wetted by saliva, which results in its rapid disintegration and dissolution to release the drug Alka et al. Citation2012. In addition, the bioavailability of sildenafil citrate exhibited an increase of more than 2.2-fold; this increase could be attributed to the decreased amount of hepatic first-pass metabolism associated with orally administered tablets, which may improve the in vivo bioavailability.
Conclusions
A Box–Behnken experimental design was utilized to formulate sildenafil citrate as a fast ODF. This novel drug delivery system provided short disintegration times and a maximum amount of released drug. The bioavailability of sildenafil citrate was enhanced by more than 2.25-fold relative to that of the marketed tablet, and the onset of action was shortened to 10 min. The use of this ODF formula may lead to the elimination of the major drawbacks associated with the conventional tablet and allow patients to tolerate the drug without fear of gastrointestinal side effects.
Acknowledgment
This work was funded by the The authors acknowledge and thank the DSR for the technical and financial support.
Declaration of interest
The authors state no conflicts of interest. This work was supported by the Deanship of Scientific Research (DSR) of King Abdulaziz University of Jeddah (Grant No. 166-005-D1433).
References
- Al-Ghazawi MA, Tutunji MS, Aburuz SM. (2010). The effect of pummel juice on pharmacokinetics of sildenafil in healthy adult male Jordanian volunteers. Eur J Clin Pharmacol 66:159–63
- Aliaa NM, Arwa SH. (2011). Characterization and optimization of orodispersible mosapride film formulations. AAPS Pharm Sci Tech 12:1384–92
- Alka T, Kiran S, Nitesh SC, et al. (2012). Formulation and evaluation of fast dissolving oral film of dicyclomine as potential route of buccaldelivery. Int J Drug Dev Res 4:408–17
- Chen G, Jiang M. (2011). Cyclodextrin-based inclusion complexation bridging supramolecular chemistry and macromolecular self-assembly. Chem Soc Rev 40:2254–66
- Cilurzo F, Cupone IE, Minghetti P, et al. (2010). Nicotine fast dissolving films made of maltodextrins: a feasibility study. AAPS Pharm Sci Tech 11:1511–17
- Dahima R, Pachori A, Netam S. (2010). Formulation and evaluation of mouth dissolving tablets containing amlodipine besylate solid dispersion. Int J Chem Tech Res 2:706–15
- El-Setouhy D, El-Malak NSA. (2010). Formulation of a novel tinapetine sodium orodispersible film. AAPS Pharm Sci Tech 11:1018–25
- Fernandes CM, Teresa VM, Veiga FJ. (2002). Physicochemical characterization and in vitro dissolution behavior of nicardipine–cyclodextrins inclusion compounds. Eur J Pharm Sci 15:79–88
- Fernandes M, Vieira T, Veiga B. (2002). Physicochemical characterization and in vitro dissolution behavior of nicardipinecyclodextrins inclusion compounds. Eur J Pharmaceut Sci 15:79–88
- Highuchi T, Connors KA. (1965). Phase solubility techniques. Adv Anal Chem Instrum 4:117–22
- Karnachi AA, Khan MA. (1996). Box–Benhken design for the optimization of formulation variables of indomethacin coprecipitates with polymer mixtures. Int J Pharm 131:9–17
- Kianfar F, Chowdhry B.Z, Antonijevic M.D. Boateng, J. (2012). Novel films for drug delivery via the buccal mucosa using model soluble and insoluble drugs. DrugDel. Ind. Pharm 38:1207–20
- Liu X, Zhu Y. (2006). Preparation and characterization of inclusion complexes of prazosin hydrochloride with beta-cyclodextrin and hydroxypropyl-beta-cyclodextrin. J Pharmaceut Biomed Anal 40:122–7
- Malke S, Shidhaye S, Desai J, Kadam V. (2010). Oral films-patient compliant dosage form for pediatrics. Internet J Ped Neonatol 11
- Misra R, Amin A. (2009). Formulation development of taste-masked rapidly dissolving films of citirizine hydrochloride. Pharm Technol 33:48–56
- Nichols DJ, Muirhead GJ, Harness JA. (2002). Pharmacokinetics of sildenafil after single oral doses in healthy male subjects: absolute bioavailability, food effects and dose proportionality. Br J Clin Pharmacol 53:5–12
- Omar L, El-Barghouthi I, Masoud A, et al. (2007). Inclusion complexation of loratadine with natural and modified cyclodextrins: phase solubility and thermodynamic studies. J Solut Chem 36:605–16
- Patel RP, Patel MM. (2007). Physico-chemical characterization and in vitro dissolution bahaviour of Simvastatin β-cyclodextrin inclusion compounds. Drug Deliv Technol 7:50–6
- Rajput G, Majmudar F, Patel J. (2011). Formulation and evaluation of mucoadhesive glipizide films. Acta Pharm 61:203–16
- Repka MA, McGinity JW. (2000). Physical-mechanical, moisture absorption and bioadhesive properties of hydroxypropylcellulose hot-melt extruded films. Bio-materials 21:1509–17
- Sheu M-T, Wu A-B, Yeh G-C, Hsia A (2003). Development of a liquid chromatographic method for bioanalytical applications with sildenafil. J Chromatogr B 791:255–62
- Veiga F, Teixeira-Dias JC, Kedzierewicz F, et al. (1996). Inclusion complexation of tolbutamide with-cyclodextrin and hydroxypropyl–cyclodextrin. Int J Pharm 129:63–71
- Walker DK, Ackland MJ, James GC, et al. (1999). Pharmacokinetics and metabolism of sildenafil in mouse, rat, rabbit, dog, and man. Xenobiotica 29:297–310
