Abstract
Objective: Preparation and characterization of curcumin solid–lipid nanoparticle (CurSLN)-loaded mucoadhesive gel for local treatment of oral precancerous lesions with low dose.
Methodology: The formulated CurSLNs were dispersed in a mucoadhesive gel matrix to be applied to the buccal mucosa. Conventional mucoadhesive gel using binary system was adopted. The prepared gels were evaluated for in vitro drug dialysis, ex vivo mucoadhesion test and ex vivo permeation study using chicken buccal mucosa. Short-term clinical evaluation was carried out on 10 patients suffering oral erythroplakia in terms of pain index and lesion size measurement.Footnote1
Results: The results showed that the loaded gel with CurSLN showed good mucoadhesion property and 25 min in vivo residence time. In addition to stability enhancement for the Cur powder. All formulae did not show any drug permeated, however, significant amount of Cur was retained within the chicken buccal mucosal tissue confirmed by histological examination. Significant reduction in pain, and complete healing was observed after 6 weeks of treatment.
Conclusion: The local use of Cur in low dose is a promising option for treatment of precancerous lesions. The lack of local anti-inflammatory compounds with reduced side effects intensifies the importance of studying natural products for this purpose.
Introduction
Oral cancer is known to be the sixth most common form of cancer worldwide (Parkin et al., Citation1993). Oral squamous carcinoma develops from exposure to different carcinogen resulting in genetic or epigenetic mutations. It is well established that the presence of an initial precursors subsequently develop into oral cancer (Reibel, Citation2003) specifically oral squamous cell carcinoma. This type of cancer is particularly devastating to patients because treatment entails removal of facial and mouth structures essential for esthetics and function. Oral lesions, oral leukoplakia, oral erythroplakia and submucous fibrosis are the major factors for cancer transformation; they contribute as high as 8–10% of the malignant transformation. Oral lesions are regarded the most challenging in oral medicine practice. They are often chronic, intensely painful and can spontaneously remit, consequently hindering normal day life activities (Gonzalez-Moles et al., Citation2003). In addition, there is no definitive clinicopathological factor or biomarker that could reliably predict malignant transformation. However, correct diagnosis and timely treatment could be a successful and promising preventive way (Amagasa, Citation2011). The easy access to the lesion allows the use of local delivery formulations to directly treat the disease without causing adverse side effects. These lesions are currently managed by invasive surgery and approximately one-third of these lesions will reoccur after surgery. Oral lesions can be effectively treated by topical therapeutic approaches, due to the ease of the oral cavity accessibility. However, formulations specifically designed for the treatment of oral mucosal diseases are few (Paderni et al., Citation2012), and most of these formulations were originally designed for dermatological application. Nevertheless, acquires a lot of limitations, whereas the oral cavity structural, environmental and functional features differ from that of the skin. Such differences are represented as the continuous saliva secretions washing out the active ingredient, swallowing, chewing, as well as phonation. All these factors result in a short retention time of the dosage forms to the oral mucosa, consequently, low therapeutic efficacy (Paderni et al., Citation2012).
Solid–lipid nanoparticles (SLNs) represent an attractive colloidal drug carrier system due to successful incorporation of hydrophobic active compounds. SLN offer an economical and patient-friendly device for administration of drugs by various routes; parenteral, oral (Hosny & Aljaeid, Citation2014), nasal and ocular (Apaolaza et al., Citation2014). During the localization of SLNs to the affected oral mucosal lesion when dealing with oral and maxillofacial region, the system development becomes more challenging. For such purpose, drug-loaded SLN should be sufficiently retained to the buccal mucosa for enough time to allow mucosal uptake.
Retentive mucoadhesive formulation intended for the oral cavity provides a promising and efficient approach for treatment of oral diseases. Unlike the conventional oral medications, they can be readily attached to the buccal cavity and retained for a longer period of time and removed at any time.
Recent developments in the field of buccal drug delivery show an increased interest toward nano buccal drug delivery systems to provide a superior drug delivery system in terms of enhanced localization and drug targeting. A very limited number of studies have been done to date in this emerging field (Karavana et al., Citation2012; Rana & Murthy, Citation2013).
Therefore, in this context, this study addresses the feasibility of formulation of SLNs into mucoadhesive dosage forms to be applied to the buccal cavity aiming at treatment of precancerous oral lesions.
Curcumin (Cur) is a polyphenol derived from the herbal remedy and dietary spice turmeric (Sharma et al., Citation2005). Cur has anti-inflammatory and antioxidant effect and a chemopreventive effect (Clark et al., Citation2010; Cai et al., Citation2015). The golden spice has been reported to be an effective option for the treatment of precancerous lesions. Rai et al. (Citation2010) previously reported its efficacy when given orally for treatment of precancerous lesions at a dose as high as 8 g/day. Clark et al. (Citation2010) have introduced the local use of Cur for oral cell carcinoma chemoprevention and treatment. Although the article proposed the possible mechanism of Cur action, it did not address the poor solubility, photosensitivity (Tønnesen et al., Citation2002) and chemical instability of Cur in physiological conditions. Cur has been previously reported to have a pH-dependant stability (Wang et al., Citation1997), where the study showed that 50% of Cur in phosphate buffer of pH 6.8 degrades in 39.7 min which is similar to the salivary condition.
In this regard, Cur was successfully encapsulated into solid–lipid-based nanoparticles. In addition, the nano-system showed to be stable and maintained the stability of Cur against degradation in phosphate buffer even in presence of mucin in an attempt to mimic the salivary physiological condition. Moreover, curcumin solid–lipid nanoparticle (CurSLN) showed good retention into the buccal mucosa upon ex vivo examination (Hazzah et al., Citation2015a).
In this domain, the current study aimed to develop and characterize a suitable mucoadhesive gel loaded with CurSLN dispersion to provide a dosage form with better adherence and efficacy. For better evaluation and comparison, mucoadhesive gel containing Cur dispersion was also prepared. Both gels (conventional Cur mucoadhesive and CurSLN-loaded gel) were characterized regarding their in vitro release, mucoadhesion, in vivo residence time and ex vivo permeation study. Clinical evaluation of CurSLN-loaded mucoadhesive gel for treatment of precancerous lesions locally at a much lower dose than the oral one; 6 mg/day was carried out. For better evaluation to prove the advantageous effect of the nanodelivery system, it was compared to simple mucoadehsive gel containing Cur.
Materials
Cur (Hebei Food Additive Co., Ltd, China) (purity >95%), Gelucire 50/13 (Kind Gift from Gattefosse, France), poloxamer 407 (PX407) (Kolliphore 407) (Kind Gift from BASF, Ludwigshafen, Germany), PEG 7-glyceryl-cocoate (Galaxy, Navi, Mumbai, India), polycarbophil (P) (Noveon® AA1 a sample gift, Lubrizol, Belgium), polyethylene glycol (PEG) 400, potassium di-hydrogen phosphate and sodium lauryl sulfate (SLS) (El-Nasr Pharmaceutical Co., Egypt) and tri-ethanol amine (TEA) (Nice Chemicals, Pvt Ltd, Kochi, India).
Methods
Preparation of mucoadhesive gels
Conventional Cur mucoadhesive gels
Six Cur mucoadhesive gel formulations were prepared using P and PX407 at different concentrations. The calculated amount of water was cooled and put into ice bath before addition of PX407, which was then refrigerated till clear solution was obtained. Polycarbophil was then added to the previously prepared solution on a magnetic stirrer (WiseStirer®, DAIHAN-Scientific Co., Ltd., Seoul, Korea). This was followed by addition of previously dispersed Cur (1.2%) in the least amount of PEG 400 (10%) with the aid of PEG-cocoate (1%). The pH was adjusted to 6.5 using TEA. Composition of the prepared mucoadhesive gels is shown in .
Table 1. Composition of conventional curcumin mucoadhesive gels.
Polymeric gel loaded with CurSLNs
Preparation of CurSLNs
The lipid (5% Gelucire 50/13) was melted at 55–60 °C followed by the addition of (0.6%) Cur. Calculated amount of aqueous phase, (water to 100%) with 8% PX407 maintained at 70–80 °C squirted gently into the lipid (oil) phase under magnetic stirring at 600 rpm. Next, the mixture underwent high-shear dispersion at 12 000 rpm for 5 min using homogenizer T18 ULTRA-TURRAX® (IKA, Germany). The emulsion obtained was cooled gradually to room temperature forming SLN.
Preparation of CurSLNs gel
The cooled CurSLN dispersion was thickened with 2% P and 2% PX407 under continuous stirring for 1 h. The pH of the gel was then adjusted to 6.5 using triethanolamine to form a polycarbophil gel matrix. The final concentration of Cur in the formulated gels was 6 mg/g gel (0.6% w/w).
Characterization of gels
Characterization was conducted for all prepared formulae as follow.
Viscosity
The viscosity of the selected gel formula was tested at 25 °C using Brookfield viscometer, (RV-TD; Brookfield Engineering Laboratories, Middleboro, Inc., MA) spindle no S15 at 20 rpm. The measurements were done in triplicates.
Spreadability test
A spreadability test was conducted by pressing 0.1 g of each prepared formula between two glass slides and left for 5 min until no more spreading was expected. The diameter of the formed circle was measured and used as comparative values for spreadability (Soliman et al., Citation2010).
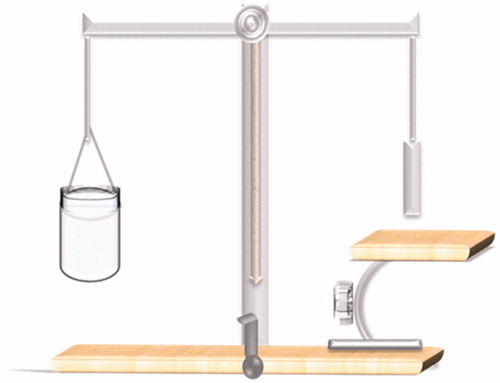
Mucoadhesion (modified balance method)
The mucoadhesive properties of the gels were evaluated by the modified balance method (Nafee et al., Citation2004). Through measuring the detachment forces between samples of the gel and from the 8% mucin in 6.8 buffer, suspension-coated filter paper surface after the 10-min contact is established. The Apparatus used was designed and assembled in our laboratories (). The device was composed of a two-armed balance. The left arm of the balance was replaced by a dosage form holder composed of a small metal lamina vertically suspended through a wire. On the same side, an adjustable/movable stage was maintained at the bottom in order to fix the model mucosal membrane. The right pan was used to add weights gradually. Results were expressed as detachment force in (dyne/cm2) required detaching the two systems (gel/filter paper membrane; average of six samples were obtained). The force required to break the adhesive bond per unit area was calculated using the following equation (Attia et al., Citation2008):
(1)
where F is the bioadhesive force in dyne/cm2, WW is the weight added on the right pan in g, g is the acceleration due to gravity in cm/s2 and A is the surface area of the gel layer in cm2
In vivo residence time
Six human healthy volunteers (22–45 years old, five females and one male) agreed to participate in this study. The mucoadhesive gels with optimized properties were selected for the in vivo evaluation. The gel was spread on the buccal mucosa between the cheek and the gingiva in the region of the upper canine. The volunteers were asked to monitor the time necessary for complete disappearance of yellow color of gel (Patel et al., Citation2012).
In vitro release testing (dialysis method)
Dialysis of an amount equivalent to 0.6 mg Cur [CurSLN dispersion, Cur conventional mucoadhesive gel (P2PX40710) and CurSLN-gel] was carried out through cellophane bag 4 cm long with a 12- to 14-kDa cut off (VISKING dialysis tubing, SERVA, electrophoresis, Germany). The bags were transferred to ambered glass bottles containing 10 ml of water:ethanol (1:1), in horizontal shaking (Horizontal shaking water bath Wisebath®, DAIHAN-Scientific Co., Ltd., Seoul, Korea) water bath at 100 strokes/min, adjusted to 37 ± 0.5 °C. The total volume was withdrawn at different time intervals (0.5, 1, 2, 3, 4 and 5 h) and replaced with an equivalent volume of a previously warmed fresh medium. The amount of Cur released was measured using UV spectrophotometer at lambda max 431 nm (El-Refaie et al., Citation2015). The percentage release was calculated on the cumulative drug released at different time intervals. All the measurements were performed in triplicates (mean ± SD).
In vitro release testing (cup method)
An accurately weighed amount of Cur-gel equivalent to 0.6 mg of Cur was placed in a stainless steel cup (10 mm diameter and 3 mm depth) and covered with polyester gauze acting as a mechanical barrier to prevent gel escape without interfering with drug release (Ismail et al., Citation2000; Ismail, Citation2006). The gauze was fixed to the cup through a specially designed stainless steel ring. The cup was placed at the bottom of 30 ml ambered glass bottle containing 20 ml of release medium (phosphate buffer pH 6.8, 0.5% SLS) which provided excellent sink condition. The bottles were placed in a thermostatically controlled water bath adjusted at 37 ± 0.5 °C shaken at 100 strokes/min. At pre-determined time intervals, total volume was withdrawn and replaced by equal, pre-warmed fresh medium. Samples were analyzed spectrophotometrically for Cur at 421 nm (Khairy, Citation2014). All the experiments were done in triplicate (mean ± SD).
Release kinetics
The mechanism of drug release from the Cur lipid nanoparticle-loaded gel and Cur conventional mucoadhesive gel was investigated using the mathematical models: Zero-order kinetics, First order, Higuchi square root of time and Peppas using DD-solver excel sheet software application.
The Peppas model has been widely used in many studies for analysis of the drug release kinetics in matrix systems. For the Fickian diffusion, n = 0.5 while for the anomalous transport, n is between 0.5 and 1 and for a case II transport (zero-order release), n = 1 (Siepmann & Siepmann, Citation2008).
Ex vivo permeation and retention test
Dissected chicken buccal mucosa (El-Samaligy et al., Citation2006) was used for drug permeation test. The open cheek pouch mucosa, with the mucosal surface facing up, was placed between two compartments of Franz diffusion cell that was assembled in our laboratory. The upper compartment contained an amount equivalent to 0.6 mg Cur of selected formulae or Cur suspension in 0.1% SLS phosphate buffer pH 6.8. The receptor compartment contained 8 ml of phosphate containing 0.1% SLS. The cells were moved to a water bath adjusted at 37 °C and 50 strokes/min. The whole volume was withdrawn at pre-determined time intervals (0.5, 1, 2 and 3 h) and replaced with an equivalent amount of fresh and pre-warmed medium. Samples were measured spectrophotometrically. The mucosa was then gently washed to remove excess formula, and entrapped Cur was extracted by soaking in acetone overnight (El-Refaie et al., Citation2015). The samples were centrifuged (Cooling centrifuge, Centurion Scientific Ltd, UK) at 4000 rpm and 4 °C for 30 min; samples were appropriately diluted and analyzed spectrophotometrically. The formulae assigned for this test were Cur conventional gel (P2PX40710), CurSLN dispersions and CurSLN-loaded gel.
Histological examinations
The previous test conditions were repeated, nevertheless the chicken buccal mucosa tissues were spared for histological examination. Tissue samples intended for staining with hematoxylin and eosin stain (H&E) were directly stored in 10% formaldehyde solution until staining. On the other hand, for examination of CurSLN entrapped into the mucosa, tissue samples were directly immersed in osmic acid stain till processing (Riemersma, Citation1963). Samples were examined using light microscope (Light Microscope, Magnüs®, MLX, New Delhi, India).
Clinical evaluation
The study was approved by the institutional review board of Alexandria University Dental Hospital, 10 cases with erythroplakia were recruited from the Department of Oral Medicine, Alexandria University. Histopathological examination was carried out to identify the type of lesion, set the base line for epithelial dysplasia, moreover, to confirm the absence of invasive cancer. The examination was also carried out after the course treatment to confirm healing of mucosal tissue.
All participating patients had general good health without other uncontrolled medical conditions. Patients with the following conditions were excluded: (1) those with a previous diagnosis of head and neck or oral cancer; (2) those having drug hypersensitivity; (3) those requiring extensive dental procedures; (4) those with a history of social or psychiatric situations interfering with study compliance and (5) those who use medications such as topical or systemic steroids, and immunomodulatory agents, antibiotics or non-steroidal anti-inflammatory were asked to cease medication for 2 weeks.
After signing an informed consent, eligible patients were randomly assigned to two groups, Group A (n = 5) and Group B (n = 5). The work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans (Association, Citation2001). A summarized characterization of patients’ condition is presented in () including age, gender and location of lesion. The treatment group (Group A) was administered conventional mucoadhesive gel P2PX40710 formula; the second group (Group B) was given CurSLN-gel at a dose 6 mg Cur/day for 6 weeks. Patient compliance was monitored through self-report during weekly follow-up visits. Clinical evaluations presented as pain index, and lesion size were made at study entry, every 2 weeks over a 6-week period and 3 months after the study period.
Table 2. Characterization of patients integrated in clinical investigation.
Each participant at day zero, baseline appointment, was asked to estimate the average level of associated pain. To evaluate the pain level, a visual analogue scale (VAS) of (0–5), where the (0) denoted no pain and the (5) denoted unbearable pain was used. Subjects were told to mark the line with a vertical line at the point that best represented the lesion pain level. To determine the size of the lesion, the distance between two opposite outside edges of the lesion border was measured using a calibrated periodontal probe with millimeter markings. Two measurements ∼90° from each other were obtained; the largest distance was used as one of the measurements. The two measurements were then multiplied to represent the cross-sectional areas of the lesion. The efficacy indices (EI) of the lesion size and pain improvement were calculated according to the following equation (Liu et al., Citation2006):
(2)
V1 and Vt referring to the values for pain and lesion size measured at different visits, while V1 referring to the baseline value measured before the study entry.
Statistical analysis
Characterization and in vitro release data were expressed as percentage or mean (±SD) and analyzed by unpaired t-test. Statistical treatment of clinical data involved the use of unpaired one-tail Student’s t-test. All tests were performed by excel software application 2010, with significance level set at 5%.
Results and discussion
Conventional Cur mucoadhesive gel
A preliminary investigation was conducted to determine the appropriate concentrations of P and PX407 for gel preparation with accepted viscosity and spreadability. Results showed that P concentration ranging from (0.5–2% w/w) when used concomitantly with 20% poloxamer provided mucoadhesive gels with acceptable viscosity, spreadability and physical appearance. Where concentrations <0.5% provided very loose gel, on the other hand, concentration >2% formed a very thick and aggregated gel. Similarly, when 10% poloxamer was used the acceptable P concentrations ranged from 1% to 3% w/w.
Viscosity
The results show that the viscosity of P gels significantly increased as a function of P concentration (). Needless to mention, formulations intentionally designed for oral cavity application for the treatment of infection, inflammation or neoplasia should optimally be easy to administer, and exhibit retention at the site of application. Following administration, these formulations should exhibit elastic properties to optimize retention and offer controlled drug release. In case of gel/semi-solid systems these two requirements present a formulation paradox. To achieve controlled drug delivery (and indeed optimal retention) a formulation that possesses high elasticity is required, however, such formulations will offer resistance to flow, rendering their application to the required site difficult (Jones et al., Citation2009). Viscosity is an important parameter for characterizing the gels as it affects the spreadability, extrudability and release of drug. Upon dispersion of P into water, the molecules will hydrate and uncoil to some extent, though the molecular chains do not achieve the greatest degree of expansion. In order to achieve maximum performance of P, neutralization step or hydrogen bonding resulting in uncoiling and extension of the polymer chain are required. As a pH-sensitive gel, the carboxyl groups in molecular chains are neutralized by adding the neutralizer (in our case triethanolamine was used). Negative charges will be produced on the backbone of the molecular chains after the polymer is ionized by the neutralizer, which may turn the molecules into an extended state with the repulsions of homogeneous charges. This reaction occurs rapidly and leads to thickening effect (Zhu et al., Citation2013).
Table 3. Physical characterization of formulated curcumin mucoadhesive gels: viscosity, mucoadhesion, spreadability and in vivo residence time.
Spreadability
As shown in , spreadability values for all prepared gel formulae ranged between 2.35 and 3.5 cm. It is worth mentioning that the larger the diameter, the better the spreadability. The results obtained revealed that using poloxamer at lower concentration showed higher spreadability, while P showed to be of little effect.
Mucoadhesion and in vivo residence time
One of the important design criteria for the drug delivery platforms is their ability to exhibit mucoadhesion. There have been several models used to assess the mucoadhesive properties of formulations (Eouani et al., Citation2001; Grabovac et al., Citation2005; Patel et al., Citation2012); the method used in this study has been used to successfully quantify their relative mucoadhesive properties using modified balance method. In vivo residence time is a reflection for the mucoadhesion force, (20–25 min) except for one formula (P1PX40710), where it was retained only for 5 min. This could be due to the fact that 10% of poloxamer and the low concentration of P were not able to provide good residence time.
Formulations containing 10% PX407 and P (1%, 2% and 3% w/w), and 20% PX407 and P (0.5%, 1% and 2% w/w) showed to possess significant mucoadhesive properties. Interestingly, at each poloxamer concentration, increasing the concentration of P significantly increase the force required to break the mucoadhesive bond.
Physico-chemical evaluation of CurSLN gel
In an attempt to increase the efficacy and stability of Cur, this study aimed at preparing CurSLN into a gel form, to maintain its stability, increase its adhesion and mucosal uptake. In addition, present the prepared CurSLN dispersion in an acceptable and easily administered formula.
Based on the results, P2PX40710 was found superior regarding viscosity, mucoadhesion, spreadability. Therefore it was selected to incorporate CurSLNs. The CurSLN-gel showed a spreadability of 3.3 cm which is considered to be acceptable compared to results shown in a study by Soliman et al. (Citation2010). Spreadability plays an important role in patient compliance and allows uniform application of gel to the affected area. The viscosity was recorded to be 13 800 cP, 25 min were the in vivo residence time and the force of mucoadhesion was 17 863.29 dyne/cm2.
In vitro release testing by dialysis method of the formulated (CurSLN-gel) compared to the conventional Cur mucoadhesive gel (P2PX40710), and (CurSLN) dispersion showed significant discrimination (p < 0.05). Results revealed that percentages Cur released from CurSLN dispersion, P2PX40710, and CurSLN-gel after 5 h was 47.2%, 27.7% and 14.2%, respectively ().
Figure 2. (A) In vitro release profiles (dialysis method) of CurSLN-gel in comparison to conventional curcumin mucoadhesive gel P2PX40710 and CurSLN dispersion [dissolution medium: 10 ml ethanol:Water (1:1)] at 37 °C. (B) In vitro release profiles (cup method) of CurSLN-gel in comparison to conventional curcumin mucoadhesive gel P2PX40710 (dissolution medium: 20 ml phosphate buffer, pH 6.8, 0.5% SLS) at 37 °C.
![Figure 2. (A) In vitro release profiles (dialysis method) of CurSLN-gel in comparison to conventional curcumin mucoadhesive gel P2PX40710 and CurSLN dispersion [dissolution medium: 10 ml ethanol:Water (1:1)] at 37 °C. (B) In vitro release profiles (cup method) of CurSLN-gel in comparison to conventional curcumin mucoadhesive gel P2PX40710 (dissolution medium: 20 ml phosphate buffer, pH 6.8, 0.5% SLS) at 37 °C.](/cms/asset/e6950f39-f13a-4b12-a00c-477bc7daa3ef/idrd_a_1065524_f0002_c.jpg)
Generally, the use of P at the studied concentration 2% retarded the release of Cur, needless to say that increasing the viscosity will increase the path diffusional length, hence decreasing the concentration gradient and amount of drug released. In addition, P2PX40710 showed faster release then CurSLN-gel. This could be attributed to the nature of dispersed Cur tested, where Cur in gel (P2PX40710), is solubilized, while Cur in CurSLN is encapsulated in a lipid matrix. Moreover, the dispersion of CurSLN in a gel matrix retarded its release. Needless to mention that Cur released form CurSLN dispersion was the highest as the nanoparticles are suspended freely ().
Another in vitro release testing by cup method was conducted using phosphate buffer pH 6.8 in order to mimic in vivo pH of saliva. However, 0.5% SLS was added to maintain the stability of released Cur (Khairy, Citation2014). The percent drug release after 5 h from P2PX40710, and CurSLN–gel was 96.4%, and 62.14%, respectively ().
In order to investigate the drug release mechanism, the release data were fitted to different release kinetics models, zero-order, first order and Higuchi square root of time and Peppas–Korsmeyer (). The r2 of both formulae was fitting the most with zero-order model (Peppas, Citation1984). Dispersion of CurSLN in gel matrix and P2PX40710 showed a zero-order release with an (r2 = 0.9969, 0.989, respectively), and “n” value nearest to “1” from applying Peppas equation came as another confirmation indicating that the drug release mechanism depended mainly on polymer swelling (Siepmann & Siepmann, Citation2008).
Table 4. Release models of formulated curcumin mucoadhesive gels.
The release mechanism showed to follow zero order, this could be justified similarly as the explanation proposed by Siepmann & Siepmann (Citation2008). The authors assumed that upon contact with the release medium, water diffuses into the delivery system. The mobility of polymer chain increases by increasing the water content and so does the drug molecule. Once a specific (polymer–water) concentration is reached the macromolecular mobility steeply increases. This phenomenon is called “polymer chain relaxation” or “glassy to rubbery phase transition”. The front at which this process takes place is called “swelling front”, which separates the swollen from non-swollen matrix. This swelling front is a moving boundary. While the initial drug concentration in the delivery system exceeds drug solubility, dissolved and non-dissolved drug co-exist within the matrix. As a result of concentration gradients and the increased mobility, dissolved drug molecules diffuse out of the swollen matrix into the release medium. This guarantees that drug molecules that are released will be replaced by the dissolution of non-dissolved drug. Eventually, when all excess drugs are dissolved, the concentration within the swollen matrix decreases. The front that separates the swollen matrix containing only dissolved drug from the swollen matrix that contains both, dissolved and non-dissolved drug, is called “diffusion front” (Siepmann & Siepmann, Citation2008). The result of our study was in agreement with Tirnaksiz & Robinson (Citation2005).
Ex vivo permeation and retention test
In this study, it was assumed that the selected formulation of CurSLN-gel would be beneficial for increasing the retention time and penetration (i.e. the enhanced permeability and retention effect) of the SLN at the application area. The ex vivo permeation studies showed that, under the study conditions, Cur was not detected in receptor phase which suggests that the prepared formulation could be a good candidate to be used as a vehicle for local delivery.
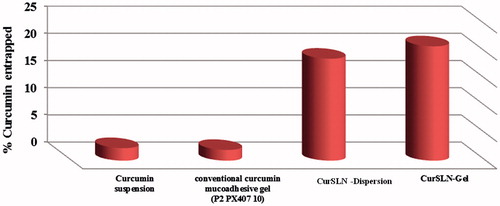
This study uniquely identified the amount of Cur uptake by mucosal tissue, where the amount of Cur that was detected in the buccal mucosa after the end of permeation test was worth mentioning. Thereby, the results showed that the amount of Cur detected in mucosa after application of Cur suspension, Cur conventional mucoadhesive gel (P2PX40710), CurSLN dispersion, CurSLN-gel at the end of the experiment were 2%, 2%, 18% and 21%, respectively, with a significant difference (p < 0.05). The results emphasize that the SLN stabilized the Cur particles against degradation in the oral mucosa where their entrapment were 18% ().
Figure 3. Amount of curcumin detected in dissected chicken buccal mucosa after 3 h of permeation test at 37 °C.
The use of poloxamer helped permeation through epithelial cells but not enough to permeate to the blood circulation, which is typically the objective for this study to localize and target the drug in the area of application for the longest possible time and permeation to the basal cells. In addition, results confirm higher adhesion of CurSLN dispersion to the mucin which results in more contact time resulting in more penetration. Applying CurSLN in a gel matrix was also beneficial in increase drug penetration between mucosal layers, due to the higher contribution of Poloxamer with a significant difference (p = 0.01). This property is explained according to the permeation enhancement ability of surfactants by perturbing intercellular lipids and altering the tight junctions of those epithelia (Lin et al., Citation2007). It is worth mentioning that Cur amount determination using UV analysis method was previously reported (El-Refaie et al., Citation2015), in addition, the blank of tissue homogenate, and homogenate spiked with similar concentration of calibration curve were also carried out to eliminate interference (if any).
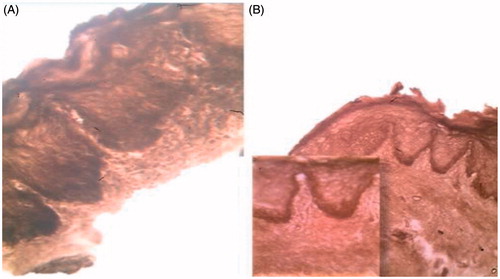
Penetration into the mucosal layers firstly investigated using simple histological examination. This was confirmed by staining mucosal tissue with osmic acid, only lipids take the stain, where the black spots confirmed penetration of the nano-system through the mucosal layers and not just being retained onto the mucosal surface (). The results obtained is live evidence to the study assumption and to the results obtained through in vitro and ex vivo characterization, where the CurSLN showed higher retention and penetration deep into the mucosal layers, allowing higher contact between the therapeutic agent and diseased area providing more profound therapeutic effect.
Clinical evaluation study
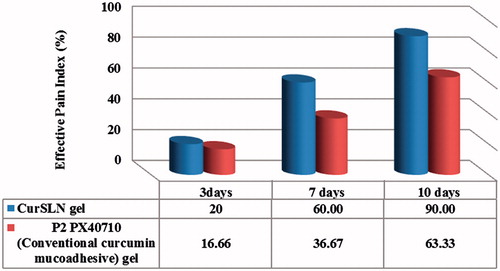
Pain and size of lesion, were the two main variables examined to evaluate the difference between the efficacy of the two selected formulae; conventional Cur mucoadhesive gel (P2PX40710) and CurSLN-gel. The CurSLN-gel showed higher rate of healing, it showed effective pain index of 20%, 60% and 90% for 3, 7 and 10 days, respectively, while P2PX40710 showed only 16.6%, 36.67% and 63.3%, respectively, with a significant difference (p < 0.05) ().
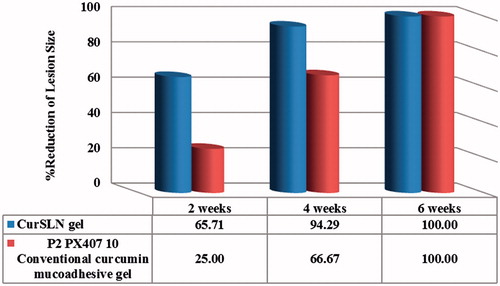
Similar observation was recorded for the % reduction of lesion size, where CurSLN-gel showed higher reduction in lesion size compared to P2PX40710. Thereby, the former showed 67.5% and 94.29%, while the latter showed only 25% and 66.67% reduction after 2 and 4 weeks, respectively, with a significant difference (p < 0.05) ().
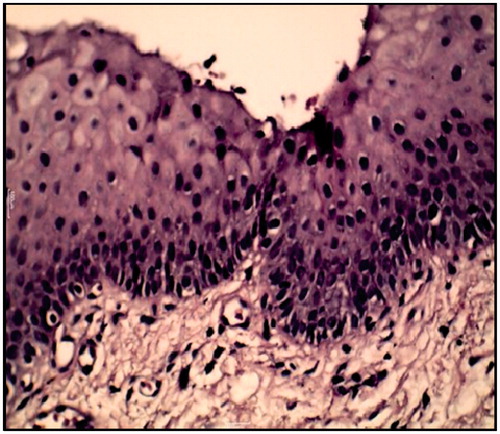
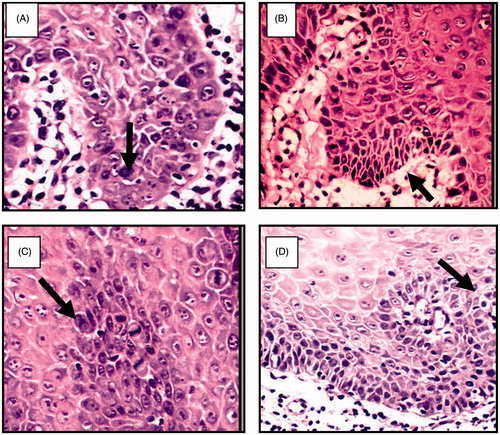
Histological sections were examined for patient at zero day treatment to identify the type of dysplasia, and after the treatment course. shows sever epithelial dysplasia before the treatment showing acanthotic epithelium with drop shape rete pege’s process. Upon higher magnification, shows large cell showing abnormal mitotic figures, shows basilar hyperplasia in basal cell layer, and shows liquification in basal cell layer and basement membrane with multiple mitotic apoptotic cell (civatte body) with clearance of cytoplasm and characteristic chromatin condensation, respectively. shows complete disappearance of epithelial dysplasia after treatment course.
Figure 7. Severe epithelial dysplasia before the treatment showing acanthotic epithelium with drop shape rete pege’s process (H&E ×200).
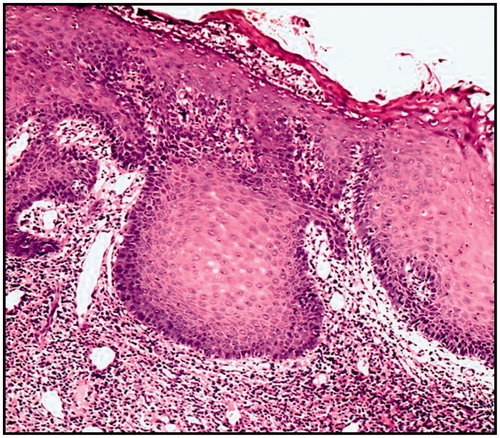
Figure 8. (A) Large cell showing abnormal mitotic figures, (B) basilar hyperplasia in basal cell layer, (C) liquification in basal cell layer and basement membrane with multiple mitotic figure and (D) apoptotic cell (civatte body) with clearance of cytoplasm and characteristic chromatin condensation (H&E ×400).
show patients with precancerous lesions before and after treatment. It was assumed that delivering Cur topically would be beneficial in the treatment of oral precancerous lesion, where it have been reported that it is effective upon oral administration, but at high daily dose 1–8 g/day (Cheng et al., Citation2001; Rai et al., Citation2010). Furthermore, it was assumed that delivering Cur in an advanced lipid nanosystem, highly stabilized and mucoadhesive formulation would be of a much greater benefit than simply dispersed Cur in a mucoadhesive matrix. For this purpose, as short-term (6 weeks) clinical evaluation was conducted. The outcome of both formulae was almost the same concerning the disappearance of lesions, after the 6-week course treatment (Hazzah et al., Citation2015b). However, the CurSLN-gel showed a more pronounced effective pain index and reduction in the lesion size.
Figure 10. Patient suffering oral erythoplakia (A) before treatment, (B) after 3 weeks and (C) after 6 weeks (treated with CurSLN-gel).
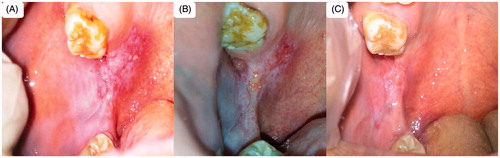
Figure 11. Patient suffering oral erythoplakia: (A) before treatment, (B) after 3 weeks, (C) after 5 weeks and (D) after 6 weeks (treated with CurSLN-gel).
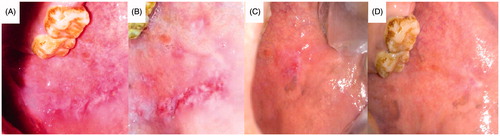
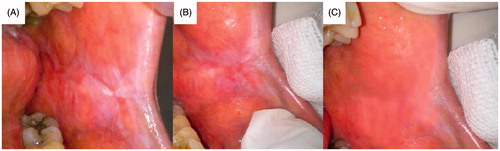
Figure 12. Patient suffering oral erythoplakia: (A) before treatment, (B) after 3 weeks and (C) after 6 weeks (treated with CurSLN-gel).
This study clearly demonstrated that topical application of Cur had remarkable therapeutic effects on precancerous oral lesion in a short-term clinical trial. Cur has shown to possess anticancer effects by blocking transformation, tumor initiation, tumor promotion, invasion, angiogenesis and metastasis. It has been demonstrated to have a dose-dependent chemo-preventive effect in animal systems of colon, duodenal, stomach, esophageal and oral carcinogenesis. Cur is a strong antioxidant compared to vitamins C and E. Rai et al. (Citation2010), showed that Cur increase the local level of vitamin C and E, while it decreased lipid peroxidation and DNA damage for patient suffered precancerous lesions, which suggested that the anti-precancerous effect is through the anti-oxidant and pro-oxidant pathways.
Recently, it has been evidently reported that Cur treatment of co-culture between oral squamous cell carcinoma (SCC-25 cells) and periodontal ligament fibroblasts (PDL) resulted in decrease of tumor cell migration and invasivity, reversal of epithelial-to-mesenchymal transition (EMT) in tumor cells and decrease of the EMT mediators’ gene expression and synthesis in fibroblasts which confirms the palliative potential of Cur in clinical application (Dudás et al., Citation2013). In addition to the study by Clark et al. (Citation2010) showing that Cur significantly inhibited migration and perforation of squamous cell carcinoma, Cur has growth inhibitory effects and prevents tumor formation in an oral carcinogen-induced model at a dose of 15 mg/day. We believe that the instability of Cur and poor water solubility is the reason why high dose as such showed effect compared to the results obtained by our study, where only 6 mg/day was found therapeutically efficient. This proofs that Cur was delivered safely and intact without degradation in the salivary pH, allowing maximum effect with lowest dose.
Conclusions
In the light of described results, it could be concluded that CurSLNs using Gelucire 50/13 and PX407 showed preferential mucosal uptake. Polymeric binary mixture of P and poloxamer provided mucoadhesive gel matrix of high mucoadhesion, high viscosity and good spreadability. The polymeric binary mixture was suitable to efficiently deliver CurSLN as a semisolid delivery system. The gel not only helped maintaining the nanosystem stability but also helped to increase mucosal uptake of CurSLN. It is worth mentioning that although in vivo residence time achieved was relatively of short duration (25 min), it was quite enough to allow lipid nanoparticles penetration into the mucosal layer. It could be also concluded that Cur is an efficient alternative for treatment for one of the most challenging oral and maxillofacial disease, precancerous oral lesions. Adopting SLNs as a delivery system to deliver Cur in a stable form effectively enhanced its activity. Incorporation of CurSLNs into mucoadhesive gel provided an efficient approach for oral mucosal targeting. Clinical study on a long term and large number of participant enrolled should be carried out.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Notes
1Cur, curcumin; P, polycarbophil; PX407, poloxamer 407.
References
- Amagasa T. (2011). Oral premalignant lesions. Int J Clin Oncol 16:1–4
- Apaolaza PS, Delgado D, del Pozo-Rodríguez A, et al. (2014). A novel gene therapy vector based on hyaluronic acid and solid lipid nanoparticles for ocular diseases. Int J Pharm 465:413–26
- Association WM. (2001). World Medical Association Declaration of Helsinki, ethical principles for medical research involving human subjects. Bull World Health Organ 79:373–4
- Attia DA, Khalid M, Ramadan MM. (2008). Optimization and characterization of bioadhesive gastroretentive amoxicillin tablets using the mixture experimental design. J Appl Sci Res 4:1328–39
- Cai Y, Sun Z, Fang X, et al. (2015). Synthesis, characterization and anti-cancer activity of Pluronic F68--curcumin conjugate micelles. Drug Deliv. [Epub ahead of print]. doi:10.3109/10717544.2015.1037970
- Cheng AL, Yunn H, Jee S, et al. (2001). Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res 21:2895–900
- Clark CA, McEachern MD, Shah SH, et al. (2010). Curcumin inhibits carcinogen and nicotine-induced mammalian target of rapamycin pathway activation in head and neck squamous cell carcinoma. Cancer Prevent Res 3:1586–95
- Dudás J, Fullár A, Romani A, et al. (2013). Curcumin targets fibroblast–tumor cell interactions in oral squamous cell carcinoma. Exp Cell Res 319:800–9
- El-Refaie WM, Elnaggar YS, El-Massik MA, Abdallah OY. (2015). Novel curcumin-loaded gel-core hyaluosomes with promising burn-wound healing potential: development, in-vitro appraisal and in-vivo studies. Int J Pharm 486:88–98
- El-Samaligy M, Afifi N, Mahmoud E. (2006). Increasing bioavailability of silymarin using a buccal liposomal delivery system: preparation and experimental design investigation. Int J Pharm 308:140–8
- Eouani C, Piccerelle P, Prinderre P, et al. (2001). In-vitro comparative study of buccal mucoadhesive performance of different polymeric films. Eur J Pharm Biopharm 52:45–55
- Gonzalez-Moles MA, Ruiz-Avila I, Rodriguez-Archilla A, et al. (2003). Treatment of severe erosive gingival lesions by topical application of clobetasol propionate in custom trays. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol 95:688–92
- Grabovac V, Guggi D, Bernkop-Schnürch A. (2005). Comparison of the mucoadhesive properties of various polymers. Adv Drug Deliv Rev 57:1713–23
- Hazzah HA, Farid RM, Nasra MMA, et al. (2015a). Targetted drug delivery systems as an effecient approach for treatment of some oral and maxillofacial diseases [Pharmaceutics PhD thesis]. University of Alexandria
- Hazzah HA, Farid RM, Nasra MMA, et al. (2015b). Curcumin: the golden spice for precancerous oral lesions treatment. Fourth Euro-Mediterranean Conference of Natural Products and Drug Discovery: Back to Mother Nature (BioNat-IV)
- Hosny KM, Aljaeid BM. (2014). Sildenafil citrate as oral solid lipid nanoparticles: a novel formula with higher bioavailability and sustained action for treatment of erectile dysfunction. Expert Opin Drug Deliv 11:1015–22
- Ismail FA. (2006). Design and in vitro evaluation of polymeric formulae of simvastatin for local bone induction. Drug Dev Ind Pharm 32:1199–206
- Ismail FA, Napaporn J, Hughes JA, Brazeau GA. (2000). In situ gel formulations for gene delivery: release and myotoxicity studies. Pharm Dev Technol 5:391–7
- Jones DS, Bruschi ML, de Freitas O, et al. (2009). Rheological, mechanical and mucoadhesive properties of thermoresponsive, bioadhesive binary mixtures composed of poloxamer 407 and carbopol 974P designed as platforms for implantable drug delivery systems for use in the oral cavity. Int J Pharm 372:49–58
- Karavana SY, Gökçe EH, Rençber S, et al. (2012). A new approach to the treatment of recurrent aphthous stomatitis with bioadhesive gels containing cyclosporine A solid lipid nanoparticles: in vivo/in vitro examinations. Int J Nanomed 7:5693
- Khairy HM. (2014). Develpment and evaluation of some drug delivery systems to the oral cavity [Master Thesis], Univeristy of Alexandria, Pharmaceutics. Masters, 140
- Lin H, Gebhardt M, Bian S, et al. (2007). Enhancing effect of surfactants on fexofenadine· HCl transport across the human nasal epithelial cell monolayer. Int J Pharm 330:23–31
- Liu J, Zeng X, Chen Q, et al. (2006). An evaluation on the efficacy and safety of amlexanox oral adhesive tablets in the treatment of recurrent minor aphthous ulceration in a Chinese cohort: a randomized, double-blind, vehicle-controlled, unparallel multicenter clinical trial. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol 102:475–81
- Nafee NA, Ismail FA, Boraie NA, Mortada LM. (2004). Mucoadhesive delivery systems. I. Evaluation of mucoadhesive polymers for buccal tablet formulation. Drug Dev Ind Pharm 30:985–93
- Paderni C, Compilato D, Giannola LI, Campisi G. (2012). Oral local drug delivery and new perspectives in oral drug formulation. Oral Surg Oral Med Oral Pathol Oral Radiol 114:25–34
- Parkin D, Pisani P, Ferlay J. (1993). Estimates of the worldwide incidence of eighteen major cancers in 1985. Int J Cancer 54:594–606
- Patel VF, Liu F, Brown MB. (2012). Modeling the oral cavity: in vitro and in vivo evaluations of buccal drug delivery systems. J Control Release 161:746–56
- Peppas N. (1984). Analysis of Fickian and non-Fickian drug release from polymers. Pharm Acta Helvetiae 60:110–11
- Rai B, Kaur J, Jacobs R, Singh J. (2010). Possible action mechanism for curcumin in pre-cancerous lesions based on serum and salivary markers of oxidative stress. J Oral Sci 52:251–6
- Rana P, Murthy R. (2013). Formulation and evaluation of mucoadhesive buccal films impregnated with carvedilol nanosuspension: a potential approach for delivery of drugs having high first-pass metabolism. Drug Deliv 20:224–35
- Reibel J. (2003). Prognosis of oral pre-malignant lesions: significance of clinical, histopathological, and molecular biological characteristics. Crit Rev Oral Biol Med 14:47–62
- Riemersma JC. (1963). Osmium tetroxide fixation of lipids: nature of the reaction products. J Histochem Cytochem 11:436–42
- Sharma R, Gescher A, Steward W. (2005). Curcumin: the story so far. Eur J Cancer 41:1955–68
- Siepmann J, Siepmann F. (2008). Mathematical modeling of drug delivery. Int J Pharm 364:328–43
- Soliman SM, Abdel Malak NS, El-Gazayerly ON, Abdel Rehim A. (2010). Formulation of microemulsion gel systems for transdermal delivery of celecoxib: in vitro permeation, anti-inflammatory activity and skin irritation tests. Drug Discov Ther 4:459–71
- Tirnaksiz F, Robinson J. (2005). Rheological, mucoadhesive and release properties of pluronic F-127 gel and pluronic F-127/polycarbophil mixed gel systems. Die Pharm Int J Pharm Sci 60:518–23
- Tønnesen HH, Másson M, Loftsson T. (2002). Studies of curcumin and curcuminoids. XXVII. Cyclodextrin complexation: solubility, chemical and photochemical stability. Int J Pharm 244:127–35
- Wang Y-J, Pan M-H, Cheng A-L, et al. (1997). Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharm Biomed Anal 15:1867–76
- Zhu Z, Zhai Y, Zhang N, et al. (2013). The development of polycarbophil as a bioadhesive material in pharmacy. Asian J Pharm Sci 8:218–27