Abstract
Galantamine hydrobromide, a promising acetylcholinesterase inhibitor is reported to be associated with cholinergic side effects. Its poor brain penetration results in lower bioavailability to the target site. With an aim to overcome these limitations, solid–lipid nanoparticulate formulation of galantamine hydrobromide was developed employing biodegradable and biocompatible components. The selected galantamine hydrobromide-loaded solid–lipid nanoparticles offered nanocolloidal with size lower than 100 nm and maximum drug entrapment 83.42 ± 0.63%. In vitro drug release from these spherical drug-loaded nanoparticles was observed to be greater than 90% for a period of 24 h in controlled manner. In vivo evaluations demonstrated significant memory restoration capability in cognitive deficit rats in comparison with naive drug. The developed carriers offered approximately twice bioavailability to that of plain drug. Hence, the galantamine hydrobromide-loaded solid–lipid nanoparticles can be a promising vehicle for safe and effective delivery especially in disease like Alzheimer’s.
Introduction
Alzheimer’s disease (AD) is a kind of dementia influencing the elderly population both economically and socially (Leon et al., Citation1998; Guttman et al., Citation1999). It is continuously deteriorating age-related disorder with its manifestations similar to that of congestive heart failure (Antuono & Beyer, Citation1999). Women are approximately 1.5 times more prone to AD than men (Gao et al., Citation1998). Neuronal degeneration is the common features of AD, which results in the loss of memory, communication skills, judgments and reasoning. Accumulation of extracellular amyloid-β (Aβ) resulting in the senile plaques development and neurofibrillary tangles formation are the important hallmarks of AD (Wimo & Winblad, Citation2001; Chopra et al., Citation2011a).
Acetylcholinesterase (AChE) inhibitors are currently the only approved class of drugs according to US food and drug administration for the symptomatic treatment of AD (Chopra et al., Citation2011b). AD pathologies are directly influenced by some of the AChE inhibitors that induce molecular and cellular changes (Takata et al., Citation2010). Galantamine hydrobromide (GH) is a reversible, competitive AChE inhibitor, which also acts as an allosterically potentiating ligand for nicotinic acetylcholine receptors (Maelicke & Albuquerque, Citation2000; Maelicke et al., Citation2001), thus exhibiting neuroprotective effects (Yanagida et al., Citation2008; Hernandez et al., Citation2013). GH has been shown to improve attention and has beneficial effects on cognitive and functional outcomes for AD. The efficacy and acceptability of GH have been proved by various clinical trials (Raskind et al., Citation2000; Wilcock et al., Citation2000). On the other hand, poor lipophilicity, the need of repeated dosing and cholinergic side effects of GH, are the major hurdles in the optimum usage of this promising drug. Although the drug manifest rapid absorption from gastrointestinal (GI) tract, but poor brain penetration and retention, makes its less bioavailable to the desired site for AD management, that is, brain. As many poorly lipophilic drugs are unable to cross the blood–brain barrier (BBB), therefore, brain targeting of such agents is one of the most tedious and challenging task.
The concept of drug targeting is traced back to Nobel laureate, Sir Paul Ehrlich (1906), who envisioned the targeting of drug to the desired site in an effective manner (Katare et al., Citation2010). Inspired by this approach, Muller discovered a novel and biocompatible nanocolloidal drug delivery vehicle known as solid–lipid nanoparticles (SLNs) (Muller et al. Citation1993). Apart from, biocompatibility, these carriers are established for passive drug targeting, bypassing of mononuclear phagocytic system (MPS), improved bioavailability, better drug payload and controlled drug release. These have been recently explored for brain delivery in various neurological disorders and fetched the scientific fraternity with promising results (Kaur et al., Citation2008). Physical association of the drug to the SLNs is quite necessary for the brain drug delivery (Sehra & Dhake, Citation2005). The studies indicate that BBB integrity remains unaltered by the SLNs, whereas polymeric-charged nanoparticles are observed to be toxic for the BBB. In the same drug concentrations, the amount of drug delivered to brain from SLNs reported to be higher than that from neutral and cationic formulations (Lockman et al., Citation2004). These carriers also possess hallmarks such as biocompatibility, ease of production and regulatory approval, which have been found to be a boon for the biomedical applications (Raza et al., Citation2013a; Singh et al., Citation2015).
Recently, several attempts have been made for effective CNS delivery of AChE inhibitors employing different polymers and methods (Wilson et al., Citation2008; Sruthi et al., Citation2015). Despite this, most of the research is based on physiochemical characterization lacking the real time evaluation of such systems in appropriate animal models (Cirrito & Holtzman, Citation2003). Taking cognisance to the challenges for better brain delivery, patient compliance and enhanced efficacy, it was envisioned to formulate SLNs loaded with GH in an attempt to overcome these concerns. Care was taken to utilize the biocompatible and eco-friendly material in a simple and scalable way. Further, the developed system was evaluated in established animal model.
Materials and methods
Materials
GH was received as a gift sample from M/s Sun Pharma (Baroda, India). Pluronic F 127 and Tween 80 were procured from M/s Sigma (St. Louis, MO). Double-distilled water, filtered using 0.22-micron nylon filter was used throughout the study. Analytical/HPLC grade chemicals and reagents were used without any further purification.
Preparation of GH-loaded SLNs (GH-SLNs)
The preparation of GH-SLNs was performed by microemulsification method (Raza et al., Citation2013a). In brief, the drug (20 mg) was dissolved in aqueous phase containing Pluronic F-127 (250 mg) as surfactant and Tween 80 as co-surfactant. Four different batches of formulations were prepared based on the varying concentrations of the cosurfactant, that is, 0.5%, 1%, 2% and 4% w/v. The solid lipid, that is, glyceryl behnate (compritol, 500 mg) was melted at 70 °C. A clear and hot microemulsion was obtained by mixing the aqueous phase and the oil phase isothermally to give a total microemulsion volume of 10 ml. The hot microemulsion was streamline added to remaining portion of water (40 ml), previously kept at 4 °C and stirred continuously at 3000 rpm for 20 min and then ultrasonicated. The microemulsion was then lyophilized to the final nanopowder form. This powder (20 mg) was dispersed in 10 ml of water to give final dispersion, that is GH-SLNs.
Characterization studies
Particle size and zeta-potential determination
The average particle size, polydispersity index (PDI) and zeta (ζ) potential of the developed SLNs were determined by Zetasizer™ installed at Department of Chemistry, Panjab University, Chandigarh, India. The mean of three consecutive measurements was considered as the final reading of each sample.
Transmission electron microscopy
Transmission electron microscopy (TEM) analysis was performed using 1% phosphotunstic acid in phosphate buffer (pH 7.4). TEM at an accelerated voltage of 80 kV was used to determine the morphology of the SLNs. The thin film of the diluted (1/20) sample was placed on a copper grid which was previously coated with carbon, viewed under TEM. SLNs observed in the view (18 000× and 30 000×) were further evaluated for the structural attributes.
Entrapment efficiency
To separate free drug from GH-SLNs dispersion, dialysis membrane having molecular weight cut off of 12 000–14 000 D (M/s Himedia Labs, Mumbai, India)) was used. SLNs dispersion (1 ml) was placed in dialysis tubing tied at both the ends and dialyzed against ethanol (100 ml) at room temperature for 2 h. The amount of drug released into the receptor medium was analyzed spectrophotometrically with appropriate dilutions. The SLNs present in the dialysis bag were distorted by appropriate dilution with a mixture of chloroform and methanol in a ratio 3:2 v/v to determine the amount of drug entrapped within SLNs (Wyss, Citation1990). Entrapment efficiency was calculated using the expression:
Fourier transform infrared (FTIR) spectroscopy
To elaborate the compatibility of the bulk material and the nanoparticles, FTIR spectrophotometer was employed. Analysis of the peaks obtained for the plain drug, that is, GH, compritol, lyophilized SLNs and physical mixture of drug/lipid (1:10) were done for any significant changes in the respective IR spectra.
Differential scanning calorimetry
Differential scanning calorimeter installed at DST-FIST laboratory at UIPS, Panjab University, was employed for these studies. The samples were sealed in aluminum pan and heated in the range of 10 °C to 250 °C under inert nitrogen atmosphere with scanning speed of 10 °C/min. Thermal analysis were done for GH, lyophilized GH-SLNs, lipid and physical mixture of drug/lipid (1:10) to infer about the physical states of the drug and excipients.
X-ray diffraction measurement
X-ray diffractometer installed at Central Instrument Facility, National Institute of Pharmaceutical Education and Research (NIPER), Mohali, was used to record XRD pattern. The samples were exposed to CuKα radiation (45 kV, 40 mA) and scanned from 5 to 50, 2θ at a step size of 0.017 and scan step time of 25 s. Samples used for XRD analysis were GH, lyophilized GH-SLNs, lipid and physical mixture of drug:lipid (1:10).
In vitro drug release
The drug release studies were performed using the Franz diffusion cell (M/s PermeGear Inc, Hellertown, PA) (Behan & Birkinshaw, Citation2001; Hu et al., Citation2003). The two compartments of the cell were separated by the dialysis membrane of 12 000–14 000 D molecular weight and 1 ml of SLNs dispersion (4.88 mg of GH/ml) was loaded onto the membrane in the donor compartment. The receptor compartment contained 30 ml of the 0.1 N HCl, maintained at 37 ± 0.5 °C with a stirring speed of 100 rpm. The sink conditions were maintained by replacing the same volume of fresh medium with the aliquots of the diffusion medium withdrawn at different times. At 280 nm, the diluted samples were analyzed for GH content spectrophotometrically.
Stability studies
Stability studies were conducted to observe the change in the size of nanoparticles formed. Total drug content of the formulation and % entrapment efficiency of GH-SLNs was also observed upon storage at refrigerated temperature of 4 °C for 3 months and at normal conditions (25 °C for 45 days). The samples requiring refrigerated conditions were withdrawn at interval of 0, 1, 2 and 3 months, whereas the one stored at 25 °C were withdrawn at 0, 15, 30, 45th day.
In vivo pharmacokinetics studies
Male New Zealand rabbits weighing 2.5 ± 0.5 kg were employed to study the pharmacokinetic profile of GH-SLNs and naive drug. Six rabbits were divided equally into two groups, that is, control group and experimental group, and were administered 2.65 mg/kg of GH and equivalent GH-SLNs by oral route, respectively. The blood samples (5 ml) from an ear vein were collected at predetermined time intervals, that is, 0 min, 30 min, 1 h, 2 h, 4 h, 8 h, 12 h and 24 h in ethylene diamine tetra acetate-coated vials. The samples were centrifuged at 10 000 rpm for 5 min to obtain the plasma and was further stored at −20 °C. The content of the drug in the plasma samples were then determined using high-performance liquid chromatography (HPLC).
HPLC analysis
The chromatograph (Shimadzu Class-VP, V6.13, Shimadzu Corporation, Kyoto, Japan) consisted of a quaternary gradient pump, a UV detector (SPD-10 A Vp, Shimadzu Corporation, Kyoto, Japan), a standard manual sampler, and a column oven. Phenomenex C18 (250 × 4.6 mm) column of 5 μm particle size and a mobile phase consisting of acetonitrile:ammonium formate buffer (2 mM, pH 9.0) (70:30), filtered under vacuum through a 0.22 -μm nylon membrane filter were used. The flow rate was set at 0.4 ml/min with the detection wavelength of 289 nm and oven temperature of 25 °C.An injection volume of 20 μl was used to analyze the sample (Mannens et al., Citation2002). In Eppendorf tube, 200 μl acetonitrile was added along with equal amount of plasma sample to precipitate all the proteins. Deionized water (1 ml) was mixed with obtained supernatant in a glass tube which was further evaporated to dryness in a water bath. Methanol (200 μl) was used to dissolve the residue and 20 μl of sample was injected in HPLC.
In vivo studies
Animals and laboratory conditions
Adult Wistar rats (250–300 g) bred in Small Animal House facility of Post Graduate Institute of Medical Education and Research, Chandigarh were used. The Institutional Animal Ethics Committee (IAEC) of PGIMER, Chandigarh approved all the experimental protocols which were also performed in accordance with the guidelines of Committee for Control and Supervision of Experimentation on Animals (CPCSEA), Government of India, (No. 57/IAEC/307 dated November 23rd, 2011).
Drugs
GH was dissolved in double-distilled water while isoproterenol was dissolved in artificial CSF (ACSF) [(2.9 mM KCl, 147 mM NaCl, 1.7 mMCaCl2, 1.6 mM MgCl2, 2.2 mM D-glucose). The dose of GH was selected according to the previous studies (Takata et al., Citation2010), while the dose of isoproterenol was standardized in the laboratory.
Intracerebroventricular (ICV) injection of isoproterenol
Method described by Wang et al. (Citation2004) was used to administer ICV injection of isoproterenol. Isoproterenol (40 mM, ICV, 2 µl) previously standardized in our laboratory was injected bilaterally into the rat brain in the hippocample region using the coordinates 4.8 mm posterior to bregma; 2.2 mm lateral to sagittal suture and 3.0 mm beneath the surface of the brain (Paxinus 1985). Only ACSF was administered ICV to the control rats in the same volume, that is 2 µl.
Experimental design and treatment schedule
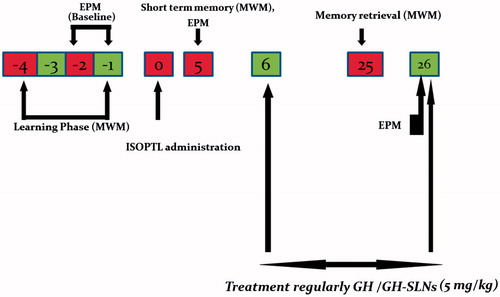
About 6–8 rats were allotted randomly in each group. Group 1: normal or naive rats; Group 2: control group, administered with equal volume of ACSF on day 1; Group 3: Sham-operated rats, underwent surgery but no isoproterenol was administered; Group 4: rats administered with ICV-isoproterenol 40 nM on day 0; Group 5: ICV-isoproterenol-treated rats administered with GH (5 mg/kg) orally for three weeks; Group 6: ICV-isoproterenol-treated rats received GH-SLNs (equivalent to 5 mg/kg) orally for three weeks ().
Figure 1. Study design; Before ICV isoproterenol administration at day 0, the rats were trained for 4 days in the Morris water for spatial memory evaluation and then assessed at days 0 (baseline), 5 and 25 after treatment with GH and its nanoformulaton (GH-SLNs). Isoproterenol was administered ICV, once at day 0 in the volume of 4 μl bilaterally (2 μl in each burr hole) and the memory was assessed on day 5. ISOPTNL = Isoproterenol.
Behavioral estimation
Morris water maze test
Morris water maze test was used to study the spatial memory of the experimental rats (Morris et al., Citation1982; Misra et al., Citation2011). A circular water tank was divided into four quadrants. Initially the water maze task was done for four days, that is, day −4 to −1, before the administration of isoproterenol with four consecutive random trainings from each quadrant having a cut off time of 90 s to reach the hidden platform. The stay period for each rat onto the platform was 20 s. The time taken by the rats to reach the platform (escape latency in seconds) was determined. After the administration of ICV-isoproterenol on day 0, escape latency was again evaluated and reported on day 5. Following the treatment with GH and GH-SLNs, memory retrieval was accessed on day 25.
Elevated plus maze test
On days −2, −1, 5 and 26, elevated plus maze test was performed for accessing memory acquisition and retention. The apparatus consisted of one closed and one open arm crossing each other. The rat under trial was placed outwardly on the open arm. Time taken by each rat to enter the closed arm was measured as mean transfer latency with cut-off time at 90 s. After 24 h, retention trials were repeated and mean transfer latency was again assessed (Misra et al., Citation2011).
Statistical analysis
Results were expressed as mean ± S.E.M. The intergroup variation was measured by one-way analysis of variance (ANOVA) followed by Tukey's test. p < 0.05 was considered as statistically significant and evaluated using the Graphpad Prism Statistical Software version 5 (San Diego, CA) .
Results and discussion
Solid–lipid nanoparticles
Based on the preliminary studies, glyceryl behenate (Compritol) was chosen as the solid lipid, as it offered stable dispersion with smaller sized particle (data not shown) (Raza et al., Citation2013b). Depending upon the ratio of solid–lipid and aqueous phase used, along with varied percentage of co-surfactant, four batches of the formulations were prepared.
Micromeritics, topography, surface charge and %entrapment efficiency
Various characterization attributes of the developed nanodispersions is listed in . GH-SLNs offered the average particle size in the range of 88.0 ± 1.89 nm to 221.4 ± 1.34 for the four batches studied with a PDI value range of 0.275 ± 0.13 to 0.380 ± 0.16. This acceptable value of PDI inferred the uniformity in the polydispersed phase (Feng & Huang, Citation2001). Reduction in the particle size is reported to elongate the retention time and bypass the MPS (Hu et al., Citation2003). The TEM images revealed that nanoparticles were spherical in shape, though with slight aggregation, which can be ascribed to the inherent properties of compritol () (Alex et al., Citation2011; Kim et al., Citation2013).
Figure 2. TEM photomicrographs of GH-SLNs at (a) 18 000x; (b) 30 000 x; (c) size distribution of GH-SLNs.
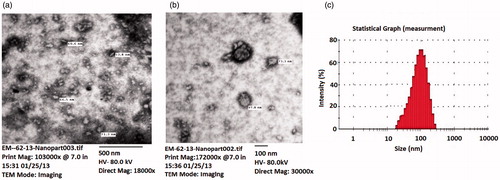
Table 1. Various characterization attributes of the developed four batches of GH-SLNs.
The ζ-potential values were found to be in the range of −18.75 ± 1.7 V to ±10.04 ± 1.9 V. These moderately higher negative ζ-potential values assured the colloidal stability due to the electrostatic repulsions, preventing the aggregation of the particles (Abrol et al., Citation2004). The developed nanoconstructs offered a high entrapment efficiency in the range of 77.29 ± 0.45% to 90.17 ± 0.78 with a higher drug payload possibly due to the slightly lipidic inclination of the drug (log p = 1.8). Apart from this, the selected microemulsification method is also reported to offer higher entrapment of the drugs with moderate water solubility-like GH (Service, Citation2003).
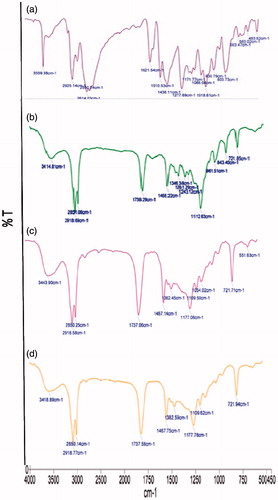
FTIR spectroscopy
As portrayed in , pure GH shows the characteristic peak at 3559.38 cm−1, 2614.03 cm−1, 1488 cm−1 and 1112 cm−1. However, in case of GH-SLNs (), there was disappearance of all these characteristic absorption bands of GH. Latter revealed the masking of these peaks of the drug due to complete encapsulation by the layers of Compritol. Further, characteristic peaks of Compritol, appearing in the spectra of GH-SLNs () at 2918.77 cm−1, 2850.6 cm−1and 721.94 cm−1, showed signified peak shifts, indicating some kind of complexation phenomenon with the drug. Additionally, the absence of any new peak in the spectra of GH-SLNs revealed the absence of some degradation and new adduct formation. This was further confirmed by the intact individual peak of each SLN component in the physical mixture of drug and Compritol ().
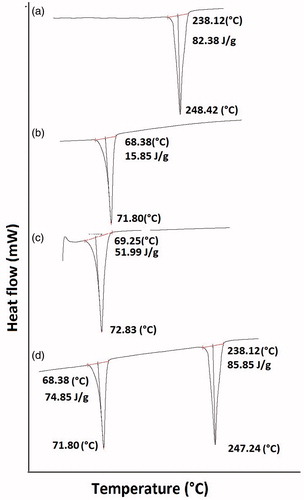
DSC analysis
As shown in , there was a sharp endotherm at 248.42 °C, indicating the melting of GH (melting range = 242–52 °C), whereas the GH-SLNs showed a sharp endothermic peak at 71.80 °C (). These findings are in consonance with the FTIR results, indicating the encapsulation of GH in the matrix of Compritol. This was further confirmed by the re-appearance of the GH melting endotherm in the physical mixture of Compritol and GH ().
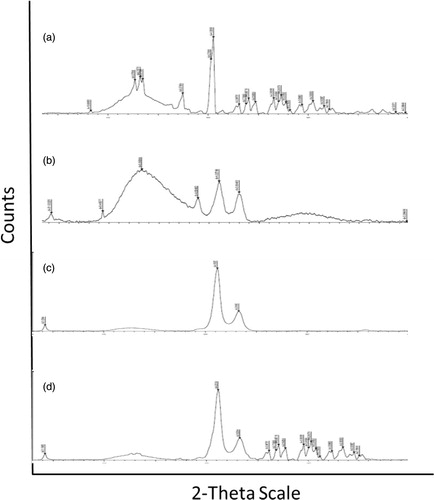
XRD pattern
XRD paradigm of GH, Compritol, lyophilized GH-SLNs and the physical mixture (GH: Compritol; 1:10) are shown in . XRD pattern of GH exhibited more than 20 sharp peaks, confirming the crystalline nature of the drug. However, these sharp peaks were absent in the GH-SLNs, indicating the loss of crystallinity and the subsequent shift toward the amorphous nature. The XRD pattern of the GH-SLNs was also completely different from the pure lipid, indicating the embedding of the drug in the interiors of the lipid matrix resulting in the amorphous mixture (Shi et al., Citation2012).
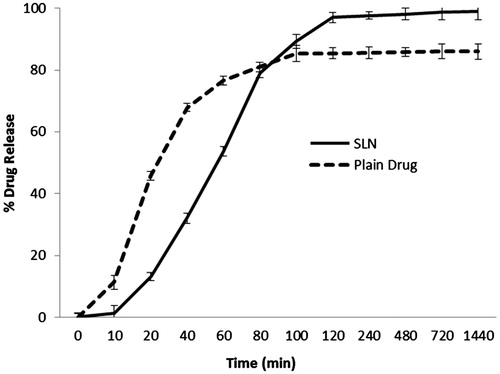
In vitro drug release evaluation
Drug release pattern of GH-SLNs and free drug for 24 h at temperature of 37 ± 0.5 °C has been portrayed in . The initial fast release from the GH-SLNs with a T50% of 6 hs might be due to the smaller particle size resulting in smaller diffusion pathway and easy access of drug molecules to the dissolution medium. This can also be ascribed to the presence of the nanonized adsorbed drug on to the SLN surfaces and the subsequent enhanced dissolution rate. Then, the decreased rate of drug release was observed, which indicated the release of incorporated drug from the interiors of the nanoparticle (Corrigan & Li, Citation2009). It is assumed that the rate of release in second episode is maintained by diffusion of drug over the lipid matrix. The release of the drug from the GH-SLNs was prolonged up to 24 h with 99.83 ± 0.11% being released in 24 h, as compared to free drug solution (almost 80.96% released in 1 h). GH, being relatively water soluble, showed a fast release, however, post-encapsulation in a lipidic layer, a controlled release profile was observed. The release flux for GH-SLNs was came out to be 0.0352 (µg/cm2/h) and for GH was 0.0573 (µg/cm2/h), which showed the controlled release pattern from GH-SLNs. The release flux depicted the most sought after release, that is, controlled release of GH from the SLNs (Genta et al., Citation1998).
In vivo pharmacokinetic studies
Patel et al., Citation2010, reported the method for the determination of GH in pharmaceutical dosage form by HPLC with the wavelengths of 289 nm. We proceeded with the same wavelength but modified the method according to our laboratory conditions which improved the sensitivity. The developed HPLC method for in vivo estimation of drug in plasma samples was found to be robust and dependable. The chromatogram of the drug in plasma sample has been shown in .
Figure 7. (a) HPLC chromatogram of blank plasma (upper) and GH control (lower) in blank plasma. (b) The mean plasma concentration-time curve of GH-SLNs and GH after oral administration in rabbits (n = 3).
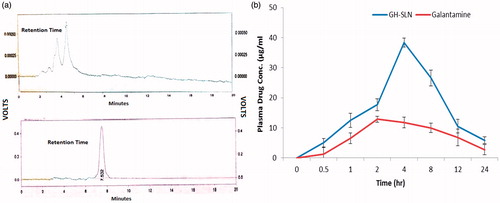
shows the various pharmacokinetic parameters obtained after fitting the data in one-compartment open body model using Microsoft excel software, while shows the plasma course of drug with respect to time. In vivo pharmacokinetic results depicted that the AUC of GH-SLN and naïve GH was 441.10 (mg/l hour) and 206.02 (mg/l hour), respectively, indicating approximately 2-fold improvement in the bioavailability of the drug as compared to the control group.
Table 2. Pharmacokinetic parameters obtained from oral administration of plain drug and GH-SLNs to rabbits (n = 3).
However, the rate of absorption of GH from GH-SLNs was slower than the plain drug, indicating involvement of some lacteal pathways instead of direct GIT absorption. These findings supported the fact of overcoming the hepatic first pass route employing lipid-based delivery vehicles (Handy et al., Citation2008). On the other hand, relatively higher Vd offered by GH-SLNs indicate some extend of tissue binding of the drug vis-à-vis the plain drug, ostensibly due to the absorption of the SLN encapsulated drug as such and afterward drug release. These findings are unique and indicate the substantial effect of SLNs on modulating the pharmacokinetics of the GH, that too in the desired manner.
Stability studies
At all the storage conditions viz. 4 °C and 25 °C, the values of drug assay were observed to fall well within the pharmacopoeial limits of 100 ± 10% (USP 32 NF 27). GH-SLNs showed a significant stability in term of maintaining the uniformity in the total drug content, entrapment efficiency and particle size as shown in .
Table 3. Table shows stability studies at 4 °C and 25 °C.
In vivo pharmacodynamic studies
Morris water maze test
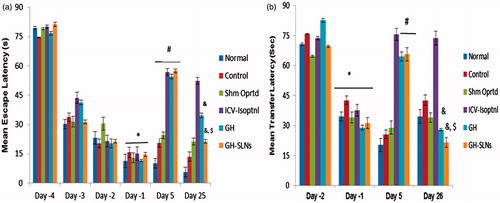
During training, on day 1 the escape latency was significantly reduced as compared to day 4 that is, first day of the training suggesting all the animals were trained well. Except control and sham operated group, on day 0, ICV-isoproterenol was administered to the rest of the groups. Following isoproterenol administration on day 5, significant increase in the escape latency was observed of all the rat treatment groups vis-à-vis control group or Sham group (<0.05) (). GH (5 mg/kg, p.o.) and GH-SLNs (equivalent to 5 mg/kg of GH, p.o.) markedly reduced the escape latency and significant difference was noticed between GH and GH-SLNs-treated groups (). At day 25, there was a significant reduction in escape latency in the groups receiving GH and GH-SLNs. However, there was noticeable reduction in GH-SLNs-treated group, as compared to isoproterenol-insulted group as well as GH-treated group (p < 0.05) (). To substantiate the finding of Morris water maze, elevated plus maze test was done. On day 1, rats showed significant reduction in the mean transfer latency to reach the closed arm as compared to day 2 (p < 0.05) in all the groups. On day 5, after isoproterenol administration, all the isoproterenol administered rats showed significant increase in mean transfer latency as compared to control and sham operated rats (p < 0.05), which was significantly improved by GH as well as GH-SLNs. Therefore, GH-SLNs was proved to be more efficacious than GH (p < 0.05) ().Thus, the results suggested reversal of cognitive deficit in isoproterenol induced rats after oral administration of GH-SLNs, indicating effectiveness of SLNs in spatial memory regaining and behavioural acquisition in cognitive deficit rats vis-à-vis plain drug.
Figure 8. (a) Morris water maze test; effect of GH and GH-SLNs (5 mg/kg, p.o.) (n = 6/group) on escape latency of memory retrieval in ICV isoproterenol-treated rats after 25 days. Data are expressed as mean ± S.E.M. *p < 0.05, as compared to day (−4) of the training; #p < 0.05, as compared to day (−1) of the training; &p < 0.05, as compared to isoproterenol group on day (25) of the training; $p < 0.05, as compared to GH group on day (25). (b) Effect of GH and GH-SLNs (5 mg/kg p.o.) treatment on mean transfer latency in elevated plus maze in ICV isoproterenol treated rats after 26 days. Data are expressed as mean ± S.E.M. *p < 0.05, as compared to day (−1) of the training; #p < 0.05, as compared to isoproterenol group on day (26) of the training. ISOPTNL = Isoproterenol.
Conclusion
The issues of lower bioavailability and poor BBB penetration are the leading factors of compromised efficacy of GH. In this study, both the concerns have been taken care with the help of promising novel carriers, SLNs. Bioavailability was enhanced by approximately 100% and significant improvement was also observed in AD-like preclinical protocol. The unique composition along with biocompatible components was able to modulate the time course of drug in vivo and release profile in vitro. The developed system provides a platform for maximum utilization of present medications by avoiding the adherent hurdles, employing drug delivery approach. However, the study presents the preclinical data with desired outcomes in animal model, with a potential promise in the clinical studies.
Acknowledgements
Authors are thankful to M/s SUN PHARMA, Baroda, India, for providing the gift sample of Galantamine hydrobromide.
Declaration of interest
Financial grant obtained from Department of Biotechnology (DBT), New Delhi, is gratefully acknowledged.
References
- Abrol S, Trehan A, Katare OP. (2004). Formulation, characterization, and in vitro evaluation of silymarin-loaded lipid microspheres. Drug Deliv 11:185–91
- Alex A, Paul W, Chacko AJ, et al. (2011). Enhanced delivery of lopinavir to the CNS using compritol-based solid lipid nanoparticles. Ther Deliv 2:25–35
- Antuono P, Beyer J. (1999). The burden of dementia: a medical and research perspective. Theor Med Bioeth 20:3–13
- Behan N, Birkinshaw C. (2001). Preparation of poly (butyl cyanoacrylate) nanoparticles by aqueous dispersion polymerisation in the presence of insulin. Macromol Rapid Comm 22:41–3
- Chopra K, Misra S, Kuhad A. (2011a). Neurobiological aspects of Alzheimer's disease. Expert OpinTher Targets 15:535–55
- Chopra K, Misra S, Kuhad A. (2011b). Current perspectives on pharmacotherapy of Alzheimer's disease. Expert Opin Pharmacother 12:335–50
- Cirrito JR, Holtzman DH. (2003). Amyloid β and Alzheimer's disease therapeutics: the devil may be in details. J Clin Invest 112:321–23
- Corrigan OI, Li X. (2009). Quantifying drug release from PLGA nanoparticulates. Euro J Pharm Sci 37:477–85
- Feng S, Huang G. (2001). Effects of emulsifiers on the controlled release of paclitaxel (Taxol) from nanospheres of biodegradable polymers. J Control Release 71:53–69
- Gao S, Hendrie HC, Hall KS, Hui S. (1998). The relationship between age, sex, and the incidence of dementia and Alzheimer's disease: a meta-analysis. Arch Gen Psychiatry 55:809–15
- Genta M, Costantini A, Asti B, Conti L. (1998). Influence of glutaraldehyde on drug release and mucoadhesive properties of chitosan microspheres. Carbohydr Polym 36:81–8
- Guttman R, Altman RD, Nielsen NH. (1999). Alzheimer disease: report of the council on scientific affairs. Arch Family Med. 8:347–53
- Handy RD, Henry TB, Scown TM, et al. (2008). Manufactured nanoparticles: their uptake and effects on fish mechanistic analysis. Ecotoxicology 17:396–409
- Hernandez CM, Kayed R, Zheng H, et al. (2013). Loss of alpha 7 nicotinic receptors enhances beta-amyloid oligomer accumulation, exacerbating early-stage cognitive decline and septohippocampal pathology in a mouse model of Alzheimer's disease. J Neurosci 30:2442–53
- Hu Y, Jiang X, Ding Y, et al. (2003). Preparation and drug release behaviors of nimodipine-loaded poly (caprolactone) poly (ethylene oxide) polylactide amphiphilic copolymer nanoparticles. Biomaterials 24:2395–404
- Katare OP, Raza K, Singh B, Dogra S. (2010). Novel drug delivery systems in topical treatment of psoriasis: rigors and vigors. Indian J Dermatol Venereol Leprol 76:612–21
- Kaur IP, Bhandari R, Bhandari S, Kakkar V. (2008). Potential of solid lipid nanoparticles in brain targeting. J Control Release 127:97–109
- Kim H, Kim Y, Lee J. (2013). Liposomal formulations for enhanced lymphatic drug delivery. Asian J Pharm Sci 8:96–103
- Leon J, Cheng CK, Neumann PJ. (1998). Alzheimer's disease care: costs and potential savings. Health Aff (Millwood). 17:206–16
- Lockman PR, Koziara JM, Mumper RJ, Allen D.D. (2004). Nanoparticle surface charges alter blood-brain barrier integrity and permeability. J Drug Target 12:635–41
- Maelicke A, Albuquerque EX. (2000). Allosteric modulation of nicotinic acetylcholine receptors as a treatment strategy for Alzheimer's disease. Eur J Pharmacol 393:165–70
- Maelicke A, Samochocki M, Jostock R, et al. (2001). Allosteric sensitization of nicotinic receptors by galantamine, a new treatment strategy for Alzheimer’s disease. Biol Psychiatry 49:279–88
- Mannens GSJ, Snel CAW, Hendrickx J, et al. (2002). The metabolism and excretion of galantamine in rats, dogs, and humans. Drug Metab Dispos 30:553–63
- Misra S, Tiwari V, Kuhad A, Chopra K. (2011). Modulation of nitrergic pathway by sesamol prevents cognitive deficits and associated biochemical alterations in intracerebroventricular streptozotocin administered rats. Eur J Pharmacol 659:177–86
- Morris RGM, Garrud P, Rawlins JNP, O'Keefe J. (1982). Place navigation impaired in rats with hippocampal lesions. Nature 297:681–3
- Muller RH, Schwarz C, Mehnert W, Lucks JS. (1993). Production of solid lipid nanoparticles for controlled drug delivery. Proc Int Symp Control Rel Bioact Mater 20:480–7
- Patel KB, Patel AV, Patel VJ, et al. (2010). Quantitative determination of galantamine hydrobromide in pharmaceutical dosage form by RP- high performance liquid chromatography. J Chem Pharm Res 2:36–43
- Service RF. (2003). American Chemical Society meeting. Nanomaterials show signs of toxicity. Science (NY) 300:243–50
- Raskind MA, Peskind ER, Wessel T, Yuan W. (2000). Galantamine in AD: a 6-month randomized, placebo-controlled trial with a 6-month extension. The Galantamine USA-1 Study Group. Neurology 54:2261–8
- Raza K, Katare OP, Setia A, et al. (2013a). Improved therapeutic performance of dithranol against psoriasis employing systematically optimized nanoemulsomes. J Microencapsul 30:225–36
- Raza K, Singh B, Singal P, et al. (2013b). Systematically optimized biocompatible isotretinoin-loaded solid lipid nanoparticles (SLNs) for topical treatment of acne. Colloid Surface B 105:67–74
- Sehra S, Dhake AS. (2005). Formulation and evaluation of sustained release microspheres of poly-lactide-co-glycolide containing tamoxifen citrate. J Microencaps 22:521–8
- Shi F, Zhao JH, Liu Y, et al. (2012). Preparation and characterization of solid lipid nanoparticles loaded with frankincense and myrrh oil. Int J Nanomedicine 7:2033–43
- Singh J, Garg T, Rath G, Goyal AK. (2015). Advances in nanotechnology-based carrier systems for targeted delivery of bioactive drug molecules with special emphasis on immunotherapy in drug resistant tuberculosis – a critical review. Drug Deliv 11:1–23
- Sruthi N, Joe F, Kumar S. (2015). Formulation and evaluation of galantamine nanoparticles for neurological disorders. IJPCBS 5:63–70
- Takata K, Kitamura Y, Saeki M, et al. (2010). Galantamine-induced amyloid-beta clearance mediated via stimulation of microglial nicotinic acetylcholine receptors. J Biol Chem 285:40180–91
- Wang DL, Ling ZQ, Cao FY, et al. (2004). Melatonin attenuates isoproterenol induced protein kinase A overactivation and tau hyperphosphorylation in rat brain. J Pineal Res 37:11–16
- Wilcock GK, Lilienfeld S, Gaens E. (2000). Efficacy and safety of galantamine in patients with mild to moderate Alzheimer's disease: multicentre randomised controlled trial. Galantamine international-1 study group. BMJ 321:1445–9
- Wilson B, Samanta MK, Santhi M, et al. (2008). Poly(n-butylcyanoacrylate) nanoparticles coated with polysorbate 80 for the targeted delivery of rivastigmine into the brain to treat Alzheimer's disease. Brain Res 1200:159–68
- Wimo A, Winblad B. (2001). Health economical aspects of Alzheimer's disease and its treatment. Psychogeriatrics 1:189–93
- Wyss R. (1990). Chromatography of retinoids. J Chromatogr B Biomed Sci Appl 531:481–508
- Yanagida T, Takeuchi H, Kitamura Y, et al. (2008). Synergistic effect of galantamine on nicotine-induced neuroprotection in hemiparkinsonian rat model. Neurosci Res 62:254–61