Abstract
To increase the anti-tumor activity of paclitaxel (PTX), novel temperature-sensitive liposomes loading paclitaxel (PTX-TSL) were developed. In vitro, characteristics of PTX-TSL were evaluated. The mean particle diameter was about 100 nm, and the entrapment efficiency was larger than 95%. The phase-transition temperature of PTX-TSL determined by differential scanning calorimetry was about 42 °C. The result of in vitro drug release from PTX-TSL illustrated that release rate at 37 °C was obviously lower than that at 42 °C. Stability data indicated that the liposome was physically and chemically stable for at least 3 months at −20 °C. In vivo study, after three injections with hyperthermia in the xenograft lung tumor model, PTX-TSL showed distinguished tumor growth suppression, compared with non-temperature-sensitive liposome and free drug. The results of intratumoral drug concentration indicated that PTX-TSL combined with hyperthermia delivered more paxlitaxel into the tumor location than the other two paxlitaxel formulations. In summary, PTX-TSL combined with hyperthermia significantly inhibited tumor growth, due to the increased targeting efficiency of PTX to tumor tissues. Such approach may enhance the delivery efficiency of chemotherapeutics into solid tumors.
Introduction
Paclitaxel (PTX) is an anticancer drug belonging to alkaloid, which stabilizes microtubules and suppresses endothelial cell proliferation, motility, and tube formation. It has been approved for chemotherapy of a variety of cancers, such as ovarian neoplasms, breast neoplasms, and pulmonary cancer (Zhang et al., Citation2009). However, the clinical therapeutic effect of PTX injection is limited because of the adverse reactions induced by polyoxyethylene castor oil: anaphylaxis, renal toxicity, and neurovirulence (Szebeni et al., Citation1998; van Zuylen et al., Citation2001). Another important adjuvant Cremophor EL also could influence the function of endothelium and vesicular muscle, consequently cause angiectasis and labored respiration (Friedland et al., Citation1993). Besides the above adverse reactions of commercial formulation for PTX, PTX itself can highly bind to plasma proteins, decreases the ability to penetrate tumor interstitium in an effective concentration and increases the toxicity toward healthy tissues (Singla et al., Citation2002). Therefore, in order to reduce toxicity, a new drug delivery system is in demand to selectively target PTX to tumor tissue.
Recently, kinds of drug delivery systems including liposomes have been studied (Wang et al., Citation2015). According to the physicochemical characteristics of the phospholipid membrane, liposome is a most hopeful nano-carrier with the opportunity to offer targeting therapy, thus increases the quality of cancer patient care (Ono et al., Citation2002; Morimoto et al., Citation2007; Asai et al., Citation2008; Abu Lila et al., Citation2009). So far, several liposomal drugs have already been authorized to treat cancer. Upon aggregation into the tumor, the liposomes mainly depend on its passive diffusion to release, which needs a relative long time. However, passive release is not able to regulate local concentration or amount of drugs in tumor. In addition, the vascular permeability and interior condition of different tumors are largely variable, which will induce unpredictable liposomal permeation. Owing to these, the liposomes in clinical only achieved the modest therapeutic index of chemotherapy. Thus, it is necessary to design a novel liposomal drug delivery system that could control content release by an ectogenous trigger in the selected location (Koning et al., Citation2010; Simona et al., Citation2013; Kneidl et al., Citation2014; Xie et al., Citation2015).
Regarding to these demands, temperature-sensitive liposomes (TSL) are being developed, based on the work of Yatvin and Weinstein since 1970s (Yatvin et al., Citation1978). TSL is composed of phospholipids with the feature of phase transition from a gel to a liquid crystalline phase at the phase transition temperature (Tm). The phase transition was able to trigger TSL to release loaded drugs generously (De Smet et al., Citation2010). The Tm of TSL applied in drug delivery ranges from 39 °C to 42 °C, in which most of content released quickly, this was anticipated to induce more drugs to aggregate into the tumor location (Kong et al., Citation2000; Needham et al., Citation2000).
In clinical, hyperthermia has been administered as an adjuvant therapy to improve the therapeutic effect combined with radiation or chemotherapy. When tumors exposed to hyperthermia, the tumor blood flow and oxygenation increased, which would improve the delivery of liposomal drugs to tumors (Karino et al., Citation1988; Horsman & Overgaard, Citation1997; Kong & Dewhirst, Citation1999). Furthermore, hyperthermia was able to increase vascular permeability of tumors, thus enhanced the amount of liposome aggregation (Gaber et al., Citation1996; Kong et al., Citation2000,Citation2001). Hyperthermia can induce synergistic effects with chemotherapy (Issels et al., Citation2010). Making use of these features, combining TSL with local heating would help us to further enhance the targeting of drugs to tumors.
To date, the most usually used drug in reported TSL was hydrosoluble doxorubicin (DOX). Except for DOX, there are few drugs used in TSL, according to the published literature (May et al., Citation2013; Simone et al., Citation2014). Our team has been studying the TSL for several years. Vinorelbine bitartrate and docetaxel were successfully encapsulated into TSL formulations by pH gradient active loading and thin film hydration, respectively. The rate of drug release changed notably with the variation of temperature, indicating that the prepared TSL had thermosensitivity. In vivo, combination TSL with hyperthermia enhanced antitumor activity of vinorelbine bitartrate or docetaxel significantly (Zhang et al., Citation2011,Citation2014).
In this work, two liposome formulations TSL loading paclitaxel (PTX-TSL) and non-temperature sensitive liposomes (NTSL) were prepared. In vivo, to compare the tumor delivery efficiency of three PTX formulations: PTX injection (PI)/NTSL/PTX-TSL, intra-tumor drug concentration was determined by HPLC-MS/MS, upon heated at 42 °C or without heating. Then, combined with hyperthermia, the therapeutic activities of the three formulations were tested on murine lung tumor models. These results revealed that PTX-TSL could delivery most PTX to tumor sites among PI, NTSL, PTX-TSL, which resulted in a further increased antitumor efficiency and decreased system toxicity. PTX-TSL combined with hyperthermia may be a potential strategy to resolve some fundamental problems of PTX in clinical applications.
Materials and methods
Materials
1,2-Distearyl-sn-glycero-3-phosphoe thanolamine-N-[methoxy (polyethyleneglycol)-2000] (DSPE-PEG2000), egg yolk phosphatidylcholine (EPC), monostearoyl phosphatidylcholine (MSPC), dipalmitoyl phosphatidylcholine (DPPC), distearoyl phosphatidylglycerol (DSPG) were purchased from Shanghai AVT Pharmaceutical Technology Limited Company and were used directly. PTX was purchased from Hui Ang Pharmaceutical Limited Company, Guilin, China. All other reagents were of analytical grade.
Methods
Preparation of liposomes
PTX-TSL composed of DPPC:MSPC:DSPE-PEG2000:DSPG = 83:3:10:4 (mass ratio) and NTSL composed of EPC:DSPE-PEG2000:DSPG =89:7:4 (mass ratio), were prepared on the basis of foregoing method (Zhang et al., Citation2005,Citation2014; Shikanov et al., Citation2008). First, chloroform solution containing phospholipids and PTX was prepared; the concentration was 100 mg/mL and 2 mg/mL, respectively. Second, a thin film emerged by evaporation of chloroform under negative pressure using a rotary evaporator. The third procedure was thin film hydration using phosphate buffer containing sucrose (25%, W/V), by rotating the flask at about 150 rpm at 50 °C until the lipid film was completely hydrated and a homogeneous dispersion was formed. Finally, the liposomes dispersion was homogenized 5 circulations under 12 000 psi pressure in high pressure homogenizer (EmulsiFlex-C3, Avestin, Canada), and then was subjected to extrusion through polycarbonate membrane (100 nm, Whatman) one cycle, using an extruder (EmulsiFlex-C3, Avestin, Canada). Thus, liposomes with uniform size were obtained. Thus, liposomes with uniform size were obtained after homogenization and extrusion of liposomes dispersion.
Quantification of PTX
Determination of PTX was performed by HPLC. The drug content analysis was performed as follow. VenusiL MP-C18 column (pore size 5 μm, 4.6 × 150 mm) was used, mixed solution composed of acetonitrile and water (55/45, v/v) was used as mobile phase with the flow rate of 1 mL/min, at 25 °C. Sample injection volume was 20 μL; UV detector was used to detect PTX under 227 nm of the wavelength.
In vitro characteristics of liposomes
Encapsulation efficiency (EE)
The EE of the prepared liposomes was determined as follow. 1.0 mL liposome solution was centrifuged at 6000 g for 8 min to separate unencapsulated PTX from the drug loaded liposomes. The supernatant was dissolved into ethanol to determine PTX concentration with HPLC above, which was encapsulated drug. At the same time, 1.0 mL liposome solution was dissolved into ethanol directly to determine PTX, which was total drug. The result of EE was calculated by the ratio of encapsulated drug (Drugafter centrifugation) to total drug (Drugbefore centrifugation), according to EquationEquation (1)(1) .
(1)
Photon correlation spectroscopy (PCS)
The particle size of the prepared liposomes was evaluated by PCS (Nanophox, Sympatec GmbH, Germany). The determination process was the same to the previous (Zhang et al., Citation2011).
Appearance of liposomes
Transmission electron microscope (TEM) was used to morphologically characterize the liposomes. After dilution with water, the lipsome suspensions were dropped on the surface of a Formvar-coated copper grid and negative stained by phosphotungstic acid solution. Before evaluated by TEM (HITACHI, H-7650, Japan), the sample was air-dried at room temperature.
Differential scanning calorimetry (DSC)
DSC (Q2000 TA Instruments, USA) analyses were performed to evaluate the phase transition of the liposomal formulations using the method reported earlier (Zhang et al., Citation2014).
Temperature triggered release of PTX
In vitro, release of PTX from PTX-TSL or NTSL as the change of temperature (37 °C, 42 °C) was performed using a dialysis technique. Briefly, liposome suspensions with a volume of 0.4 mL were diluted with 10 mL phosphate-buffered saline (PBS, pH 7.4) containing 0.5 M sodium salicylate. The dilution was added into a dialysis bag (MWCO 8–14 kDa) and was sealed. The dialysis bag was immersed into 30 mL PBS and followed by stirring with a magnetic mixer at 100 rpm under the designed temperature. After certain interval, 0.5 mL sample was taken and simultaneously replaced with an equal volume of PBS. Concentration of PTX in sample was analyzed by HPLC above.
Stability studies of PTX-TSL
In vitro, stability of PTX-TSL was evaluated by determining the change of parameters at store temprature −20 °C. Drug content, pH, diameter, and EE of PTX-TSL were assayed as a function of the storage time. Meanwhile, the stability of PTX-TSL was monitored by a Turbiscan Lab® Expert (Formulaction, L’Union, France) (Yang et al., Citation2014), an implement able to reflect the negligible variation of colloidal solution. At appointed time points, 10 mL PTX-TSL placed into tailor-made sample cell was exposed to Turbiscan Lab® Expert and measured.
In vivo studies
Animals and tumor model
Male KM mice, 4 weeks old, were purchased from the center of laboratory animal, Academy of Military Medical Sciences, China. In this research, Lewis lung carcinoma (LLC) cell line was used to establish tumor model on mice. 0.2 mL of cell solution (1 × 107/mL) was inoculated s.c. under the right axilla of the mouse. Tumors were allowed to grow to a volume of approximately 100 mm3 before treatment.
Concentration of PTX in solid tumor
The mice were randomly divided into six treatment groups: PI (PTX injection, Hai Zheng Pharmaceutical Company, Zhe Jiang, China), NTSL, and PTX-TSL with or without hyperthermia. All groups received intravenous injection of 10 mg/kg PTX (PI or NTSL, PTX-TSL). Before administration, mice were anesthetized with sodium pentobarbital (50 mg/kg) by intraperitoneal injection. Then mice received administration. After this, the mice in three groups (PI with hyperthermia, NTSL with hyperthermia, PTX-TSL with hyperthermia) were put on insulation board with holes controlled by water bath about 42 °C. Tumor locations were exposed to the holes one to one for 30rese (Hauck et al., Citation1997). In this process, only tumor was heated to about 42 °C, meanwhile body was about physiological temperature checked by a rectal temperature probe. At the same time, the mice in other three groups (PI without hyperthermia, NTSL without hyperthermia, PTX-TSL without hyperthermia) were not given other treatment, beside administration. After treatment, overall mice were sacrificed by cervical dislocation and tumors were surgically excised immediately.
The analysis of the PTX in tumor tissue was performed as follows (Shikanov et al., Citation2008). The tumor tissues (0.1–0.2 g) were weighed and homogenized in 5-fold purified water with dispersion machine (IKA, HAD-IKAT25, Germany) 10 000 rpm, tissue suspension was produced. The 0.5 mL suspension was transferred to a clean tube and 10 μL of docetaxel solution (1 μg/mL) as an internal standard was added to the solution. After that 3 mL Tert-Butyl methyl ether (TBME) was added, vortexed for 3 min and centrifuged at 13 000 rpm for 10 min. After that the organic layer (about 3 mL) was transferred to a clean tube and evaporated to dryness at 37 °C. The residue in the tube was reconstituted with 100 μL of mobile phase and 5 μL were injected onto the column. HPLC-MS/MS (Agilent, USA) system was composed of a G1367C Binpump, G1314C UV detector, G1379B Degasser, G1367C Sample injector, G6410 Mass detector and Agilent MassHunter data analysis program, using a C18 reverse phase column (Agilent SB C18, 2.1 × 50 mm, 5 μm. A mixture of 70% methanol: 30% water at a flow rate 0.3 mL/min was used as mobile phase. The retention time for docetaxel and PTX were 1.91 and 1.66 min, respectively.
Antitumor activity of PTX formulations
By tumor volume, the mice were randomly divided into four treatment groups: control group (5% glucose), PI, NTSL, PTX-TSL group. At the first step of the experiment, mice were anesthetized with sodium pentobarbital (50 mg/kg) by intraperitoneal injection. Then four groups were given three sequential injections every 72 h at a dose of 10 mg/kg of PTX (glucose, free drug or liposome encapsulated) by tail vein injection. After administration, the mice were exposed to local hyperthermia above for 30 min; meanwhile body was about physiological temperature checked by a rectal temperature probe. In the course of treatment, tumor volume (mm3) and tumor inhibition rate (IR) was used to evaluate the antitumor activity (Kim et al., Citation2008). Tumor dimensions were determined everyday using a caliper. Three days after final treatment, mice were sacrificed by cervical dislocation. Tumors were deprived and weighed. Tumor volume (EquationEquation 2(2) ) and tumor IR (EquationEquation 3
(3) ) were individually calculated using the following formulas:
(2)
a: major diameter, b: minor diameter.
(3)
mt: average weight of tumor in other experimental groups, m: average weight of tumor in control group.
Pharmacokinetics studies
Pharmacokinetics of PTX-TSL and NTSL were performed and the same concentration of PI (PTX injection, Hai Zheng Pharmaceutical Company, Zhe Jiang, China) was used as a control, using male Sprague-Dawley rats with body weights ranging from 200 to 250 g. PI, PTX-TSL or NYSL was administrated through the vein at the PTX dose of 7.0 mg/kg rats. Blood samples were taken from the retroorbital plexus at various times (5 min, 10 min, 15 min, 30 min, 1 h, 2 h, 4 h, 6 h, 8 h, 12 h, 24 h, 36 h, 48 h, 72 h), and centrifuged immediately (10 min, 5000× g). Plasma samples were stored at −70 °C and subsequently analyzed for PTX. Before quantitation of PTX, the 0.1 mL plasma sample was transferred to a clean tube and 10 μL of docetaxel solution (1 μg/mL) as an internal standard was added to the solution. After that, 400 μL TBME was added, vortexed for 3 min and centrifuged at 13 000 rpm for 30 min. The other handling and analysis were similar to the section “Concentration of PTX in solid tumor”. The pharmacokinetic parameters of each formulation were attained using the 3P97 pharmacokinetics calculation software.
Statistical analysis
Statistical analysis was performed with a two-tailed unpaired t-test and one-way ANOVA. All values are expressed as mean ± SD. p < 0.05 was considered statistically significant.
Results
In vitro characteristics of liposomes
PTX-TSL and NTSL exhibited an uniformitarian particle size of approximately 100 nm as determined by PCS and was subtranslucent with calamine blue opalescence, which was similar to the our previous research (Zhang et al., Citation2011,Citation2014). The polydispersity index for PTX-TSL and NTSL was about 0.82 and 0.84, respectively. The concentration of PTX was approximately 2.0 mg/mL and EE of the two liposomal systems were over 95% ().
Table 1. Physicochemical property of liposomal formulations.
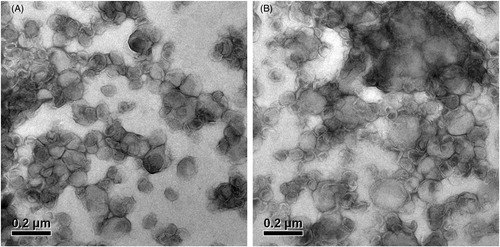
Compared with PTX-TSL, NTSL had no difference in appearance and particle size as shown in and . The TEM images of PTX-TSL and NTSL were presented in . The particle size was comparable to the results obtained from particle size measurement using PCS technique.
Figure 1. Particle size distribution of liposomes (A: PTX-TSL; B: NTSL), which were determined using PCS (Nanophox, Sympatec GmbH, Germany). Liposome suspension was diluted with distilled water, laser intensity was adjusted to 50–60%, desired temperature was 25 °C, measuring mode was cross correlation.
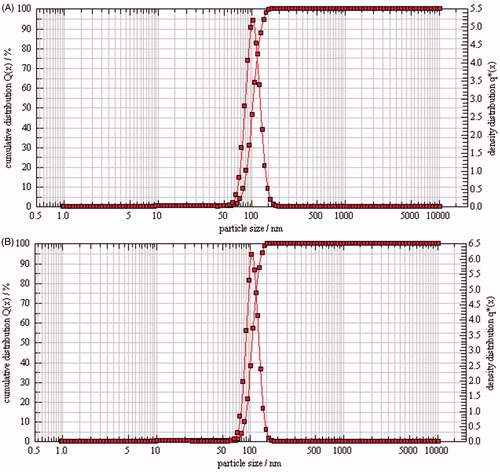
Figure 2. Transmission electron microscope photos of PTX-TSL (A) and NTSL (B), which show the morphology of the liposome dropped on a copper grid and stained with 2% phosphotungstic acid, then observed by TEM (JEM-1010, JEOL Ltd., Tokyo, Japan).
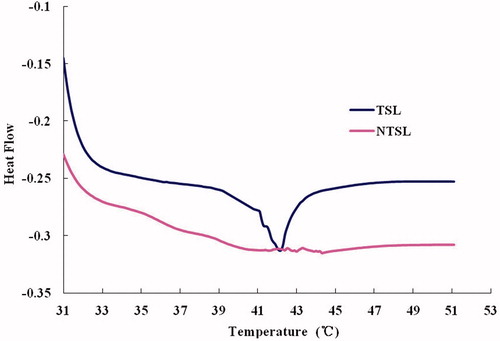
DSC data () showed that PTX-TSL had obvious phase change within 30 °C to 50 °C, when it was 38 °C PTX-TSL has began phase transition, the Tm of PTX-TSL was approximately 42 °C. Meanwhile, NTSL had no notable variation from 30 °C to 50 °C.
Figure 3. DSC of liposomal formulations, which shows phase transition of the TSL and NTSL, determined by differential scanning calorimetry (Q2000 differential scanning calorimeter, TA Instruments, USA).
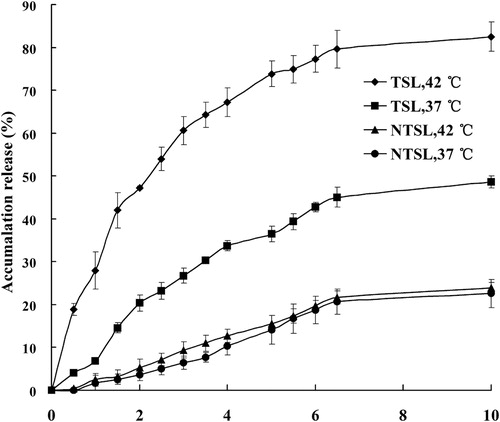
The release of PTX from PTX-TSL and NTSL at 37 °C or 42 °C was depicted in . The results indicated that PTX release from PTX-TSL exhibited thermosensitive characteristic, only about 20% PTX released from PTX-TSL within 2 h at 37 °C. However, when temperature increased to 42 °C, within 2 h, about 47% drug released from PTX-TSL. As for NTSL, the release did not exhibit thermosensitive characteristic. Regardless of low or high temperature, about 5% PTX released from NTSL after 2 h. In addition, at the same low temperature (37 °C), PTX-TSL showed a little higher release profile than NTSL.
Figure 4. In vitro, drug release from PTX-TSL and NTSL in vitro, determined at 37 °C and 42 °C, respectively. An aliquot of liposomal dispersion (0.4 mL) was dispersed into 10 mL release medium, i.e. PBS (pH 7.4) containing 0.5 M sodium salicylate. Then the suspension was placed into a dialysis tube immersed in release medium, samples were taken at predetermined time. Content of PTX released was determined by HPLC.
PTX-TSL exhibited long term stability at −20 °C for 3 months (). The concentration of PTX was in the scheduled specifications (95–105% of the original) for up to 3 months. Particle diameter, pH, and EE had no notable variations in the period of research. At the same time, the storage stability of PTX-TSL was determined using Turbiscan Lab® Expert. The changes of PTX-TSL in transmission or backscattering profiles during 3 months were shown in . The data exhibited the changes in transmission or backscattering intensity of light on each sample were less than 3%, indicating that PTX-TSL had no obvious aggregation or sedimentation emerged during the storage, compared the original sample.
Figure 5. Transmission and backscattering profiles of liposomes by using Turbiscan Lab® Expert, an implement able to reflect the negligible variation of colloidal solution by determination of transmission and backscattering. At appointed time points, 10 mL PTX-TSL placed into tailor-made sample cell was exposed to Turbiscan Lab® Expert and measured.
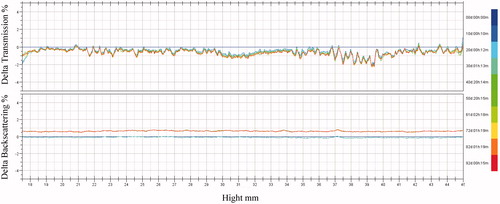
Table 2. Stability data of PTX-TSL, at −20 °C.
In vivo studies
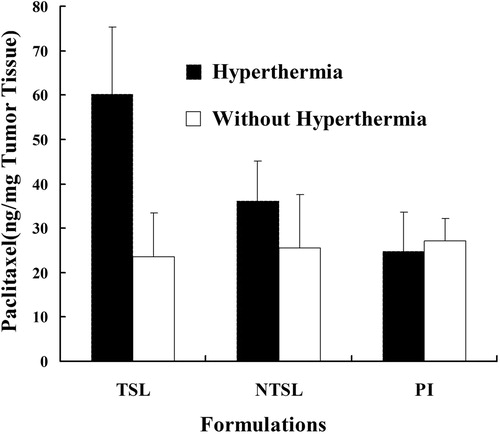
Intratumoral accumulation of different PTX formulations was shown in . At physiological temperature (without hyperthermia), the PTX accumulating into tumor of all the groups had no significant difference. The content of PTX in tumor ranged from 23 ng/mg to 27 ng/mg. Meanwhile, at 42 °C (hyperthermia), PTX content (60.1 ng/mg) in PTX-TSL group was higher than NTSL (30.1 ng/mg, p < 0.05) and PI (24.7 ng/mg, p < 0.01) group. PTX-TSL achieved higher PTX content in tumor at 42 °C than at 37 °C (p < 0.05), probably owing to the temperature triggered release.
Figure 6. In vivo, tumor accumulation of paclitaxil in tumor bearing mice, which were randomly divided into 6 treatment groups: PI (PTX injection), NTSL, and PTX-TSL with or without hyperthermia. All groups received intravenous injection of 10 mg/kg PTX (PI or NTSL, PTX-TSL).
Tumor volume and IR were used to evaluate the antitumor effects of three PTX formulations (PTX-TSL, NTSL, PI), combined with hyperthermia, at 42 °C for 30 min. showed the change of tumor volume after treatment, which indicated that all PTX formulations were significantly more effective than control group (hyperthermia alone, 2080.2 mm3, p < 0.05). The rank order of growth suppression was PTX in PTX-TSL (868.2 mm3) > PTX in NTSL (1324.9 mm3) or free PTX in PI (1274.7 mm3) (p < 0.05). The tumor volumes of NTSL and PI were not statistically different. Furthermore, the data of IR in showed the similar results to tumor volume. PTX-TSL had the highest IR (64.9%), while IR of PI and NTSL were 42.5% and 47.8%, respectively.
Figure 7. Antitumor efficiency of various paclitaxil formulations on tumor-bearing mice (n = 9), which were randomly divided into four treatment groups: control group (5% glucose), PI, NTSL, PTX-TSL group. The groups were given three sequential injections every 72 h at a dose of 10 mg/kg of PTX, the mice were exposed to local hyperthermia above for 30 min.
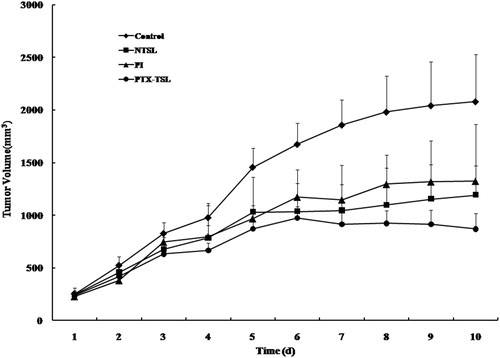
Table 3. Volume, mass, and inhibition rate of tumor (n = 10).
and reported the plasma PTX concentration/time curves and the pharmacokinetic parameters for the PTX-TSL, NTSL, and PI, based on the total PTX levels in plasma measured by HPLC-MS/MS. After administration, compared with PTX-TSL and NTSL, PI followed a biphasic pattern with a rapid distribution phase (t1/2α 2.74 h) and a rapid terminal elimination phase (t1/2β 12.58 h). Encapsulation of PTX in liposomes produced a significant change in pharmacokinetic parameters: after administration of conventional liposomes, the drug was distributed (t1/2α 3.58, 3.74) and eliminated slowly (t1/2β 15.79, 15.32). The mean clearance value of PI(0.264) was twice greater than that of PTX-TSL and NTSL (0.119, 0.134). There were no obvious differences between PTX-TSL and NTSL.
Figure 8. Concentration/time profiles of PTX formulations (PTX-TSL, NTSL, and PI). Each formulation was injected at a dose of 7.0 mg/kg, based on the total paclitaxel levels in plasma measured by HPLC-MS/MS.
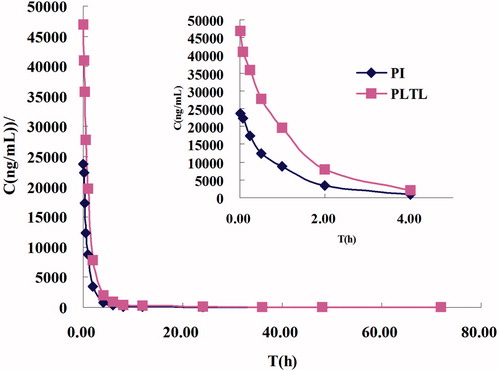
Table 4. Pharmacokinetics parameter of PTX-TSL, NTSL, and PI.
Discussion
PTX is water-insoluble, so the liposomes containing PTX were prepared by thin film hydration method, which was the same as the preparation of PTX-TSL containing docetaxel (DTX-TSL) (Zhang et al., Citation2014). Both processes fabricated the liposomes with EE over 95%, but the ratio of drug to lipid was significantly different. In the process of preparation liposomes: the ratio of PTX to lipid was 1:50, while the ratio of DTX to lipid was 1:25. Due to their water insolubility, PTX and DTX would insert into the bilayer of liposomes, which was depended on the drugs affinity to lipids. As DTX-TSL had a larger drug-lipid ratio than PTX-TSL, the lipids affinity of DTX might be higher than PTX. Furthermore, the preparation of PTX-TSL is different form the process reported by Sharma et al. (1998), who developed PTX thermosensitive liposomes by reverse phase evaporation and pH gradient method.
The DSC indicated that Tm of PTX-TSL was about 42 °C, and the width of the peak was broader than TSL containing water-soluble drug we previous reported (Zhang et al., Citation2011). This result is similar to DTX-TSL and indirectly suggests that the PTX molecule is inserted into the bilayer of liposomes, which disturbed the hydrocarbon chain conformation and caused a widening of lipid phase transition (Zhao et al., Citation2004). Meanwhile, the result was similar to the previous PTX thermosensitive liposomes (Sharma et al., 1998).
The data of drug release from liposomes indicated that PTX-TSL had significant thermosensitivity, while NTSL had no obvious variation with the change of medium’s temperature. This result was consisted with the data obtained from DSC. Compared with TSL containing water-soluble drugs (De Smet et al., Citation2010; Zhang et al., Citation2011), the rate of drug release from PTX-TSL was larger when the temperature was 37 °C; but when the temperature increased to 42 °C the rate of drug release became slower in the similar release conditions. At the physiological temperature (37 °C), PTX-TSL released about 48% of PTX within 10 h, while DTX-TSL released 40% of DTX. Within 10 h, when the medium’s temperature enhanced to 42 °C, over 80% of PTX released from PTX-TSL; while about 65% of DTX released from DTX-TSL. Therefore, regardless of the medium temperature (lower or higher than Tm), the release of PTX was both quicker than DTX (Zhang et al., Citation2014). The above results demonstrated that both PTX and DTX had certain affinity to lipids, but the lipids affinity of PTX was weaker than DTX.
In addition, under the same conditions (37 °C), release rate of PTX-TSL was significantly higher than NTSL. Within 10 h, TSL released about 48%; meanwhile, NTSL released approximately only 20%. The major component of PTX-TSL and NTSL was saturated phospholipids DPPC and unsaturated phospholipids EPC, respectively. Previous research indicates that the PTX affinity to saturated phospholipid is lower than to unsaturated phospholipid (Zhao et al., Citation2004). Thus, the difference of release rate could be ascribed to the different composition of liposome bilayer.
The stability of liposomes is an important factor to be taken into account when a clinical application is intended. PTX-TSL had a good stability: over 95% of PTX were still remained in liposomes for up to 3 months. There were no obvious variations on particle diameter, pH, and drug concentration in the stability research. In addition, the aggregation of liposomes is another factor of instability to be considered. In order to discover the physical stability change of liposomes as soon as possible, Turbiscan Lab® Expert was used to determine the stability of PTX-TSL. This method can detect the negligible variation by transmission or backscattering profiles before other instability phenomena emerge, thus shorting the lapse of time necessary for the identification of instability (Coradini et al., Citation2014). Variation over 10% in the graphical profiles of backscattering or transmission is deemed as the signal of instability (Marianecci et al., Citation2010). According to this, the results obtained () were reminiscent of observations that all the liposomes were stable in the storage. The stable PTX-TSL would be favorable for their further applications in clinical.
The data in indicated that PTX formulations without heat could not improve drug accumulation in the target site; while combined with hyperthermia, PTX-TSL could achieve more local delivery to the tumor site, compared with other formulations. Hyperthermia was able to increase vascular permeability, increase vascular pore size or tumor pore size, thus increased tumor liposome accumulation (Kong et al., Citation2000). Therefore, PTX content of tumor tissue in PTX-TSL or NTSL group was higher than the PI group. Although heat might induce additional delivery of NTSL to the tumor tissue (Kong et al., Citation2000), but NTSL could not be triggered to release drug quickly by heat. That was why both NTSL and PTX-TSL had the same size but PTX-TSL had a higher local drug concentration in the tumor tissue than NTSL. In a word, PTX-TSL combined with hyperthermia might cause drug targeted release at tumor sites. Consequently, in the following antitumor efficacy test, all formulations were exposed with 42 °C hyperthermia during the treatment.
The antitumor effects of three PTX formulations (PTX-TSL, NTSL, PI) were demonstrated in and . PTX-TSL combined with hyperthermia achieved higher therapy than NTSL or PI. After PTX-TSL was intravenously injected, they would accumulate into tumor because liposome preferentially extravasated from pores in tumor vessel walls, the size of which would increase with hyperthermia (Koning et al., Citation2010). Moreover, PTX-TSL was able to release drugs more quickly than NTSL upon their arrival on the heated tumor area, resulting in higher PTX concentration in the local region of tumor. This was corresponded with the results of drug concentration in tumor. Combined with hyperthermia, the in vivo drug release rate of PTX-TSL might become faster than in vitro because of the existence of plasma proteins.
The pharmacokinetic parameters for the PTX-TSL, NTSL, and PI indicated that PTX in PI was rapidly eliminated from systemic circulation with the largest values. On the other hand, PTX-TSL and NTSL exhibited longer blood circulating properties and higher AUC. The longer circulation and higher AUC of liposomes might lead to the disposition of liposomes into tumor, which probably was the reason for higher PTX concentration of PTX-TSL and NTSL. But, NTSL could not be triggered release by hyperthermia, so the therapy of NTSL with hyperthermia was less than PTX-TSL with hyperthermia.
The clinical efficiency of various cancer chemotherapeutic regimens was limited by the associated toxicity for normal tissues. In this study, there were no cases of weight loss than the initial weight exceeded 15% in the PTX-TSL group, and in most cases the animals gained weight during the study. It suggested that combined with hyperthermia, PTX-TSL could overcome the drawbacks of PTX and thus extend its clinical application.
Conclusion
In this report, we entrapped most PTX into liposomes and antitumor activities of liposomes were investigated. The obtained liposomes were uniform and DSC data showed that PTX-TSL had thermosensitivity at about 42 °C. The results of pharmacodynamics indicated that PTX-TSL could more remarkably suppress tumor growth, presumable as a result of delivering the PTX at tumor sites combined with hyperthermia, compared with NTSL and injection. Based on the above results from a rodent trial, PTX-TSL combined with hyperthermia may have potential to be a new therapy method for solid tumor.
Declaration of interest
All authors state that there were no conflicts of interest for this manuscript. We thank Important National Science & Technology Specific Projects of China (grant no. 2012ZX09301003-001-009), National Natural Science Foundation of China (No. 81202466) and Beijing Natural Science Foundation (No. 7144229) for financial support.
References
- Abu Lila AS, Kizuki S, Doi Y, et al. (2009). Oxaliplatin encapsulated in PEG-coated cationic liposomes induces significant tumor growth suppression via a dual-targeting approach in a murine solid tumor model. J Control Release 137:8–14.
- Asai T, Miyazawa S, Maeda N, et al. (2008). Antineovascular therapy with angiogenic vessel-targeted polyethyleneglycol-shielded liposomal DPP-CNDAC. Cancer Sci 99:1029–33.
- Coradini K, Lima FO, Oliveira CM, et al. (2014). Co-encapsulation of resveratrol and curcumin in lipid-core nanocapsules improves their in vitro antioxidant effects. Eur J Pharm Biopharm 88:178–85.
- De Smet M, Langereis S, van den Bosch S, Grüll H. (2010). Temperature-sensitive liposomes for doxorubicin delivery under MRI guidance. J Control Release 143:120–7.
- Friedland D, Gorman G, Treat J. (1993). Hypersensitivity reactions from taxol and etoposide. J Natl Cancer Inst 85:2036–45.
- Gaber MH, Wu NZ, Hong K, et al. (1996). Thermosensitive liposomes: extravasation and release of contents in tumor microvascular networks. Int J Radiat Oncol Biol Phys 36:1177–87.
- Hauck ML, Coffin DO, Dodge RK, et al. (1997). A local hyperthermia treatment which enhances antibody uptake in a glioma xenograft model does not affect tumor interstitial fluid pressure. Int J Hyperthermia 13:307–16.
- Horsman MR, Overgaard J. (1997). Can mild hyperthermia improve tumour oxygenation? Int J Hyperthermia 13:141–7.
- Issels RD, Lindner LH, Verweij J, et al. (2010). Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre study. Lancet Oncol 11:561–70.
- Karino T, Koga S, Maeta M. (1988). Experimental studies of the effects of local hyperthermia on blood flow, oxygen pressure and pH in tumors. Jpn J Surg 18:276–83.
- Kim JH, Kim YS, Park K, et al. (2008). Antitumor efficacy of cisplatin-loaded glycol chitosan nanoparticles in tumor-bearing mice. J Control Release 127:41–9.
- Kneidl B, Peller M, Winter G, et al. (2014). Thermosensitive liposomal drug delivery systems: state of the art review. Int J Nanomedicine 9:4387–98.
- Koning GA, Eggermont AM, Lindner LH, ten Hagen TL. (2010). Hyperthermia and thermosensitive liposomes for improved delivery of chemotherapeutic drugs to solid tumors. Pharm Res 27:1750–4.
- Kong G, Anyarambhatla G, Petros WP, et al. (2000). Efficacy of liposomes and hyperthermia in a human tumor xenograft model: importance of triggered drug release. Cancer Res 60:6950–7.
- Kong G, Braun RD, Dewhirst MW. (2001). Characterization of the effect of hyperthermia on nanoparticle extravasation from tumor vasculature. Cancer Res 61:3027–32.
- Kong G, Braun RD, Dewhirst MW. (2000). Hyperthermia enables tumor specific nanoparticle delivery: effect of particle size. Cancer Res 60:4440–5.
- Kong G, Dewhirst MW. (1999). Hyperthermia and liposomes. Int J Hyperthermia 15:345–70.
- Marianecci C, Paolino D, Celia C, et al. (2010). Non-ionic surfactant vesicles in pulmonary glucocorticoid delivery: characterization and interaction with human lung fibroblasts. J Control Release 147:127–35.
- May JP, Ernsting MJ, Undzys E, Li SD. (2013). Thermosensitive liposomes for the delivery of gemcitabine and oxaliplatin to tumors. Mol Pharm 10:4499–508.
- Morimoto K, Kondo M, Kawahara K, et al. (2007). Advances in targeting drug delivery to glomerular mesangial cells by long circulating cationic liposomes for the treatment of glomerulonephritis. Pharm Res 24:946–54.
- Needham D, Anyarambhatla G, Kong G, Dewhirst MW. (2000). A new temperature sensitive liposome for use with mild hyperthermia: characterization and testing in a human tumor xenograft model. Cancer Res 60:1197–201.
- Ono A, Takeuchi K, Sukenari A, et al. (2002). Reconsideration of drug release from temperature-sensitive liposomes. Biol Pharm Bull 25:97–101.
- Shikanov A, Shikanov S, Vaisman B, et al. (2008). Paclitaxel tumor biodistribution and efficacy after intratumoral injection of a biodegradable extended release implant. Int J Pharm 358:114–20.
- Simona M, Julien N, Patrick C. (2013). Stimuli-responsive nanocarriers for drug delivery. Nat Mater 12:991–1003.
- Simone L, Jasmin H, Rebecca S, et al. (2014). Gemcitabine treatment of rat soft tissue sarcoma with phosphatidyldiglycerol-based thermosensitive liposomes. Pharm Res 31:2276–86.
- Singla AK, Garg A, Aggarwal D. (2002). Paclitaxel and its formulations. Int J Pharm 235:179–92.
- Szebeni J, Muggia FM, Alving CR. (1998). Complement activation by Cremophor EL as a possible contributor to hypersensitivity to paclitaxel: an in vitro study. J Natl Cancer Inst 90:300–6.
- van Zuylen L, Karlsson MO, Verweij J, et al. (2001). Pharmacokinetic modeling of paclitaxel encapsulation in Cremophor EL micelles. Cancer Chemother Pharmacol 47:309–18.
- Wang XL, Song YZ, Su YQ, et al. (2015). Are PEGylated liposomes better than conventional liposomes? A special case for vincristine. Drug Deliv. [Epub ahead of print]. doi:10.3109/10717544.2015.1027015.
- Xie X, Yang Y, Yang Y, et al. (2015). A photo-responsive peptide- and asparagines-glycine-arginine (NGR) peptide-mediated liposomal delivery system. Drug Deliv. [Epub ahead of print]. doi:10.3109/10717544.2015.1008707.
- Yang YF, Yang Y, Xie XY, et al. (2014). PEGylated liposomes with NGR ligand and heat-activable cell-penetrating peptide-doxorubicin conjugate for tumor-specific therapy. Biomaterials 34:368–81.
- Yatvin MB, Weinstein JN, Dennis WH, Blumenthal R. (1978). Design of liposomes for enhanced local release of drugs by hyperthermia. Science 202:1290–3.
- Zhang H, Wang ZY, Gong W, et al. (2011). Development and characteristics of temperature-sensitive liposomes for vinorelbine bitartrate. Int J Pharm 414:56–62.
- Zhang H, Gong W, Wang ZY, et al. (2014). Preparation, characterization, and pharmacodynamics of thermosensitive liposomes containing docetaxel. J Pharm Sci 103:2177–83.
- Zhang JA, Anyarambhatla G, Ma L, et al. (2005). Development and characterization of a novel Cremophor EL free liposome-based paclitaxel (LEP-ETU) formulation. Eur J Pharm Biopharm 59:177–87.
- Zhang Q, Huan XE, Gao LL. (2009). A clinical study on the premedication of paclitaxel liposome in the treatment of solid tumors. Biomed Pharmacother 63:603–7.
- Zhao L, Feng SS, Go ML. (2004). Investigation of molecular interactions between paclitaxel and DPPC by Langmuir film balance and differential scanning calorimetry. J Pharm Sci 93:86–98.
