Abstract
There is a strong desire to develop docetaxel (DTX) formulation with good therapeutic effectiveness in view of serious adverse reactions of the commercial formulation of DTX (Taxotere®). In this study, a redox-responsive DTX-vitamin E prodrug was successfully formulated into liposomes with the drug loading of 4.14% ± 0.10%. Compared with DTX liposomes, the DTX prodrug liposomes (DPLs) showed good stability for 30-d shelf life and during dilution with different media. In vitro antitumor activity of DPLs on human prostatic carcinoma PC-3 cells and human lung cancer A549 cells was evaluated using cytotoxicity and apoptosis assays. In spite of a decrease in in vitro antitumor activity, the in vivo pharmacokinetic study reveals that DPLs exhibit significantly longer DTX plasma half-life (t1/2, 1.38-fold) and higher bioavailability (AUC0−t, 14.49-fold) compared with DTX liposomes. The antitumor activity of DPLs to the A549 tumor xenograft model showed selective accumulation in tumor tissue, significant inhibition the growth of the tumors and a much lower toxicity as seen in body weight loss, compared with DTX-Solution. Taken together, the results showed that DPLs is a promising strategy for DTX antitumor delivery.
Introduction
Docetaxel (DTX), a semisynthetic chemotherapeutic drug, is widely used for solid tumors, especially for breast, gastric, prostate, and non-small cell lung cancers, etc. (Ganesh, Citation2007). DTX is currently regarded as one of the most promising antitumor drugs because of unique mechanism of promoting the polymerization of tubulin into stable microtubules, then the cell division is blocked at the G2/M phase, which induces cell apoptosis (Ganesh, Citation2007). However, the poor water-solubility and low tissue-specificity limit its clinical application (Kim et al., Citation2014). Taxotere®, the commercial formulation of DTX with Tween 80 and 13% (w/w, ethanol/water) solution of ethanol as solvents caused serious adverse reactions such as hypotension and bronchospasm (Park et al., Citation2014).
In order to avoid the side effects of Tween 80-based vehicle, various approaches including liposomes, polymer micelles and DTX conjugates have been tried for DTX delivery (Etrych et al., Citation2010; Zhigaltsev et al., Citation2010; Wang et al., Citation2014). Compared with other nanotechnology-based drug delivery systems, liposomes have obtained significantly development both in clinically approved products and new experimental applications, due to its various unique characteristics. Therefore, the improvement of the compatibility between DTX and lipid should be beneficial to the preparation of DTX liposomal formulation.
It is a favorable method to improve the lipid solubility of DTX through chemical modification. Various kinds of DTX prodrugs have been reported (Chu et al., Citation2014; Wang et al., Citation2014; Wohl et al., Citation2014), here in a redox-responsive DTX prodrug containing disulfide bond between DTX and vitamin E was synthesized (Supplementary Figure S1). Given that DTX could be released from DTX prodrug due to the cleavage of disulfide bond triggered by the high concentration of glutathione (GSH) in tumor tissue, the reduced toxicity was expected to be achieved (Ai et al., Citation2014; Wang et al., Citation2014).
In this work, DTX prodrug liposomes (DPLs) were first prepared and its GSH-dependent behavior and antitumor activity were accessed. DTX liposomes were also prepared under the same condition. The physicochemical properties of DPLs including hydrodynamic size, zeta potential, drug encapsulation efficiency (EE), drug-loading (DL), stability, and drug release behavior were evaluated in detail. Cytotoxicity assay and apoptosis assay were performed to evaluate its antitumor activities in vitro. Pharmacokinetic behavior, tumor accumulation, systematic toxicity, and in vivo antitumor effectiveness of DPLs were investigated.
Materials and methods
Materials
1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE-PEG2000) was bought from Shanghai Advanced Vehicle Technology Pharmaceutical Ltd (Shanghai, China). DTX were purchased from Nanjing Jingzhu Bio-technology Co. Ltd (Nanjing, China). N,N′-dicyclohexylcarbodiimide (DCC), 4-dimethylaminopyridine (DMAP) were all purchased from Aladdin Industrial Corporation (Shanghai, China). Thiodiglycolic acid VE ester (TA-VE) was synthesized in this laboratory. Cholesterol was purchased from Bodi Chemical Co. Ltd. (Tianjin, China). Soya lecithin was provided by Shanghai Taiwei Pharmaceutical Co. Ltd. (Shanghai, China). Sephadex G-50 was purchased from RujiBio-technology Co. Ltd (Shanghai, China). DTX prodrug was synthesized in our laboratory.
The human prostatic carcinoma PC-3 cells and human lung cancer A549 cells were acquired from Chinese Academy of Sciences Cell Bank (Beiijng, China). Roswell Park Memorial Institute (RPMI-1640), Dulbecco’s modified Eagle’s medium (DMEM, high glucose), trypsin, fetal bovine serum (FBS), and MTT were purchased from Gibco (Beijing, China). Hoechst 33258 was purchased from Sigma (St. Louis, MO). Lyso-Tracker was purchased from Invitrogen (Carlsbad, CA). All other reagents and solvents were of analytical grade, and used without further purification.
Animals
Sprague-Dawley rats (200–250 g) were obtained from Liao Ning Chang Sheng Biotechnology Co. Ltd (Liaoning, China; license SCXK (liao) 2010-0001). Nude mice (18–22 g) were obtained from Beijing Huafukang Bioscience Technology Co. Ltd (Beijing, China; license SCXK (jing) 2014-0004). All animal studies were performed according to an approved protocol for the Care and Use of Laboratory Animals, which was approved by Shenyang Pharmaceutical University Committee of Ethics.
Synthesis and characterization of DTX prodrug
DTX prodrug was synthesized according to our previous work with some modification (Wang et al., Citation2014). Thiodiglycolic acid VE ester (TA-VE) was added to a solution of DCC, DMAP, and DTX in anhydrous dichloromethane. The mixture was stirred for another 48 h. The precipitate was filtered off and the filtrate was purified by a column chromatography. The DTX prodrug was confirmed by Fourier transform ion cyclotron resonance mass spectrometer (FT-ICR MS), 1H nuclear magnetic resonance (1H NMR), and Fourier transform infrared spectroscopy (FT-IR). The synthetic route of DTX prodrug was showed in Supplementary Figure S1.
Preparation of liposomes
DPLs were prepared using the thin film hydration method. Briefly, soybean lecithin (64.8 mg), cholesterol (9.6 mg), DSPE-PEG2000 (31.2 mg) and DTX prodrug (8.0 mg) were dissolved in 5 mL chloroform. The organic solvent was removed by rotary evaporation at 30 °C to obtain the lipid film. The lipid film was hydrated with 4 mL distilled water through vigorous hand shaking for 5omin, followed by rotating for another 15 min at 40 °C. The suspensions were sonicated by a probe sonicator for 5 min. Then the prepared liposome was centrifuged for 10 min at 3000 rpm g to get rid of the untrapped DTX prodrug before any assay. Finally, the solution was extruded through a 0.22 μm pore-sized polyether sulfone membrane and stored at 4 °C.
Characterization of liposomes
Size and zeta potential
The hydrodynamic size, zeta potential, and polydispersity index (PDI) of the liposomes were assessed by a Zetasizer (Nano ZS, Malvern, UK). The diameter and zeta potential of DPLs were measured by dynamic light scattering (DLS) and electrophoretic mobility measurements respectively.
Cryogenic-transmission electron microscopy
The morphology of the liposomes was characterized by cryogenic-transmission electron microscopy (cryo-TEM, Hitachi, Japan). Briefly, the samples were prepared in a controlled environment vitrification system, where the temperature and relative humidity were maintained at 25 °C and 100% respectively. Prepared DPLs (5 μL) was deposited on 200-mesh perforated carbon-coated copper grids. The excess liquid was blotted with filter paper. The specimen was plunged into a liquid ethane bath which was cooled with liquid nitrogen, and was observed in a FEI Tecnai F20 electron microscope employing a Gatan cryo-holder at about −180 °C. The imaging of the samples were operated at 120 kV, and recorded in a low-dose electron mode with a Gatan UltraScan 1000 cooled-CCD camera (Gatan, Pleasanton, CA).
Encapsulation efficiency and drug-loading
A modified mini-column centrifugation method was employed to determine the EE and DL of DPLs (Fry et al., Citation1978). Briefly, Sephadex G-50 was swelled in distilled water at room temperature for 24 h. A piece of filter paper was placed in the bottom of a 2.5 mL plastic syringe, which was filled with Sephadex G-50. The mini-column was centrifuged at 1800rifu for 2omin to remove the excessive water. Subsequently, DPLs suspensions (62.5 μL) were added on the top of the mini-column. Then the mini-column was centrifuged at 1800 rpm for 1omin. After that, 100 μL distilled water was slowly added on the mini-column, then centrifuged at 2000 rpm for 3 min. The same procedure was repeated twice. Finally, the eluted DPLs were collected and ruptured through acetonitrile. The encapsulated DTX prodrug was analyzed using the ultra high-performance liquid chromatography (Waters ACQUITY UPLC) on a reverse ODS Cosmosil-C18 column (150 mm × 4.6 mm, 5 μm) at a column temperature of 40 °C, with acetonitrile and redistilled water (96:4, V/V) at a flow rate of 1.2 mL/min. The detector was kept at 230 nm. The EE and DL were calculated as the following equations:
where Wpe was the amount of prodrug encapsulated in liposomes after centrifugation and Wpt was the total amount of prodrug used in the fabrication before centrifugation.
where Wpe was the weight of DTX encapsulated in liposomes and Wpl was the total weight of DPLs in the fabrication.
Stability study
To evaluate the dilution stability of DPLs at room temperature for 24 h, DPLs (200 μL) were diluted with 2 mL of saline. At predesigned time intervals the particle size and EE of samples were measured by Zetasizer and UPLC, respectively. To evaluate the long-term stability study of DPLs, samples were kept at 4 °C and taken for analysis at 0, 12, 20, and 30 d. Changes in particle size and EE were used to assess the stability of DPLs.
To evaluate the stability of DPLs in rat plasma, DPLs were introduced into rat plasma at the desired concentration (50 μg/mL). Subsequently, the samples were placed in an air bath under the shaking of 100 rpm at 37 °C for 24 h. At defined time intervals, 50 μL samples were taken and processed with the same operation steps as mentioned in pharmacokinetic study section in which UPLC-MS/MS was used to determine the content of DTX.
In vitro drug release
In vitro drug release study was performed by the dialysis method (Wang et al., Citation2012, 2015b). DPLs (200 μL) were placed into a dialysis bag (MWCO of 12 000–14 000) and suspended in 30 mL of release media with different components: (1) phosphate buffer solution (PBS, pH 7.4), (2) PBS with 0.002 mM GSH, (3) PBS with 0.01 mM GSH, (4) PBS with 0.1 mM GSH, (5) PBS with 1 mM GSH, (6) PBS with 10 mM GSH. The samples were filled with nitrogen and shaken at the speed of 100 rpm in a water bath at 37 °C. At the defined time intervals, aliquot samples were withdrawn and replaced with the same volume of fresh release medium, then the samples were quickly sealed with nitrogen. The DTX content was analyzed by UPLC using a reverse ODS Cosmosil-C18 column (150 mm × 4.6 mm, 5 μm) at 230 nm with a mobile phase of acetonitrile and redistilled water (55:45, V/V). The flow rate was 0.8.mL/min, and the column temperature was set at 35 °C.
In vitro cell assays
Cells culture
The A549 cells and PC-3 cells were grown in DMEM and RPMI-1640 medium containing 10% (V/V) heat-inactivated FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin, respectively. Cells were maintained in an incubator with a 95% humidified atmosphere containing 5% CO2 at 37 °C.
Cytotoxicity assay
MTT assay was used to evaluate the cytotoxicity of DTX-Solution (DTX-Sol) and DPLs. Briefly, cells were seeded into 96-well plates (2 × 103 cells per well). After cultured for 24 h, the cells were exposed to series concentrations of DTX-Sol and DPLs at 37 °C for 48 h, 72 h and 96 h, respectively. The cells incubated with culture medium were selected as the negative control. The cytotoxicity of blank vehicle was also treated in the same way. At designated intervals, cells were rinsed twice with PBS, followed by addition of 200 μL of fresh culture medium and 50 μL of MTT solution (2 mg/mL). After incubating at 37 °C with 5% CO2 for 3.5 h, the culture medium was removed and 200 μL dimethyl sulfoxide (DMSO) was added into 96-well plates to dissolve the purple blue formazan crystals. The absorbance of formazan at 570 nm was determined using a BioRad icroplates reader (Model 500, USA). The cell growth inhibition rate was calculated as following equation:
where Asamples was sample absorbance, and Acontrol was negative absorbance. Half-maximal inhibitory concentration (IC50) was obtained by Compusyn software (ComboSyn).
Cell apoptosis assay
Hoechst 33258 staining of nuclei was carried out to evaluate the cell apoptosis qualitatively. Briefly, A549 cells and PC-3 cells were plated in 12-well plates at a density of 1 × 105 cells per well and allowed to incubate for 24 h. The cells were then treated with DTX-Sol and DPLs (at the same concentration of DTX about 1.17 μg/mL) and incubated in a standard incubator at 37 °C for 12 h and 24 h respectively. The cells were fixed, washed with PBS thrice and stained with Hoechst 33258 for 20rmin in the dark, then observed under fluorescence inversion microscope (IX71S8F, Olympus, Japan).
Annexin V-PI staining assay was performed according to the FITC Annexin V apoptosis detection kit (BD Biosciences). A549 cells and PC-3 cells (2 × 105 cells/well) were seeded in 6-well plates for 24 h. The negative control group was incubated without drugs. After incubation, the culture medium was removed and replaced with DTX-Sol (1.17 μg/mL) and DPLs (2 μg/mL) for 24 h. Then, the cells were harvested and washed with 4 °C PBS twice, and 1 × 105 cells resuspended in 100 μL binding buffer were stained with 5 μL Annexin V-FITC and 5 μL PI in the dark for 15 min at room temperature. Subsequently, 400 μL of binding buffer was added. The samples were determined by FACS Calibur system within 1 h.
Pharmacokinetic study
All animal experiments were carried out according to the Guidelines for the Care and Use of Laboratory Animals approved by the Ethics Committee of Animal Experimentation of Shenyang Pharmaceutical University, China. The SD rats weighing 200–250 g were fasted overnight and randomly divided into two groups (6 rats each), then injected with DTX-Sol and DPLs (DTX dose, 12 mg/kg) via tail vein respectively. Blood samples were obtained from the orbital plexus and put into heparinized tubes at specific time interval, centrifuged at 12 000 rpm for 10 min. Then the plasma samples were stored at −20 °C. The DTX content in blood was determined by UPLC-MS/MS. Data were analyzed by using statistical moment theory (DAS version 2.0).
Tissue distribution study
A549 cells (3 × 106) were injected subcutaneously into the right hind leg of nude mice. When the tumors grew to 100–150 mm3, the mice were randomly divided into two groups (n = 3 per group). DTX-Sol and DPLs were intravenously administered to the A549 tumor-bearing nude mice at the dose of 10 mg/kg and 17 mg/kg respectively. At 5 h post-treatment, the blood samples (300 μL), tumor, heart, liver, lungs, kidneys, and spleen were harvested from each mouse. The samples were frozen at −20 °C before analysis. UPLC-MS/MS was used to measure the concentration of DTX in plasma and tissue samples.
In vivo antitumor activity
The antitumor activity of DTX-Sol and DPLs were investigated on A549 tumor-bearing nude mice model. When A549 tumor reached a size about 100–200 mm3, the nude mice were randomly divided into four groups (n = 6 per group), and treated with saline (control), DTX-Sol (10 mg/kg), DPLs (17 mg/kg) via the tail vein for 4 times every other day. Then tumor volume , where width (w) and length (l) are the shortest and longest diameter of tumor, respectively) measured by caliper and body weights were recorded to evaluate the toxicity of each preparation. At the end of experiment, the excised tissues (tumor, heart, liver, spleen, lungs, and kidney) were harvested and fixed in 10% formal saline for immunohistochemical staining, which were stained by hematoxylin and eosin (H&E). The prepared organ sections were observed under fluorescence microscopy. Survival curve of each group was drawn using SPSS software 21.0.
Statistical analysis
All the experiments were carried out in triplicate and the results were expressed as mean ± standard deviation (SD). The statistical analysis was performed using SPSS software 21.0 (Chicago, IL). A value of p < 0.05 was considered significant, and a very significant difference was defined if p was less than 0.01.
Results and discussions
Synthesis and characterization of DTX prodrug
A redox-responsive DTX prodrug containing disulfide bond between DTX and VE was successfully synthesized (Supplementary Figure S1). The results of FT-ICR MS (Supplementary Figure S2), 1H NMR (Supplementary Figure S3), and FT-IR (Supplementary Figure S4) indicated a redox-responsive prodrug of disulfide-linked between DTX and VE was successfully synthesized.
Characterizations of DPLs
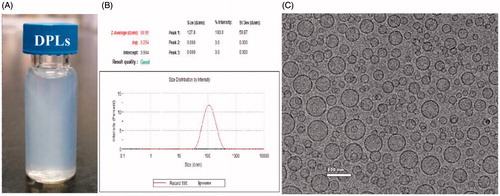
The mean diameter and zeta potential of DPLs were 93.06 ± 0.72 nm and −18.2 ± 0.89 mV, respectively. The results of photograph, DLS and cryo-TEM were shown in , which indicated that the prepared DPLs gave out light blue opalescence and exhibited uniform and spherical morphology.
The EE and DL of DPLs calculated by the mass of DTX were about 97.60 ± 0.03% and 4.14 ± 0.10%, respectively. At the same time, DTX liposomes were prepared under the same condition. The EE and DL of the DTX liposomes were 44.71 ± 0.17% and 1.88 ± 0.71%, respectively. Poor compatibility between DTX and lipids leads to the significantly decreased EE and DL of DTX liposomes (Letchford & Burt, Citation2012; Wang et al., Citation2015a). Furthermore, DTX liposomes demonstrated inferior in vitro shelf stability, precipitation occurred after 12 h. On the contrary, this PEGylated liposomal prodrug delivery displayed its unique advantages in improving the EE and DL of DTX. Therefore, DTX liposomes were not an appropriate control for keeping the same administrated dose and volume with DPLs.
Stability of the DPLs
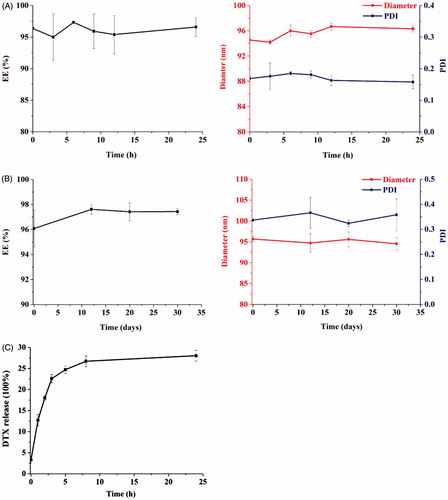
Because DPLs would be administered to the patient by intravenous infusion, it was important to investigate the effect of dilution and 30-d shelf life. DPLs diluted with saline at the ratio of 1:10 (V/V) showed a good stability, there was no significant difference in EE and size before and after dilution (p > 0.05) as shown in . DPLs were physically and chemically stable within 30 d, and no precipitation was observed upon storage at 4 °C (). The release of DTX was up to 26.72% after 8 h incubation, and the cumulative release amount of DTX hardly changed between 8 h and 24 h (28.02%; ).
In vitro drug release of DTX
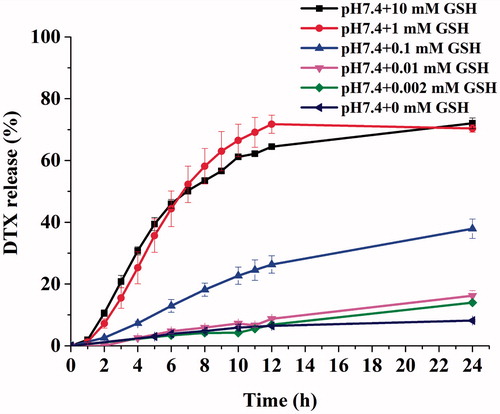
The GSH-triggered release of DTX from DPLs was investigated in PBS with different concentration of GSH at 37 °C during 24 h (Chen et al., Citation2012). The cumulative release percentages were shown in . The results showed that the release of DTX from DPLs depended upon the concentration of GSH in PBS. The 70.35% and 72.03% of DTX were released within 24 h incubation at pH 7.4 PBS with 1 mM and 10 mM concentration of GSH, respectively. This indicated that most of the DTX could be converted from DPLs at the high concentration of GSH simulating the tumor microenvironment. In contrast, the release rate of DTX from DPLs was found to be significantly slower in PBS with GSH at the concentration of 0.1 mM, 0.01 mM, 0.002 mM after 24 h. also showed that a slight release of DTX from DPLs was found in pH 7.4PBS without GSH. The DTX prodrug was incubated in pH 7.4 PBS with different concentration of GSH to access the stability of DTX prodrug in PBS. DTX was not released from DTX prodrug in pH 7.4 PBS, indicating that DTX prodrug possessed good stability in pH 7.4 PBS (Supplementary Figure S5). Considering the good stability of prodrug in pH 7.4 PBS and EE (97.60%), it is probably due to some lipid components which lead to the hydrolysis of DTX prodrug, so the developed formulation should be lyophilized.
In vitro cell assay
Cytotoxicity assay
The cytotoxicity of DPLs was evaluated in A549 cells and PC-3 cells by MTT assay with DTX-Sol as control. The results illustrated that blank liposomes showed no apparent toxicity on A549 cells and PC-3 cells, whereas the blank vehicle of DTX-Sol showed slight toxicity (Supplementary Figure S6). Furthermore, the constituents of the blank liposome were very normal lipid materials. Therefore, it was feasible not to take the blank liposome as control in in vivo experiments (Agardan et al., Citation2015; He et al., Citation2015; Li et al., Citation2015; Marzban et al., Citation2015; Peng et al., Citation2015).
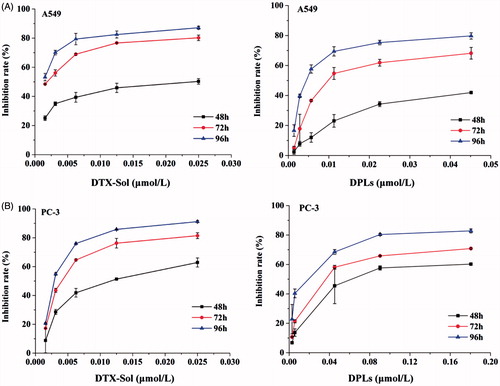
As shown in , the cytotoxicity of DTX-Sol and DPLs presented time and concentration-dependent cytotoxic profiles. After 96 h incubation, DTX-Sol (0.025-μmol/L) and DPLs (0.045-μmol/L) exhibited the strongest anti-proliferation activities on A549 cells with inhibition rates of 87.14% and 79.74%, respectively (). As for PC-3 cells, the inhibition rates were 91.19% for DTX-Sol (0.025 μmol/L) and 82.82% for DPLs (0.18 μmol/L) (). Furthermore, IC50 value of DTX-Sol in A549 cells and PC-3 cells was 3-fold and 5.66-fold of that from DPLs, respectively (Supplementary Table S1). The decreased cytotoxicity of DPLs might be attributed to the DTX prodrug encapsulated in liposomes needed to be released and reduced to DTX to endow its cytotoxicity (Duan et al., Citation2013).
Cell apoptosis assay
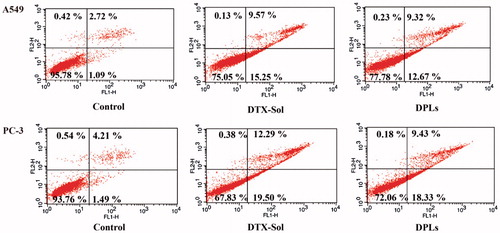
The cells were exposed to DTX-Sol or DPLs at the equivalent DTX concentration of 1.17 μg/mL for 12 h and 24 h (Supplementary Figure S7A1, B1), then the morphological changes of A549 cells and PC-3 cells were observed by fluorescence microscope. The morphological changes of both cells exhibited time-dependent profiles such as loss of intercellular contact, cell shrinkage to a rounder and smaller shape, and the formation of apoptotic bodies, whereas the shape of the cells of the control group was not changed. Further, A549 cells and PC-3 cells treated with DTX-Sol and DPLs at the same conditions mentioned above were stained with Hoechst 33258 to evaluate the nuclear morphology. Both cells treated with DTX-Sol and DPLs presented condensed, irregular, and fragmented nuclei (Supplementary Figure S7A2, B2), suggesting that both DPLs and DTX-Sol induced cell apoptosis and the populations of A549 cells and PC-3 cells with apoptotic nuclei increased along with time. Moreover, apoptotic activation was also investigated using Annexin V and PI double staining method. As shown in , apoptosis rate of A549 cells treated with DTX-Sol and DPLs for 24 h was 15.25%, and 12.67%, and apoptosis rate of PC-3 cells was about 19.50% and 18.33%, respectively. Comparing with DTX-Sol, the apoptosis rate of DPLs was slightly lower, indicating that the release of DTX from DPLs triggered by the high concentration of GSH in tumor cells was important to the exert of the cytotoxicity of prodrug. In summary, these results demonstrated that DPLs induced cell apoptosis in the same manner with DTX-Sol.
Pharmacokinetic and biodistribution study
The pharmacokinetic profiles of the DPLs in SD rats were investigated at the DTX dose of 12 mg/kg. The mean DTX plasma concentration versus time profiles after the injection of DTX-Sol and DPLs via tail vein were presented in . The main pharmacokinetic parameters were showed in Supplementary Table S2. The AUC0 − 24 h and t1/2 for DPLs were 50,488.03 μg/mL·h and 3.40 h, which were 14.49-fold and 1.38-fold higher than that of DTX-Sol, respectively (Supplementary Table S2, p < 0.01). These results demonstrated that the retention time of DTX released from DPLs in blood was prolonged remarkably, that would be beneficial to the drug accumulation in the tumor (Jain et al., Citation2010).
Figure 6. (A) Plasma concentration-time curves of DTX following intravenous administration of DTX-Sol and DPLs at a dose of 12 mg DTX/kg to rats (mean ± SD, n = 5). (B) Biodistribution of DTX in A549 tumor-bearing nude mice at 5 h after intravenous injection of DTX-Sol and DPLs (**p < 0.01 versus DTX-Sol group).
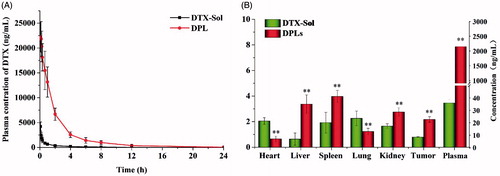
The A549 tumor-bearing nude mice were used to investigate the tissue distribution of the DPLs in comparison with DTX-Sol. The content of DTX in plasma and various tissues was determined using UPLC-MS/MS, and the results were illustrated in At 5 h after injection, the content of DTX released from DPLs in plasma was much higher than that of DTX-Sol, which was consistent with the pharmacokinetic study. The results showed that the accumulation of DTX in the tumor was 2.17 μg/g (2.75-fold of DTX-Sol) (p < 0.01). The liposomes significantly changed the cargo’s distribution and leaded to much higher accumulation of drug in tumor due to the enhanced permeability and retention effect.
In vivo antitumor activity
The antitumor effects of DPLs and DTX-Sol in vivo were compared using the xenograft model. Mice bearing A549 tumor were injected intravenously every other day with saline, DTX-Sol (10 mg/kg) or DPLs for 4 consecutive injections. The measurements of the body weight and tumor volume were performed every other day, and the results were presented in . It could be observed that there was significant difference of the tumor volumes among saline group, DTX-Sol group, and DPLs group (**p < 0.01), and the tumor volumes of DPLs group was much lower than that of DTX-Sol group (#p < 0.05) (). As shown in DTX-Sol treatment group decreased the body weight compared with that of saline group, and there was a significant difference of body weight between two groups (**p < 0.01). On the contrary, the body weight loss of DPLs group was not observed compared with the saline group (p > 0.05). The results suggested that DPLs could not only inhibit the tumor growth but also reduce the toxicity.
Figure 7. In vivo antitumor efficiency of different treatment groups in A549 tumor-bearing nude mice. (A) the changes of tumor volume; (B) the body weight variations; (C) the tumor weight and the calculated inhibition rate of tumor growth at the end of experiment.
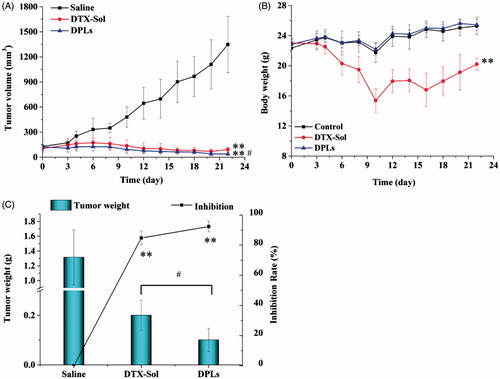
At the end of the study on 22th day, the excised tumor tissues were weighed and the inhibition rates were calculated, which were showed in . The mean weight of tumor tissues of saline, DTX-Sol and DPLs groups was of 1.32 ± 0.37 g, 0.20.± 0.060 g, and 0.09.± 0.027 g, respectively, corresponding to the inhibition rate of 84.78 ± 4.57% (DTX-Sol group) and 93.23 ± 2.06% (DPLs group). To investigate the toxicity of the two formulations on different tissue sections, we further performed a histopathological analysis of various tissues by H&E staining assay. The red arrow indicated the damaged domains. It could be concluded that DTX-Sol treatment induced remarkable necrosis in the hepatic tissue (), which meant that DTX-Sol had a severe hepatic toxicity compared with Saline group. And the degree of tumor cells necrosis in DPLs group was higher than that in DTX-Sol group, indicating that the necrosis induced by DPLs treatment was enhanced compared with DTX-Sol treatment at the same dose. The toxicity of DTX-Sol (Taxotere®) might be attributed to the usage of surfactant Tween 80 (Liu et al., Citation2010; Park et al., Citation2014).
Figure 8. Histological examination of heart, liver, spleen, lungs, kidney, and tumor slices excised from A549 tumor-bearing nude mice on the 22th day after the respective treatment with saline, DTX-Sol and DPLs.
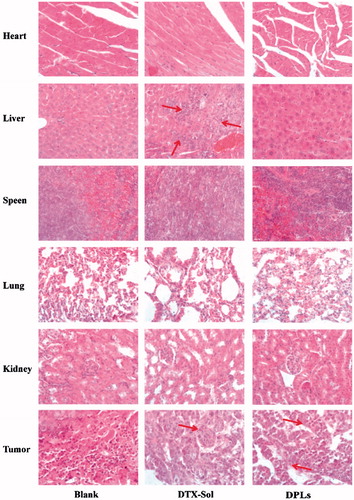
In summary, DPLs treatment showed nearly complete tumor suppression, indicating that DPLs could effectively deliver the prodrug to tumor. Then theredox-responsive prodrug was hydrolyzed with the aid of high concentration of GSH. Subsequently, the released DTX induced cell apoptosis and death.
Conclusions
In this study, the synthesized redox-responsive prodrug with disulfide linkage between DTX and VE was successfully encapsulated in liposomes by the thin film hydration method with high DL and good stability. Although DPLs showed inferior in vitro antitumor activity compared with DTX-Sol, pharmacokinetic and biodistribution studies revealed prolonged circulation in blood and enhanced accumulation in the tumor, which led to the enhanced antitumor activity and lower adverse effects. DPLs represented a promising option for effective DTX delivery.
Supplementary material available online
supporting_information.docx
Download MS Word (8.1 MB)Declaration of interest
The authors report no academic and commercial conflicts of interest.
This work was supported by the National Nature Science Foundation of China (No. 81273450), Nature Science Foundation of Liaoning Province (No. 2014020079), and the General Project in Education Department of Liaoning Province (No. L2014396).
References
- Agardan NB, Degim Z, Yilmaz S, et al. (2015). The effectiveness of raloxifene-loaded liposomes and cochleates in breast cancer therapy. AAPS PharmSciTech. [Epub ahead of print]. doi:10.1208/s12249-015-0429-3
- Ai X, Sun J, Zhong L, et al. (2014). Star-shape redox-responsive PEG-sheddable copolymer of disulfide-linked polyethylene glycol-lysine-di-tocopherol succinate for tumor-triggering intracellular doxorubicin rapid release: head-to-head comparison. Macromol Biosci 14:1415–28
- Chen H, Wu J, Sun M, et al. (2012). N-trimethyl chitosan chloride-coated liposomes for the oral delivery of curcumin. J Liposome Res 22:100–9
- Chu KS, Finniss MC, Schorzman AN, et al. (2014). Particle replication in nonwetting templates nanoparticles with tumor selective alkyl silyl ether docetaxel prodrug reduces toxicity. Nano Lett 14:1472–6
- Duan X, Xiao J, Yin Q, et al. (2013). Smart pH-sensitive and temporal-controlled polymeric micelles for effective combination therapy of doxorubicin and disulfiram. ACS Nano 7:5858–69
- Etrych T, Šírová M, Starovoytova L, et al. (2010). DTX-HPMA copolymer conjugates of paclitaxel and docetaxel with pH-controlled drug release. Mol Pharm 7:1015–26
- Fry DW, White C, Goldman ID. (1978). Rapid separation of low molecular weight solutes from liposomes without dilution. Anal Biochem 90:809–15
- Ganesh T. (2007). Improved biochemical strategies for targeted delivery of taxoids. Bioorg Med Chem 15:3597–623
- He X, Li L, Su H, et al. (2015). Poly(ethylene glycol)-block-poly(ɛ-caprolactone)-and phospholipid-based stealth nanoparticles with enhanced therapeutic efficacy on murine breast cancer by improved intracellular drug delivery. Int J Nanomedicine 10:1791–804
- Jain JP, Jatana M, Chakrabarti A, et al. (2010). Amphotericin-B-loaded polymersomes formulation (PAMBO) based on (PEG)3-PLA copolymers: an in vivo evaluation in a murine model. Mol Pharm 8:204–12
- Kim H, Lee Y, Lee IH, et al. (2014). Synthesis and therapeutic evaluation of an aptide-docetaxel conjugate targeting tumor-associated fibronectin. J Control Release 178:118–24
- Letchford K, Burt HM. (2012). Copolymer micelles and nanospheres with different in vitro stability demonstrate similar paclitaxel pharmacokinetics. Mol Pharm 9:248–60
- Li XT, He ML, Zhou ZY, et al. (2015). The antitumor activity of PNA modified vinblastine cationic liposomes on Lewis lung tumor cells: in vitro and in vivo evaluation. Int J Pharm 487:223–33
- Liu D, Wang L, Liu Z, et al. (2010). Preparation, characterization, and in vitro evaluation of docetaxel-loaded poly(lactic acid)-poly(ethylene glycol) nanoparticles for parenteral drug delivery. J Biomed Nanotechnol 6:675–82
- Marzban E, Alavizadeh SH, Ghiadi M, et al. (2015). Optimizing the therapeutic efficacy of cisplatin PEGylated liposomes via incorporation of different DPPG ratios: in vitro and in vivo studies. Colloids Surf B Biointerfaces 136:885–91
- Park MH, Keum CG, Song JY, et al. (2014). A novel aqueous parenteral formulation of docetaxel using prodrugs. Int J Pharm 462:1–7
- Peng PC, Hong RL, Tsai YJ, et al. (2015). Dual-effect liposomes encapsulated with doxorubicin and chlorin e6 augment the therapeutic effect of tumor treatment. Lasers Surg Med 47:77–87
- Wang D, Fu Q, Tang J, et al. (2015a). Molecular-matched materials for anticancer drug delivery and imaging. Nanomedicine (Lond) 10:3003–13
- Wang J, Li L, Du Y, et al. (2015b). Improved oral absorption of doxorubicin by amphiphilic copolymer of lysine-linked ditocopherol polyethylene glycol 2000 succinate: in vitro characterization and in vivo evaluation. Mol Pharm 12:463–73
- Wang J, Sun J, Chen Q, et al. (2012). Star-shape copolymer of lysine-linked di-tocopherol polyethylene glycol 2000 succinate for doxorubicin delivery with reversal of multidrug resistance. Biomaterials 33:6877–88
- Wang Y, Liu D, Zheng Q, et al. (2014). Disulfide bond bridge insertion turns hydrophobic anticancer prodrugs into self-assembled nanomedicines. Nano Lett 14:5577–83
- Wohl AR, Michel AR, Kalscheuer S, et al. (2014). Silicate esters of paclitaxel and docetaxel: synthesis, hydrophobicity, hydrolytic stability, cytotoxicity, and prodrug potential. J Med Chem 57:2368–79
- Zhigaltsev IV, Winters G, Srinivasulu M, et al. (2010). Development of a weak-base docetaxel derivative that can be loaded into lipid nanoparticles. J Control Release 144:332–40