Abstract:
Objective: To study the effect of programmed cell death 5 (PDCD5) on apoptosis of rheumatoid arthritis fibroblast-like synoviocyte (RAFLS) induced by triptolide. Method: Cultured synovial cells in vitro from RA patients were transfected with Ad-PDCD5. In protein level, expression of PDCD5 protein in Ad-PDCD5 transfected RAFLS was detected by Western blot. RAFLS transfected with Ad-PDCD5 were cultured in presence or absence of triptolide and RAFLs apoptosis was determined by flow cytometry. Result: Transfection of RAFLS with increasing concentration of Ad-PDCD5 (50-300 MOI) resulted in dose-dependent increase of PDCD5 production. Apoptotic cells percentage of no transfection group, Ad-null group and Ad-PDCD5 group were, respectively, (22.41 +/– 3.87)%, (28.77 +/– 12.97)%, and (48.87 +/– 12.69)%. Alternatively, transfection without triptolide stimuli had no effect. The data showed that gene transfection of PDCD5 alone without triptolide was not sufficient to activate RAFLS apoptosis; PDCD5 acted as an enhancer rather than inductor of apoptosis. Conclusion: Overexpression of PDCD5 could enhance apoptosis of RA FLS induced by triptolide; PDCD5 may be a potential therapeutic target to RA.
INTRODUCTION
The main pathological characteristics of rheumatic arthritis are abnormal synoviocyte proliferation, thickening of lining layer, and infiltration of inflammation cells, which form rheumatic synovial pannus and erode cartilage and bone, eventually destroying the joint [Citation1]. RA fibroblast-like synoviocyte (RAFLS) is thought to be the main joint synovial cell and can release many kinds of cytokine. RAFLS proliferate like tumor cells with RA joint microenvironment change, and have abnormal apoptosis and proliferation [Citation2]. RAFLS of synovial tissue is extensively activated, especially in pannus eroding cartilage and bone. Activated RAFLS manifest resistance to apoptosis induced by the apoptic stimulus signal, which is also called the apoptosis defect [Citation3]. Programmed cell death factor 5 (which is derived from apoptosis up-regulation gene of TF21 cell clone) can enhance apoptosis induced by different apoptotic stimulus [Citation4], and is extensively expressed in many kinds of tissue [Citation5]. In recent years, some studies show that PDCD5 expression is down-regulated in RA pannus and cultured RAFLS in vitro. Down-regulated PDCD5 is evenly distributed in the RA synovial membrane. PDCD5 protein mainly locates in cytoplasm of RAFLS [Citation6,Citation7]. During the process of RAFLS apoptosis, PDCD5 expression is up-regulated, and PDCD5 protein nuclear translocation appears in the early stage of apoptosis [Citation7]. Until now, the key factor that regulates apoptotic sensitivity of RAFLS is not clear. Although PDCD5 expression level and location change during RAFLS apoptosis is clear, intrinsic effect caused by PDCD5 overexpression is not clear. Because PDCD5 is down-regulated in RA synovial membrane and RAFLS, then PDCD5 overexpression may be very important. Our experiment is to study the apoptotic effect of PDCD5 overexpression on RAFLS through Ad-PDCD5 adenovirus transfection.
MATERIALS AND METHODS
Reagent
DMEM culture medium of American Life Technologies Corporation, fetal bovine serum of American Gibcol Corporation, Adenovirus vector Ad-PDCD5\ Ad-null\ Ad-eGFP of Peking University Hematonosis Research Institute, BCA Protein Assay kit of American Pierce Corporation, Anti-mouse IgG labeled with second antibody IRDye800 of Rockland Corporation of USA, rat monoclonal antibody IgG (3A3) for human PDCD5 of Peking University Human Disease Research Institute, Collagenase I and rat monoclonal antibody for β-actin of American Sigma Corporation, Annexin-VFITC cell apoptosis test kit of Nanjing Biological Science & Technology Corporation.
Specimen
Synovium specimen was acquired from RA patients (n522) who had received total knee replacement or synovectomy operations at the Arthritis Clinical & Research Center Peking University People's Hospital from 2005–9 to 2006–9. All these patients were diagnosed with rheumatic arthritis according to1987 American Rheumatology College criteria [Citation8] and gave informed consent.
1.3 Cell Culturing
Synovial membrane specimen of RA patient was incubated in an aseptic test tube containing culturing medium, rinsed with iced PBS for 5-6 times, minced thoroughly and PBS containing 0.125% trysin was added, then incubated for 2 hours in 37°C constant temperature, and centrifuged 1500 r/min, washed with PBS solution for three times to remove trypsin, then DMEM culturing medium (10% volume fraction fetal bovine serum, 25 mmol/l HEPEs, 100 U/ml penicillin, 100 mg/L Streptomycin) was added. It was incubated for 48∼72 hours in 37°C constant temperature and 5% volume fraction CO2 and we changed the culturing medium. About 5∼7days later, cultured cells were passaged with1:3 ratio when cell confluence reached 85% in cultural flask. After three passages, homogenous fibroblast-like synovial cell were acquired.
Adenovirus Vector Transfection
PDCD5 recombinant adenovirus vector was constructed by Benyuanzhengyang Gene Corporation Ltd using the Canadian AdMAXTM adenovirus vector packaging system, in which PDCD5 gene expression box contain CMV promoter/PDCD5cDNA/SV40polyA signal sequence and was inserted into E1 region of adenovirus genome with E3 region loss. In control group, Ad-eGFP (containing green fluorescent protein gene, GFP gene) and Ad-null (simple vector without target gene) were constructed with the same method. When RAFLs cell growth confluence reached 85%, DMEM culturing medium containing 0.15% (volume fraction) fetal bovine serum was added. RAFLs cells were cultured for another 24 hours, then were infected for 36 hours with different multiplicity infection (MOI) (50/100/200/300), and rinsed with PBS solution for two times. Then DMEM culturing medium containing 10% fetal bovine serum was added. Infected RAFLS were cultured and processed with different procedures.
Observation Through Fluoroscopy
RAFLs were transfected by Ad-eGFP with different MOI, and were observed in reversed fluoroscopy. Transfection efficacy of recombinant adenovirus vector was primarily measured through analyzing the CCD system image with image software analysis.
Measurement of Adenovirus Transfection Efficacy for RAFLs
RAFLs were transfected by Ad-eGFP with different MOI (50/100/200/300). After 36 hours, RAFLs were digested with trysin and collected. 1 × 105 − 5 × 105 RAFLS cells were rinsed with PBS solution twice, and re-suspended in Annexin-Vbuffer solution (10 mmol/L HEPS, 140 mmol /L NaCl, 2 mmol/LMgCl2, 5 mmol/L KCl, 2.5 mmol/L CaCl2, pH7.4), to acquire unicellular suspension solution. 2 L Annexin-V-FITC solution was added and mixed equably, and then 5 L propidium iodide was added and mixed equably. RAFLS cells were cultured for 20 minutes at room temperature without light shedding, and measured with flow cytometry. Every sample contained about 1 × 104 cells, and was analyzed with CELLQuest software (BD Bioscience, USA). Transfection efficacy was figured with form of percentage.
Detection of PDCD5 Protein in Ad-PDCD5 Transfected RAFLs through Western Blotting
RAFLS cells were, respectively, transfected with adenovirus Ad-null (200 MOI) (as negative control group) and Ad-PDCD5(50-300 MOI), and cultured for 36 hours, then digested with 0.25% trypsin (mass fraction) and centrifuged to deposit. Precolded lysis buffer solution was added, and placed on ice for 30 minutes, then centrifuged with 18000 g force for 20 minutes in 4°C. Protein concentration in supernatant was measured according to the instruction book of BCA Kit (Pierce Corporation), using bovine serum albumin as standard. 40 g protein were added into 5× sample buffer to be denatured, then electrophoresis was processed in 15 % (mass fraction) SDS-PAGE. Gel protein band was transmembraned onto PVDF membrane (OSMONIC Corporation), and blocked with 50 % (mass fraction) skim milk for 1 hour at room temperature. Anti-human PDCD5 polyclonal mouse antibody (1:400) and anti-β-actin monoclonal mouse antibody were added, and incubated in 4°C overnight. The membrane was washed for 10 minutes 3 times; we added a second antibody IRdye 800 labelled anti-mouse IgG (1:8000), and incubated for 1 hour at room temperature without light shedding. Then the membrane was washed 3 times, detected and analyzed with the Odyssey imaging system (LI-COR Bioscience Corporation).
Detection of triptolide-induced RAFLs Apoptosis Enhanced by Overexpression of PDCD5
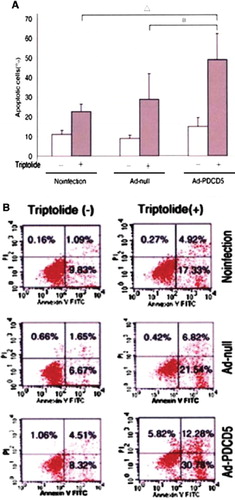
Phosphatidylserine (PS) translocation from the inner layer to outer layer of plasma membrane is an important biochemical marker [Citation9]. Eversed PS can be specifically combined by Annexin-V. Using fluorescein-labelled Annexin-V and flow cytometry, eversed PS can be quantitatively analyzed [Citation10], and cell apoptosis is determined according to fluorescence intensity change. Integrality of cytomembrane is determined through PI staining. 50 nmol/L trip-tolide were added into the Ad-null group and Ad-PDCD5 group with MOI 200 to induce cell apoptosis. After 24 hours, cells were collected and detected with flow cytometry through Annexin-V-FITC/PI double staining.
Statistical Analysis
Statistical analysis was performed with SPSS 11.0 software. K independent samples test was performed for quantitive data. P>0.05 between groups shows statistical difference.
RESULTS
RAFLs Adenovirus Infection Effect
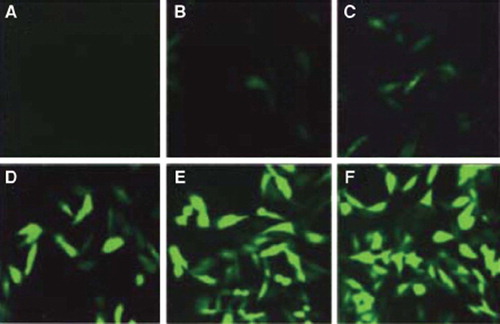
Fluorescence microscope examination of RAFLs infected by Ad-eGFP (). Fluorescence was observed first after RAFLs transfection, which mainly distribute in cytoplasma and cellular nucleus. After 36 hours, fluorescence intensity reach peak, and number of fluorescent cells reach highest, about 70∼85%. RAFLs were infected with 50/100/200 and 300 different MOI. RAFLs were infected with 200 MOI, and after 36 hours incubation about 85% fluorescent cells were acquired.
Figure 1. RA FLS cells infected by Ad-eGFP (×400) (A) control; (B) 12 h 200 MO I; (C) 36 h 50 MO I; (D) 36 h 100 MO I; (E) 36 h 200 MO I; (F) 36 h 300 MO I.
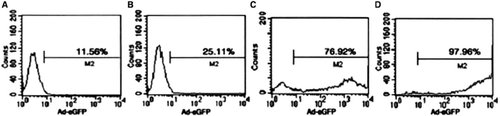
Infection efficacy (). RAFLs were transfected by Ad-eGFP adenovirus for 36 hours. Through flow cytophtometry measurement, Ad-eGFP transfection efficacy was, respectively, 11.56%, 25.11%, 76.92, and 97.76% for 50/100/200 and 300 MOI.
PDCD5 Protein Expression in RAFLs Infected by Ad-PDCD5 ()
Figure 3. Western blot analysis of kinetics of PDCD5 protein expression in RA FLS following infection with Ad-PDCD5 for 36 hours.

Immunoblotting detection result shows that PDCD5 protein band density become stronger after RAFLS transfetion by Ad-PDCD5 for 36 hours with MOI increase. That is to say, PDCD5 protein expression level is dose-dependent. Adenovirus systems can effectively cause overexpression of PDCD5 in RAFLS.
Effect of PDCD5 Overexpression on Triptolide-induced RAFLS Apoptosis ()
There was no statistical difference between the non-infection group, Ad-null vector group, and Ad-PDCD5 group without triptolide induction. RAFLs apoptosis ratio was, respectively, (22.51 ± 3.87) %, (28.77 ± 12.97), and (48.87±12.69)% for the non-infection group, Ad-null vector group, and Ad-PDCD5 group with triptolide induction. Annexin-V positive RAFLS percentage in Ad-PDCD5 group is obviously higher than in the other groups. Simple triptolide induction or simple PDCD5 transfection can't obviously induce RAFLs apoptosis, but overexprssion of PDCD5 together with triptolide induction can obviously induce RAFLs apoptosis.
DISCUSSION
In our experiment, through the immunoblotting technique we proved that overexpression of PDCD5 protein in Ad-PDCD5 transfected RAFLs, which were treated with different dose triptolide, is dose-dependently up-regulated. Through flow cytophotometry we found that RAFLS, which was only transfected by Ad-PDCD5 but without triptolide induction, has no apoptosis enhancement. So we conclude that overexpression of PDCD5 can enhance triptolide-induced RAFLs apoptosis sensitivity. A previous study in the cell line has shown that the action of PDCD5 on apoptosis was enhancement but not induction [Citation4], which was also proved by our experiment. Overexpression of PDCD5 without triptolide-induction cannot activate RAFLs apoptosis. A similar effect was also observed in fibroblast-like synoviocyte of osteoarthritis synovium, which shows that apoptosis enhancement of PDCD5 keep the same action in a different cell. According to these results, we infer that overexpression of PDCD5 can decrease the anti-rheumatic drug-resistance of RA patients. PDCD5 gene is a broad-spectrum apoptosis enhancement activity gene, which has extensive action in enhancement of programmed cell death. But until now the exact signal mechanism has not been clear. Recent research found that PDCD5 may directly or indirectly promote Bax transition from cytoplasm to outer membrane of mitochondria, increase cytochrome C release into cytoplasm, promote caspases activation, and so enhance apoptosis [Citation11]. However, PDCD5 not only participates in the endogenesis pathway of cell apoptosis, but also has action in the signal pathway mediated by cytomembrane death receptor. PDCD5 protein increase was detected in a Jurket cell processed by Fas monoclonal antibody [Citation12]. Fas is a typical cytomembrane receptor delegate of exogenesis cell apoptosis signal transduction. As a promoter of cell apoptosis PDCD5 may participate in both endogenesis and exogenesis signal transduction pathways. PDCD5 not only participates in programmed cell death in apoptosis, but also participates in programmed cell death in paraptosis through promoting over-expression of the independent tumor necrosis factor super-family member TAJ/TROY [Citation13]. In RA, the pathway through which PDCD5 regulates apoptosis and whether PDCD5 interacts with abnormally expressed TNF2α, NF2κB and XIAP apoptosis molecule needs further research.
In summary, our experiment shows that overexpression of PDCD5 through Ad-PDCD5 transfection may enhance triptolide-induced RAFLs apoptosis, which provides a potential therapeutic target for rheumatic arthritis.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This paper was first published online as an Early Online article on 5 January 2010.
REFERENCES
- Firestein, G.S. (2003). Evolving concepts of rheumatoid arthritis. Nature 423:356–361.
- Pap, T., Muller Launder, Gary R.E. ,. (2000). Fibroblast biology: Role of synovial fibroblasts in pathogenesis of rheumatoid arthritis. Arthritis Res 2:361–367.
- Baier, A., Meineckel, I., Gap, S., . (2003). Apoptosis in rheumatoid arthritis. Cur Op in Rheumatol 15:274–2799.
- Liu, Hongtao, Wang, Yugang, Zhang, Yingmei. (1999). TRAP19, a novel apoptosis-related gene cloned from human leukemia cell line TF21, could enhance apoptosis of some tumor cells induced by growth factor withdrawl. Biochem Biophys Res Commun 254:203–210.
- Chen, Yingyu, Zhang, Yingmei, Sun, Yonghua. (2000). Preparation and identification of monoclonal antibody for human apoptosis-related protein TRAP19. Acta Acdemiate Medicinae Sinecae 22:502–504.
- Chen, Zhankun, Lv, Houshan, Wang, Ning. (2008). Expression of apoptotic gene PDCD5 in rheumatoid arthritis synovium. Chinese Journal of Rheumatology 12:36–39.
- Chen, Zhankun, Lv, Houshan, Wang, Ning. (2008). Upregulation of PDCD5 in rheumatoid arthritis fibroblast-like synoviocyte. Chinese Journal of Biochemistry and Molecular Biology 24:563–568.
- Arnett, F.C., Edworthy, S.M., Bloch, D.A. (1988). The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324.
- Fadok, V.A., Bratton, D.L., Frasch, S.C. (1998). The role of phosphatidylseine in recognition of apoptotic cells by phagocyte. Cell Death Differ 5:551–562.
- Smith, T.D., Waelde, C., Horwitz, A. (2002). Evaluation of the role of phosphatidylserine translocase activity in ABCA1-mediated lipid effhex. J Biol Chem 277: 17797–17803.
- Pope, R.M. (2002). Apoptosis as a threrapeutic tool in rheumatoid arthritis. Nat Rev Immunol 2:527–535.
- Li, Li, Chen, Yingyu, Zheng, Rui. (2000) TFAR19 expression in Jurket cell apoptosis induced by several modes. Chinese Journal of Experimental Hematology 8:81–84.
- Wang, Ying, Li, Xianting, Wang, Ling. (2004). An alternative form of paratosis-like cell death, triggered by TAJ/TROY and enhanced by PDCD5 overexpression. J Cell Sci 117:1525–1532.