Abstract
An amperometric biosensor based on zucchini (Cucurbita pepo) tissue homogenate is presented. The zucchini tissue homogenate was crosslinked with gelatine using glutaraldehyde and fixed on a pretreated teflon membrane. The zucchini tissue contained the enzyme ascorbate oxidase and this enzyme catalyzed the oxidation of ascorbic acid in the presence of dissolved oxygen. The principle of the measurements was based on the determination of the decrease in the dissolved oxygen level. Determinations were carried out by standard curves, which were obtained by the measurement of the decrease in the oxygen level related to ascorbic acid concentration.
Optimization and characterization studies of the biosensor were carried out in detail. First of all, the amounts of zucchini tissue homogenate, gelatin, and glutaraldehyde percentage were optimized. Experimental parameters such as buffer system, pH, buffer concentration, and temperature were also optimized carefully. Thermal stability, storage stability, and repeatability of the biosensor were investigated. A linear response was observed from 5×10−6 M to 1.2×10−3 M ascorbic acid. Finally, the results of some plant and drug samples analyzed with the presented biosensor compared with the spectrophotometric method (Tillman reagent) used as a reference.
INTRODUCTION
Ascorbic acid (Vitamin C) is an essential nutrient that the human body cannot manufacture from other compounds. Ascorbic acid is a potent reducing and antioxidant agent that functions in fighting bacterial infections, in detoxifying reactions, and in the formation of collagen in fibrous tissue, teeth, bones, connective tissue, skin, and capillaries [Citation1, Citation2]. It is also a good anti-oxidant, preventing damage from oxygen-free radicals. Ascorbic acid is added to many foods for its nutritive value, but is also used extensively as an anti-oxidant, to prevent flavors and colors from being damaged by oxidation [Citation3]. It is often used in canned or frozen fruits to prevent the browning that accompanies oxidation.
In order to monitor ascorbic acid a lot of different determination methods such as chromatographic methods [Citation4] and [Citation5], spectrophotometric methods [Citation6] and [Citation7], electrochemical methods [Citation8–12] have been reported. From these techniques, electrochemical ones have long been used to detect ascorbic acid either directly or combined with other techniques. Moreover, an HPLC method has been used to monitor Vitamin C levels in serum or plasma [Citation13]. In addition, Tulley reported an automated enzymatic method for the Beckman Synchron CX5 analyzer [Citation14]. The measurement principle was based on the condensing dehydroascorbic acid with OPDA to form a quinoxaline derivative that was absorbed light at 340 nm [Citation15]. Recently, Ihara and coworkers have also reported a new method for ascorbic acid using an analyzer [Citation16].
Because ascorbate oxidase is relatively costly and the HPLC procedure is labor-intensive and difficult to automate, we developed a new and inexpensive biosensor. The enzymatic methods are known to be very sensitive, specific, simple and useful, in which the immobilizations of enzymes are generally used. However, in the present study, we used a plant, zucchini tissue homogenate as a biorecognition element. Zucchini contained abundant ascorbate oxidase.
Ascorbic acid was oxidized to dehydroascorbic acid in the presence of oxygen by the enzyme of ascorbate oxidase. The principle of the biosensor was based on this reaction. That is to say that when this reaction was made to occur on the sensing element of a conventional Clark dissolved oxygen electrode containing zucchini tissue homogenate, there was a local oxygen depletion, causing the voltage response of the electrode to decrease. This principle was used for the construction of a biosensor for ascorbic acid determination, wherein a zucchini (Cucurbita pepo) rich in ascorbate oxidase was used in association with a Clark oxygen electrode. The aim of this work was to use a natural system, plant tissue, as the biosensing part and to monitor ascorbic acid in some pharmaceutical preparations and fruits. In the study several optimization and characterization studies of the biosensor were investigated. Finally, the biosensor was validated by a reference method spectrophotometrically.
EXPERIMENTAL
Materials
Glutaraldehyde (Grade II, 25 % aqueous solution), gelatin (type 3, 225 Bloom), ascorbic acid, sodiumdodecylsulfate (SDS) were obtained from Sigma (St. Louis, MO, USA). Glucose, sitric acid, hydroquinon, succinic acid, aspartic acid, phenol, ethanol, catechol, sodium hydroxide, potassium dihydrogen phosphate were purchased from E. Merck (Buchs, Germany). Bidistilled water was used to prepare the solutions. All reagents were analytical grade. Fruit samples were obtained from a Turkish commercial source.
Apparatus
YSI 54 A model oxygen meter and YSI 5700 series dissolved oxygen (DO) probes, YSI (Ohio, USA) were used. A water bath was used for preparation of bioactive material (Stuart Scientific, Redhill, UK). All the measurements were carried out of constant temperature using a thermostat (Haake, JF, Germany). Magnetic stirrer (Janke&Kunkel, Staufen, Germany) and pH meter with electrode (Inolab, Weilheim, Germany) for preparing buffer solutions were used. The temperature was maintained constant in the reaction cell by circulating water at appropriate temperature around the cell comportment during the experiment.
Procedure
Dissolved oxygen probe. To construct the biosensor a dissolved oxygen probe was covered with high-sensitive teflon membrane by using on O-ring and then the teflon membrane, which is selective for oxygen, was pretreated with 0.5 % sodium dodecylsulphate in phosphate buffer (50 mM, pH 7.5) to reduce the tension on the membrane surface.
Preparation of the bioactive layer material. Zucchini tissue that was close to the skin of the fruit was first homogenized by a manual glass homogenizer. 10 mg gelatin was weighed and added to a test tube. Then 200 l zucchini tissue homogenate was pipetted into the test tube. After this step, 50 l phosphate buffer (pH 7.5 and 50 mM) was added to the tube. This mixture was incubated at 38 C for 5-10 minutes to dissolve gelatin.
Biosensor preparation. 200 μl of the gelatin-zucchini tissue homogenate mixture was dispersed over the dissolved oxygen probe membrane surface and allowed to dry at 4 C for 45 minutes. For crosslinking with glutaraldehyde, the probe carrying bioactive layer was immersed into 2.5 % (v/v) glutaraldehyde solution (in phosphate buffer, 10 ml, 50 mM and pH 7.5) and was allowed to wait 5 minutes. At the end of this time, the biosensor was washed with distilled water and it was ready to use.
In order to prevent drying of the bioactive layer of the biosensor, it was stored in a flask that contained some distilled water at 4 C. The biosensor was not in contact with distilled water. This condition provided a moisture medium for the biosensor.
Measurement procedure. The biosensor based on zucchini tissue homogenate was put into the thermostatic reaction cell containing working buffer (pH 7.5, 50 mM phosphate buffer) and the magnetic stirrer was fixed at a constant speed. A few minutes later, dissolved oxygen concentration was equilibrated because of the diffusion of dissolved oxygen between working buffer and dissolved oxygen probe. At this time, ascorbic acid was injected into the thermostatic reaction cell. The dissolved oxygen concentration started to decrease and a few minutes later it reached constant dissolved oxygen concentration due to the enzymatic reaction equilibration below.
At this moment, dissolved oxygen concentration was recorded. Measurements were carried out by the change of dissolved oxygen concentration related to ascorbic acid concentration added to the reaction cell.
RESULTS AND DISCUSSION
Effect of Zucchini Tissue Homogenate Amounts on the Biosensor
In the optimization studies of the biosensor, first of all, the amount of the plant tissue immobilized on the dissolved oxygen probe was investigated. Standard graphs related to ascorbic acid are shown in .
Figure 1. The effect of zucchini tissue homogenate amount [▴–▴: 50 μL, –•–•–: 100 μL, –▪–▪–: 200 μL. The amount of glutaraldehyde and gelatin were kept constant at 2.5% and 10mg, respectively. Working conditions: Phosphate buffer, 50 mM, pH 7.5, T:35 °C].
![Figure 1. The effect of zucchini tissue homogenate amount [▴–▴: 50 μL, –•–•–: 100 μL, –▪–▪–: 200 μL. The amount of glutaraldehyde and gelatin were kept constant at 2.5% and 10mg, respectively. Working conditions: Phosphate buffer, 50 mM, pH 7.5, T:35 °C].](/cms/asset/7176e89b-9ed5-459d-904d-78017478d129/ianb19_a_479080_f0001_b.gif)
As can be seen from the figure, more increase in the homogenate amount caused increase in the activity. In lower homogenate amounts than 200 μL, the biosensor response for ascorbic acid was decreased. This was probably caused by the lower enzyme content on the biosensor when we used lower amounts of plant tissue homogenate. However, the detection limit of the biosensors was the same as 5×10−6 M of ascorbic acid. In addition, the higher amount of the zucchini tissue homogenate than 200 μL couldn’t be tested because the area of the Clark electrode was not feasible for more than a volume of 200 μL. The best biosensor signals for ascorbic acid were obtained with the biosensor that contained 200 μL tissue homogenate. As a result, in further measurements 200 μL zucchini tissue homogenate was used.
Effect of Gelatine on the Biosensor
The effect of quantity of gelatine in the bioactive layer was determined. In these studies 5, 7.5, and 10 mg gelatine amounts were tested on biosensors. Optimization studies for the gelatine amount showed that there were slight changes in the biosensor activities related to gelatine amount. shows the results of gelatine amount effect on the biosensor.
Figure 2. The effect of gelatin amount on the biosensor [–▪–▪–: 10 mg, –•–•–: 7.5 mg, and ▴–▴: 5 mg. The amount of zucchini tissue homogenate and glutaraldehyde were kept constant as 200 μL and 2.5%, respectively. Working conditions: Phosphate buffer, 50 mM, pH 7.5, T:35 °C].
![Figure 2. The effect of gelatin amount on the biosensor [–▪–▪–: 10 mg, –•–•–: 7.5 mg, and ▴–▴: 5 mg. The amount of zucchini tissue homogenate and glutaraldehyde were kept constant as 200 μL and 2.5%, respectively. Working conditions: Phosphate buffer, 50 mM, pH 7.5, T:35 °C].](/cms/asset/94ef851e-ab66-4a24-b88e-f702461192cd/ianb19_a_479080_f0002_b.gif)
With amounts lower than 10 mg of gelatine (5 and 7.5 mg) the biosensor signals didn’t alter seriously. Moreover, gelatine amounts higher than 10 mg couldn’t be investigated. The viscosity of the biorecognition membrane was very low when we used amounts higher than 10 mg. Consequently, it was not possible to build up a good membrane. At the end of these studies 2.5 mg of gelatine was chosen as the optimum gelatine amount.
Effect of Glutaraldehyde Percentage on the Biosensor
In the investigation of the effect of differing glutaraldehyde percentages on the biosensor for ascorbic acid monitoring, experiments were carried out by keeping the amount of zucchini tissue homogenate (200 μL) and gelatin (10 mg) constant while changing the amount of glutaraldehyde.
As it is obvious from , although the highest biosensor response was observed when 2.5 % glutaraldehyde was used, there was no considerable change in the biosensor signals by altering the glutaraldehyde concentrations. The response of the biosensor decreased as the glutaraldehyde percentage was increased. This result may be attributed to excessive cross-linking between the immobilization materials, where the active sites of the enzyme were blocked and thus a decrease in the activity of the immobilized enzyme. Moreover, the decrease in the biosensor signal may also be caused by the negative diffusion barrier effects. However, when glutaraldehyde concentration was decreased the biosensor signals were decreased again, because the physical stability of the membrane formed with 1.25 % glutaraldehyde was insufficient. Finally, the optimum glutaraldehyde percentage was 2.5 % for the biosensor.
Optimum Temperature and Thermal Stability of the Biosensor
One of the most important parameters for biosensors is the optimum temperature. In the temperature range 20–45 °C, the biosensor signals increased with increasing temperature. The highest biosensor response was obtained when working with 45 °C. At higher temperatures than 45 °C, the biosensor response started to decrease. The optimum temperature graph is shown in .
Figure 4. Optimum temperature of the biosensor [the amount of zucchini tissue homogenate, glutaraldehyde percentage and gelatin were kept constant at 200 μL, 2.5%, and 10mg, respectively. Working conditions: Phosphate buffer, 50 mM, pH 7.5. Ascorbic acid concentration used was of 6×10−4 M.].
![Figure 4. Optimum temperature of the biosensor [the amount of zucchini tissue homogenate, glutaraldehyde percentage and gelatin were kept constant at 200 μL, 2.5%, and 10mg, respectively. Working conditions: Phosphate buffer, 50 mM, pH 7.5. Ascorbic acid concentration used was of 6×10−4 M.].](/cms/asset/d76d51d0-c018-435a-b185-4f0a6bf400c1/ianb19_a_479080_f0004_b.gif)
Although the highest response was obtained at 45 °C, to avoid as much as possible the denaturation process of ascorbate oxidase in the bioactive layer of the biosensor, 35 °C was chosen as the working temperature. Moreover, the signal difference between the temperature of 45 and 35 °C was just 20 %.
In the experiments of thermal stability of the biosensor, it was tested by the help of incubation of the biosensor at 35 °C and then making measurements. The biosensor showed no thermal deactivation in the temperature of 35 °C at the end of the incubation period of 7 hours. On the other hand, the biosensor retained 94 % of its initial activity at the end of the period of 9 hours.
Optimum pH, Appropriate Buffer System, and Buffer Concentration
The pH value of the working buffer was one of the most important parameters that could dramatically affect the biosensor response. An optimum pH scan was done in the range of pH 6–8. shows the optimum pH graph.
Figure 5. pH effect on the biosensor response [the amount of zucchini tissue homogenate, glutaraldehyde percentage and gelatin were kept constant at 200 μL, 2.5%, and 10mg, respectively. All buffers were 0.05 M and pH 6, 6.5, 7, 7.5 and 8 were of phosphate buffers, T = 35°C. Ascorbic acid concentration used was of 6×10−4 M].
![Figure 5. pH effect on the biosensor response [the amount of zucchini tissue homogenate, glutaraldehyde percentage and gelatin were kept constant at 200 μL, 2.5%, and 10mg, respectively. All buffers were 0.05 M and pH 6, 6.5, 7, 7.5 and 8 were of phosphate buffers, T = 35°C. Ascorbic acid concentration used was of 6×10−4 M].](/cms/asset/3c30de7e-c8fc-408e-b58b-995630b27d32/ianb19_a_479080_f0005_b.gif)
These results showed that the best biosensor signal was obtained at pH 7. As can be seen clearly from the figure, for a pH of 6.5 the biosensor signal was about 7 % lower than that found at pH 7 and the signal decreased for pH values higher than 7.
The buffer system can result in the change of the capacity of the buffer or ion strength and can change the biosensor response. For this purpose, five different buffer systems that were adjustable to pH 7, Tris/HCl, Na2HPO4/Citric acid, KH2PO4/NaOH, Hepes/NaOH, and triethanolamine/HCl were used to investigate the effect of the buffer system on the biosensor. The results showed that the buffer systems altered the biosensor response considerably. The best biosensor response was obtained with the KH2PO4/NaOH buffer system. Consequently this buffer system was accepted as the working buffer.
The increase of buffer concentration often contributes to the reproducibility of biosensor response together with the decay of the response value and the lifetime of the immobilized enzyme or tissue. So we also investigated optimum concentration of the working buffer. An increase in buffer concentration resulted in a signal increase. The highest biosensor signal was obtained at a concentration of 0.2 M. These results are given in .
Figure 6. Optimum buffer concentration [a – the effect of buffer concentration on the biosensor response (Ascorbic acid concentration used was of 6×10−4 M), b– calibration graphs obtained with different buffer concentrations (–▪–▪–: 50 mM, –•–•–: 200 mM phosphate buffer). The amount of zucchini tissue homogenate, glutaraldehyde percentage and gelatin were kept constant at 200 μL, 2.5%, and 10mg, respectively. T= 35 °C].
![Figure 6. Optimum buffer concentration [a – the effect of buffer concentration on the biosensor response (Ascorbic acid concentration used was of 6×10−4 M), b– calibration graphs obtained with different buffer concentrations (–▪–▪–: 50 mM, –•–•–: 200 mM phosphate buffer). The amount of zucchini tissue homogenate, glutaraldehyde percentage and gelatin were kept constant at 200 μL, 2.5%, and 10mg, respectively. T= 35 °C].](/cms/asset/a7721a2b-11ff-40fe-ba45-36b18890c457/ianb19_a_479080_f0006_b.gif)
In order to determine the correct buffer concentration two calibration graphs for ascorbic acid were drawn, one with 0.05 M buffer and the other with 0.2 M buffer.
As can be clearly seen from , there was a serious deviation from linearity when worked with the concentration of 0.2 M. Consequently, the appropriate buffer concentration was accepted as 0.05 M.
Calibration Graph for Ascorbic Acid
A calibration curve for ascorbic acid is presented in . The biosensor showed a good linear calibration graph for ascorbic acid between 5×10−6 and 1.2×10−3 M.
Figure 7. Calibration graph for ascorbic acid [the amount of zucchini tissue homogenate, glutaraldehyde percentage, and gelatin were kept constant at 200 μL, 2.5%, and 10mg, respectively. Phosphate buffer, 50 mM, pH 7.5, T:35 °C].
![Figure 7. Calibration graph for ascorbic acid [the amount of zucchini tissue homogenate, glutaraldehyde percentage, and gelatin were kept constant at 200 μL, 2.5%, and 10mg, respectively. Phosphate buffer, 50 mM, pH 7.5, T:35 °C].](/cms/asset/f7ba845e-4618-4a03-b967-03257c400d25/ianb19_a_479080_f0007_b.gif)
The minimum detectable activity amount of β–galactosidase was estimated to be 5×10−6 M. This detection limit was perfect for the biosensor–based zucchini tissue homogenate. The linear range, the lowest and the highest limits of the biosensor were perfect for analytical aspects.
Repeatability
Repeatability is another factor that must be determined, especially for a biosensor. The signal changes of the biosensor were investigated when it was alternately exposed to a 6×10−4 M ascorbic acid standard solution for 8 times. R2 was 0.9975 and repeatability of the measurements was very good considering that the correlation coefficient on measurements was 2.74 %, and average value and standard deviation were calculated as 5.9×10−4 M, and ± 1.6×10−5 M, respectively. The results showed that the biosensor exhibited a fairly desirable analytical feature of repeatability.
Specificity of the Biosensor
The biosensor was applied to 8 different compounds that had possible interference effects to the biosensor and ascorbic acid. Analytical results for each compound obtained from the experiments are summarized in .
Table 1. Results obtained from the measurements of some compounds by the biosensor
As seen in , ascorbic acid showed the highest activity. The activity of catechol was recorded as 20 %. Catechol activity was followed by hydroquinone. The other compounds’ activities were not considerably higher. The activity of the biosensor towards phenolic compounds such as phenol, hydroquinone, and catechol was not surprising, because it has been reported that ascorbate oxidase catalysed the aerobic oxidation of a variety of catechol and aminophenol derivatives [Citation17–19].
Storage Stability of the Biosensor
The purpose of the storage stability test was to provide evidence on how the performance of the biosensor based on zucchini tissue homogenate for ascorbic acid varies with time under the influence of environmental factors such as temperature and humidity.
The biosensor was stored at +4 °C for 11 days. The biosensor response was constant for the first 5 days. After 8 days storage period the biosensor lost 12.5 % of its initial activity. At the end of the 9th and 11th days, the remaining activity of the biosensors was 81.25 and 75, respectively.
Real Sample Analysis by the Biosensor
To establish the viability of the biosensor for real sample analysis, vitamin C tablet, lemon and grapefruit juices, verified with Tillman reference method, were tested with the biosensor utilizing zucchini tissue homogenate as a biorecognition element. shows the results of the analyses performed on real samples, in comparison to the Tillman reference method.
Table 2. Ascorbic acid contents of some plant samples and a commercial Vitamin C tablet obtained by Tillman's Method and the present biosensor-based zucchini tissue homogenate
Deviations from the reference method, Tillman, for vitamin C tablet, lemon and grapefruit juices were −3.6 %, 5.11 %, and 3.05 %, respectively. Thus, it can be concluded that the biosensor is considerably accurate and reliable.
CONCLUSIONS
In the literature, several studies have been reported for ascorbic acid determination. For instance, Wang and coworkers presented a biosensor utilizing an enzyme micelle membrane [Citation20]. A concentration range of 5×10−6 to 4×10−4 M was observed for the biosensor. Messina et al. also reported a method for continuous-flow/stopped-flow system for determination of ascorbic acid [Citation21]. Although a linear range from 1.2×10−8 to 3.5×10−6 M was obtained by the method, its preparation process was long and tiring. In addition, real-time detection of ascorbic acid employing a reversed sequential differential measuring technique of the SIRE (Sensors based on Injection of the Recognition Element)-technology-based biosensor was also reported by Kriz et al. [Citation22]. The linear determination range for ascorbic acid was obtained between 0 and 3×10−3 M. Fernandes and coworkers reported a potentiometric biosensor based on Cucumis sativus L., which was the natural source of ascorbate oxidase [Citation23]. The biosensor exhibited a linear range between 8.0×10−6 and 4.5×10−4 M for ascorbic acid. In this study some steps were needed, such as enzyme extraction. The sensor presented by Volotovsky and Kim had a dynamic range for ascorbic acid between 2.5×10−4 and 2×10−3 M [Citation24]. Moreover, for ascorbic acid monitoring a PtAu hybrid film modified electrode was also constructed [Citation25]. In this system, ascorbic acid could be detected in the range of 2.4×10−5−3.84×10−4 M.
Ascorbic acid could be measured with high accuracy and reliability by means of the biosensor presented. It was an advantage to construct the biosensor easily with the help of a very simple immobilization procedure. The biosensor could be prepared in an hour completely. Moreover, the biosensor had a good and wide linear relationship with a correlation coefficient of 0.9975 for ascorbic acid. A wide linear detection range for ascorbic acid was obtained over the concentration range of 5.10−6 M and 1.2.10−3 M ascorbic acid. In addition, the simplicity of the zucchini tissue homogenate immobilization and the use of a very cheap tissue in comparison with commercial ascorbate oxidase enzyme demonstrate a considerable economic advantage. However, the most important disadvantage of the biosensor was insufficient storage stability. The insufficient storage stability of the biosensor can be staved off via its economical advantages.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Voet, D., and Voet, J.G. (1990). Biochemistry, Wiley, New York, 160F, 251F, 536F.
- Tolbert, B.M., Ward, J.B. (1982). Ascorbic acid, chemistry, metabolism and uses. P.A. Seib, B.M. Tolbert, Advances in Chemistry 200, American Chemical Society, Washington, DC.
- Clemetson, C.A.B. (1989). Vitamin C, CRC Press, Boca Raton, FL.
- Lopes, P., Drinkine, J., Sacier C. and Glories, Y. (2006). Determination of l-ascorbic acid in wines by direct injection liquid chromatography using a polymeric column. Analitica Chimica Acta, 555: 242–245.
- Hernandez, Y., Lobo, M.G., and Gonzalez, M. (2006). Determination of vitamin C in tropical fruits: A comparative evaluation of methods. Food Chemistry, 96(4): 654.
- Tang, B., Wang, Y., Du, M., Ge J., and Chen, Z. (2003). Catalytic spectrophotometric determination of ascorbic acid in tea drink with 1,5-bis(p-Hydroxybenzaldene) thiocarbohydrazone as the substrate for horseradish peroxidase. Journal of Agricultural Food Chemistry. 51(15): 4198–4201.
- Andreu, Y., Marcos, S.D., Castillo, J.R., and Galban, J. (2005). Sensor film for Vitamin C determination based on absorption properties of polyaniline. Talanta. 65(4): 1045–1051.
- Ijeri, V.S., Jaiswal P.V., and Srivastava, A.K. (2001). Chemically modified electrodes based on macrocyclic compounds for determination of Vitamin C by electrocatalytic oxidation. Analitica Chimica Acta.439(2): 291–297.
- Bae, Z., Park, J., Lee S., and Chang, H. (1999). Nickel(II) tetraaza macrocycle modified electrodes for the electrocatalytic determination of L-ascorbic acid by the flow injection method. Journal of Electroanalytical Chemistry468(1): 85–90.
- Ijeri, V.S., Algarra, M., and Martins, A. (2004). Electrocatalytic determination of vitamin C using calixarene modified carbon paste electrodes. Electroanalysis 16:2082–2086.
- Uchiyama, S., Kobayashi, Y., Suzuki, S., and Hamamoto, O. (1991). Selective biocoulometry of vitamin C using dithiothreithol, N-ethylmaleimide, and ascorbate oxidase. Analytical Chemistry. 63(20): 2259–2261.
- Teixeira, M.F.S., Ramos, L.A., Fatibello, O., and Cavalheiro, E.T.G. (2003). Carbon paste electrode modified with copper(II) phosphate immobilized in a polyester resin for voltammetric determination of l -ascorbic acid in pharmaceutical formulations. Analytical and Bioanalytical Chemistry 376(2): 214–219.
- Washko, P.W., Welch, R.W., Dhariwal, K.R., Wang, Y., and Levine, M. (1992). Ascorbic acid and dehydroascorbic acid analyses in biological samples. Analytical Biochemistry. 204(1): 1–14.
- Tulley, R. (1992). New enzymatic method for the analysis of vitamin C in plasma and automation on the Beckman CX5. Clinical Chemistry.38: 1070.
- Okamura, M. (1985). Vitamin C analysis in biological fluids. Katsura, E., Fukui, S., eds., Methods in Vitaminology, Tokyo-Kagaku-Dozin, Tokyo, 4–9 [Japanese].
- Iharaa, H., Matsumotoa, N., Shinoa, Y., Aokia, Y., Hashizume, N., Nanba, S., and Urayama, T. (2000). An automated assay for measuring serum ascorbic acid with use of 4-hydroxy-2,2,6,6-tetramethylpiperidinyloxy, free radical and o-phenylenediamine. Clinica Chimica Acta. 301(1–2): 193–204.
- Dawson, C. R. (1966). The Biochemistry of Copper (Peisach, J., Aisen, P. and Blumberg, W. E., eds.) 305–337, Academic Press, New York.
- Marchesini, A., Cappelletti, P., Canonica, L., Danieli, B., and Tollari, S. (1977). Evidence about the catecholoxidase activity of the enzyme ascorbate oxidase extracted from Cucurbita pepo medullosa. Biochimica et Biophysica Acta,. 484: 290–300.
- Casella, L., Gullotti, M., and Marchesini, A. (1985). The interaction of ascorbate oxidase with L-dopa, L-tyrosine and 3,4-dihydroxycinnamic acid. Evidence for irreversible damage of the enzyme during catechol oxidase activity. Inorganica Chimica Acta. 107: 19–22.
- Wang, X., Watanabe, H., and Uchiyama, S. (2008). Amperometric l-ascorbic acid biosensors equipped with enzyme micelle membrane. Talanta. 75(5): 1681–1685.
- Messina, G.A., Torriero, A.A.J., De Vito, I.E., and Raba, J. (2004). Continuous-flow/stopped-flow system for determination of ascorbic acid using an enzymatic rotating bioreactor. Talanta. 64(4): 1009–1017.
- Kriz, K., Anderlund, M., and Kriz, D. (2001). Real-time detection of L-ascorbic acid and hydrogen peroxide in crude food samples employing a reversed sequential differential measuring technique of the SIRE-technology based biosensor. Biosensors and Bioelectronics. 16(6): 363–369.
- Fernandes, J.C.B., Kubota, L.T., and Oliveira Neto, G. (1999). Potentiometric biosensor for L-ascorbic acid based on ascorbate oxidase of natural source immobilized on ethylene–vinylacetate membrane. Analytica Chimica Acta. 385(1–3): 3–12.
- Volotovsky, V., and Kim, N. (1998). Determination of glucose, ascorbic and citric acids by two-ISFET multienzyme sensor. Sensors and Actuators B: Chemical. 49(3): 253–257.
- Thiagarajan, S., and Chen, S.M. (2007). Preparation and characterization of PtAu hybrid film modified electrodes and their use in simultaneous determination of dopamine, ascorbic acid and uric acid. Talanta.74(2): 212–222.
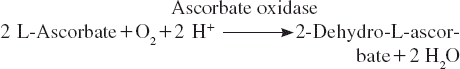
![Figure 3. The effect of glutaraldehit percentage [▴–▴: 1.25 %, –▪–▪–: 2.5 %, –•–•–: 5%. The amount of zucchini tissue homogenate and gelatin were kept constant at 200 μL and 10mg, respectively. Working conditions: Phosphate buffer, 50 mM, pH 7.5, T:35 °C].](/cms/asset/df714627-a9f4-46f5-a896-0fde74b5e409/ianb19_a_479080_f0003_b.gif)