Abstract
Abstract: Nanotechnology has potential in the development of novel and effective delivery of drugs within lungs. Different strategies have been utilized for pulmonary delivery of drugs, including the use of lipid-based delivery systems (liposomes, ISCOMs, SLNs), use of polymeric matrix (PLGA, poly caprolactone, cynoacrylates, gelatin), development of polysaccharide particulates (chitosan, alginates, Carbopol, etc.), biocompatible metallic inorganic particles (iron, gold, zinc), etc. This paper reviews various nanopaticulate approaches in the form of lipids, polymers, metals, polysaccharides, or emulsions based for pulmonary drug delivery that could provide an increased biological efficacy and better local and systemic action.
INTRODUCTION
Lungs offer a large surface area for absorption, rich blood circulation, better permeability, and limited proteolytic activity, which makes it an ideal route for non-invasive administration of therapeutics. Localized delivery shows great promise not only in the treatment of respiratory diseases, such as asthma, tuberculosis, influenza, cystic fibrosis, chronic obstructive pulmonary disease (COPD), etc., but also reduces the systemic toxicity. Alternatively, systemic drug delivery can be achieved by targeting the alveolar region where the drug can be absorbed through a thin layer of epithelial cells and into the systemic circulation. This leads to enhanced permeability, a rapid onset of action, and avoidance of first-pass metabolism. Moreover, recent advances shows immense potential for efficient pulmonary delivery of proteins that cannot be taken orally and require parenteral delivery [Citation1].
In the process of breathing, the lungs are continuously exposed to materials of various sources and sizes, such as pollen (20–90 μm), bacteria (0.2–200 μm), and tobacco smoke (0.01–1 μm). These airborne particles deposit along the respiratory tract from the conducting upper airways (with the oropharynx, trachea, main bronchi, and terminal bronchioles) down to the respiratory region of the lower airways (with respiratory bronchioles and alveolar sacs). In upper airways, particles get rapidly cleared by cilia, where they are swallowed and metabolized. The pulmonary epithelium is thick (50– 60 μm) in the trachea and poses a barrier to absorption. Towards the lower airways, the epithelium of the lung diminishes to a thickness of 0.2 micron at the alveoli; thus, it provides an appropriate platform for the gas to exchange and absorb [Citation2].
MECHANISM OF DEPOSITION OF PARTICLE IN THE LUNGS
Dynamics in the respiratory tract are the basis upon which particle deposition is built. The nature of the dynamics involved in the pulmonary delivery depends on the incompressibility, non-dimensional analysis, flow patterns, turbulence, interaction with mucus, temperature, and the aerodynamic diameter. Particles having a diameter of more than 5μm can deposit by means of inertial impaction in the mouth and upper parts of the respiratory tract. Smaller particles of 1 to 5μm diameter deposit in the deeper areas of the respiratory tract by inertial impaction. The deposition of particles having diameter less than 1 nm occurs by diffusion to alveoli. Particles with a diameter of 1-3μm offer effective deposition [Citation1].
In general, there are no mathematical equations that establish a relationship between particle velocity and its deposition behavior. Thus, determination of particle trajectories, inertia, and diffusion under the synchronized conditions are essential to envisage the distribution pattern of inhaled particles [Citation2]. The deposition of particle in lungs is shown in .
Inertial Impaction
Inertial impaction offers a potential mechanism of deposition for the particles between 2μm to 5μm. Inertial impaction delivers the drug in the complex geometry of the upper respiratory tract and conducting airway bifurcations. Stoke's number explains the probability of the particle to diverge from streamline of carrier gas:
Stk = (ρpd2pu)/(18μd)
Dp and ρp are particle diameter and density
u = μ are the linear velocity and dynamic viscosity of carrier gas.
D is the diameter of the air space
More Stk leads to an efficient deposition of the particle by inertial impaction.
a) Gravitational Sedimentation: Settlement of the particle under the action of the gravity covers the gravitational sedimentation mechanism for the deposition of the particle to the lungs. Particles sized between 0.5μm to 5μm have been settled by this mechanism. An effective deposition by this mechanism occurs in the small airways and the alveoli but the deposition also occurs in the upper respiratory tract. The terminal settling velocity for the spherical particles is expressed by
Vs = (ρpd2pg)/18μ
g is gravitational acceleration
The probability of the particle deposition by this mechanism is proportional to the terminal settling velocity. The probability increases with increases in particle size, density, and increasing residence time.
b) Brownian Diffusion: Deposition by Brownian diffusion occurs by the collisions of the particle with gas molecules. It is a potential mechanism of the deposition for the particles having less than 0.5μm diameter and in the acinar region of the lungs. The particles below 0.01μm are effective in nose, mouth, and pharyngeal airways. The diffusion rate is proportional to Brownian diffusion coefficient
Db = (ckT)/(3π μdp)
k is Boltzmann's constant
T is absolute temperature
c = Cunningham Correction factor
c) Electrostatic Precipitation: Electrostatic precipitation results when charged particles approaches oppositely charge surfaces onto airways.
d) Interception: Interception acts as a potential mechanism of the particle deposition in small airways and alveoli. Interception involves the deposition of an elongated particle in the gas space while one of its ends touches the alveolar wall under the influence of gravity.
PARTICLE CLEARANCE
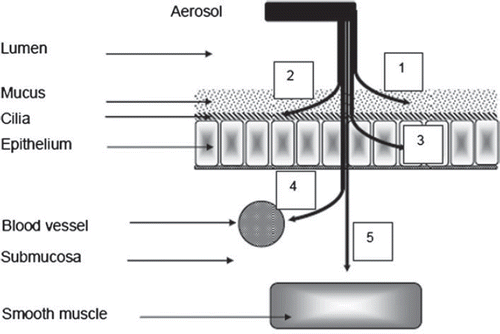
Elimination of inhaled particulate compounds deposited in the respiratory tract is necessary for the clearance. Clearance mechanisms are different in different regions of the respiratory tract and also depend on the physicochemical characteristics of deposited material. The particle clearance from the respiratory tract includes the clearance from nasopharyngeal, tracheo-bronchial, and pulmonary compartments. Clearance from the nasopharyngeal compartment includes the mucociliary and mechanical clearance with absorption into the circulation. The trachea-bronchial clearance includes mucociliary clearance, endocytosis into the peribronchial region, and absorption into circulation. Clearance from the pulmonary compartment occurs by alveolar macrophage-mediated clearance, endocytosis by lung epithelial cells into the interstitum, and absorption into circulation (). Factors like age, exercise, influenza, and pneumonia significantly affect the clearance rate of deposited particles (). The rate of clearance decreases with age while exercise increases the rate of clearance. Clearance may be delayed up to three months to one year in cases of influenza and pneumonia.
Figure 2. Fate of aerosolised drugs delivered to the lungs (1-Interaction with mucus layer; 2-Removed by mucociliary escalator; 3-Have limited access via epithelium and be complexed/biotransformed by epithelium-associated components; &/or enzymatic digestion; 4-Diffused into submucosal blood vessels before reaching its target; 5-The target cell, airway smooth muscle cell).
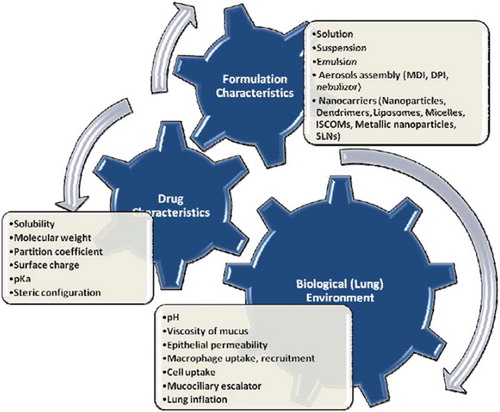
FACTORS AFFECTING DRUG BIOAVAILABILITY VIA PULMONARY
Fate and bioavailability of administered drug via pulmonary depend on a multitude of factors. Various physiochemical, biological, and formulation parameters have played an important role in the absorption of drugs through the lungs (). Diffusion and absorption of drugs from the lungs’ interface depends on the molecule's lipid solubility, size, degree of ionization, and the area of absorptive surface. It is likely that lipid-soluble drugs will diffuse more rapidly due to the lipoidal nature of the cell membrane. Small molecules tend to penetrate membranes more rapidly than larger ones. Most drugs are weak organic acids or bases, existing in un-ionized and ionized forms in an aqueous environment. The un-ionized form is usually lipid soluble (lipophilic) and diffuses readily across cell membranes. The ionized form has low lipid solubility (but high water solubility—i.e., hydrophilic) and high electrical resistance and thus cannot penetrate cell membranes easily. The proportion of the un-ionized form present (and thus the drug's ability to cross a membrane) is determined by the pH and the drug's pKa (acid dissociation constant). At the physiological pH (7.4), drugs with lower pH have more uncharged molecules, which are free to cross the alveolar mucosal membranes and producing faster absorption than with higher pKa drugs [Citation3–6]. Lipophilic basic amines, such as imipramine, propranolol, lidocaine, fentanyl, and verapamil, have been reported to have an extensive first-pass pulmonary uptake and to partition into pulmonary tissue [Citation7–11].
Alveolar mucosal membrane is highly vascular, permitting the drug to enter the systemic circulation rapidly and to reach its target organ with minimal delay. Drugs may also be administered directly into the target organ, resulting in site-specific therapeutic action of drugs. This is important for the delivery of bronchodilators, anti-inflammatory agents, mucolytics, antiviral agents, anticancer agents, and phospholipid-protein mixtures in critical and chronic conditions like asthma, bronchiectasis, tuberculosis, cystic fibrosis, and others. Further, particle size of drugs has also affected the absorption, dissolution, and deposition of drugs in the epithelial lining fluid. Smaller nanosize particles will show better bioavailability, faster dissolution, and different deposition patterns compared to larger-size particles [Citation4,Citation6]. Many studies have shown that 0.5 and 5 microm mass median aerodynamic diameters (MMAD) have efficiently deposited particles within the lungs. Further, nanoparticles, slow inhalation, method and condition during administration will be decisive factors for deeper deposition of particles in lungs [Citation12]. Recently, 5- fluorouracil drug particles have been prepared by a supercritical antisolvent process and demonstrated regular shape with nano size (avg. 248 nm), smooth surfaces with a high surface area. Developed nanoparticles had shown better aerodynamic performance in presence of lactose [Citation13]. Zeman et al. have reported that deposition of inhaled therapeutic drugs depends on both the particles’ aerodynamic size and the patient's breathing pattern. Results indicated that the conducting airway deposition of large particles (9.5-μm MMAD) and smaller particles (5-μm MMAD) was 35% (± 8%) and 27% (± 11%), respectively [Citation14]. Similarly, high azithromycin loading powders has been prepared by spray-drying techniques for pulmonary adminstration. Results indicated that optimized powder had better flowability, aerodynamic diameter (3.82 microm), and in vitro deposition (51%). Studies revealed that developed powder had better drug targeting index via intratracheal route than intravenous injection [Citation15].
Various physiological factors can affect the absorption and deposition of drugs, such as lung surface, lungs morphology, airway caliber, lungs surfactant, mucus, breathing pattern, inhalation time, and disease status. The lung has a large, highly perfused surface area and is larger than the surface of a person's skin. Inhalation of highly diffusive agents may lead to undesirable systemic spillover, bioavailability, and pharmacokinetics, which essentially mirror the intravenous infusion in such cases. Further studies have demonstrated that efficient absorption and lower metabolism of small molecular drugs through lungs compared oral delivery (gastric environment and fast pass metabolism) [Citation6, Citation16].
Various physiological mechanisms have demonstrated the absorption of drugs via the lung such as active transport, passive transport, paracellular, transcellular, receptor mediated endocytosis, and transcytosis pathways. Studies revealed that sugar, proteins, and peptides follow paracellular, transcellular, endocytosis and transcytosis pathways, while lipids and active drugs follows receptor-mediated endocytosis and pinocytosis pathways. Further studies suggested that small peptides (i.e., 40 kDa) paracellular transport may dominate, while for larger proteins (i.e., 40 kDa) transcytosis may be more important. There is some evidence for receptor-mediated transcytosis of albumin. Generally, large macromolecules are nonspecifically absorbed by airspace using a combination of transcytosis and endocytosis transport [Citation6, Citation17].
Mucociliary clearance has also been a major constraint for pulmonary absorption of drugs. The volumes and composition of epithelial lining fluid of alveolar epithelial cells are also contributory factors for absorption of drugs. This fluid is, in turn, covered by a monolayer of lung surfactant. The epithelial cell layer forms the major barrier to absorption of drug molecules. Alveolar epithelium plays an important role in absorption of inhaled drugs within lungs. The absorption of drugs from mucus barriers is mainly dependent on particle charge, solubility, lipophilicity, and size [Citation18, Citation19]. The caliber of the airway through which the aerosol passes to reach the more distal parts of the lungs is very vital. Airway caliber may alter lung deposition and bioavailability of inhaled drugs as a result of narrowed airways reducing peripheral drug delivery. The baseline airway caliber significantly alters the early lung absorption profile of salbutamol in patients with severe asthma [Citation20]. Airway obstruction is a respiratory problem that reduces the amount of drug absorption due to the deposition is more in the central large airways rather than the peripheral regions comprising of small airways and alveoli. Under normal breathing conditions, submicronic particles are exhaled. However, particles of ultrafine sizes (0.01 μm) can be deposited via diffusion. Holding of breath or slowing of respiration rate increases the dwell time, decreases turbulent flow, and permits more time for these particles to deposit by sedimentation and diffusion [Citation21].
Deposition of inhaled aerosol particles can be affected by types of drug formulation and method of administration. Droplet size, shape of particles, velocity of particles, and type of particulate systems have affected the bioavailability and distribution of drugs within lungs. Further, novel colloidal carriers can be employed to control the duration of local or systemic drug activity by modulating the solute-release characteristics. Pulmonary delivery of pharmaceuticals can be achieved by powder formulation using dry powder inhalers, aqueous formulations for nebulizers, or solvent formulations for meter dose inhalers [Citation6,Citation22]. These devices require mechanical energy for the generation of aerosols particulates like micronization of powder in DPIs, breakdown of liquid droplet in nebulizers, and evaporation of dispersing solvent in MDIs [Citation23]. Major challenges confronted in pulmonary drug delivery are the poor efficacy of conventional device to deliver dry powder of small molecules, peptide and protein to the lower parts of respiratory tract. Low inertia particles get exhaled and large macromolecules drugs may yield poor bioavailability as they get deposited on the oropharynx and larynx. Pulmonary drug delivery shows improper dosing reproducibility and it is often difficult to adjust dose as per patient need [Citation24].
DEVICES FOR PULMONARY DELIVERY
Appropriate devices are used to deliver the drug to the lungs effectively (). Selection of devices depends upon physicochemical and dosing demands associated with the drug substances to be inhaled. A variety of technology is under development to overcome problems associated with conventional aerosol device intended for dry powder inhalation system and liquid inhalation system. The aerosol devices used to deliver the drug substance to lung are as follows.
Table 1. Advantages and disadvantages of aerosol devices.
Jet Nebulizers
Jet nebulizers are the most common type of inhalation device. These are the subset of the more general twin fluid atomizers. The operating procedure includes a pressurized air source that supplies high-pressure air, which flows through a nozzle where the air acceleration takes place. The pressure near the nozzle is designed so that high-speed air flows over a short section of the liquid surface supplied by the liquid feed tube. This is the site where the production of liquid droplet takes place.
The governing parameters are geometric parameters like nozzle shape, dynamic non-dimensional parameters like Reynold's number in gas jet (Reg = Uρgdg/μg), Reynold's number in liquid surrounding gas jet (Rel = Udlρl/μl), Mach number in gas jet (Mag = U/c), Gas Weber number = (Weg = ρgU2dg/σ), viscosity ratio (μg/μl), density ratio(ρg/ρl)
where v is velocity
ρ is density
c is speed of sound in gas
μ is viscosity
U is velocity of air-water interface
σ is stress
The primary determinants of both efficiency and output rate are the ability of the nebulizer to return droplets to nebulizer reservoir for renebulization. A major determinant of the output rate in a jet nebulizer is the rate at which primary droplets are created, which increases with increase in the flow rate of liquid into droplet production site with all other variables unchanged [Citation2].
Dry Powder Inhalers
The purpose of DPI is to insert a prescribed dose of powder aerosol into air inhaled by the patient during a single breath while the powder is prevented from being exposed to ambient air until the patient is ready to inhale. Coughing might be induced by inhalation of large amounts of powder; total amounts of inhaled powder are usually less than 10-20 mg. The powder to be inhaled from DPI must be swept up by the air being inhaled, a process known as “fluidization.” The mechanics of dry powder inhalers are complex for irregularly shaped and rough particles.
Most DPI formulations consist of micronized drug blended with large carrier particles which enhance flow, reduce aggregation, and aid in dispersion. A combination of intrinsic physicochemical properties, size, shape, area, and morphology affects forces of interaction and aerodynamic properties which determine fluidization, dispersion, and delivery to lungs and deposition in peripheral airways. On actuation, the formulation gets fluidized and enters the airway. The drug separates from the carrier and gets carried deep into the lungs. The larger carrier particles impact on the oropharyngeal surfaces and get cleared. If cohesive forces are strong, shear does not separate carrier from drug and thus results in low deposition efficiency [Citation2].
Metered Dose Inhalers
The mechanics of MDI involve a transient, cavitation turbulent fluid that flashes into rapidly evaporating droplets. MDIs remain a standard aerosolized delivery system which can produce an aerodynamic particle size of less than 5 microns with HFA propellants. HFA-propelled MDIs are easily portable, tamper-proof, and multidose. They protect the remaining product from oxidation, light, and moisture, while providing a simple and economical technology with accurate liquid actuation by volume. The aerosol drug dose exits the MDI mouthpiece as a rapidly moving large droplet cloud. However, at about the distance of the back of the throat, the droplet diameter is reduced due to propellant evaporation, and a reasonable proportion of the “polydispersed” aerosol cloud is now small enough to penetrate the lung. A proportion of each “metered dose” is lost in the actuator mouthpiece, and a further proportion is lost in the oropharynx due to inertial impaction of the “ballistic portion” of the spray [Citation25]. demonstrates the operation of metered dose inhalers.
NANOPARTICLES FOR PULMONARY DELIVERY
Different strategies have been developed and patented to facilitate and enhance the mucosal pulmonary absorption for localized and/or systemic effects, including the use of lipid-based delivery systems (i.e., liposomes, chochleated, immune stimulating complexes, solid lipid nanoparticles), entrapment/encapsulation of drugs into polymeric matrix (poly(lactide-co-glycolide), chitosan, alginates, carbopol, gelatin, etc.), admixing of drugs with preformed nanoparticles (metals, sugars), and use of colloidal carriers like suspension and emulsion. An overview of different nanotechnological approaches is shown in .
LIPID-BASED NANOPARTICULATE DELIVERY SYSTEM
Solid Lipid Nanoparticles
Solid lipid nanoparticles are submicron-sized nano-carriers made up of lipids. These consist of phospholipid coating associated with a solid hydrophobic core of high melting point with the material to be encapsulated. They are structurally different form other lipid-based vesicular systems as they consist of a solid hydrophobic core having a monolayer of phospholipid coating. SLNs have acted as a potential carrier of therapeutic agents for systemic or localized delivery. Many approaches on SLNs have been utilized for lung delivery. Many studies investigated the potential of SLNs as a suitable pulmonary delivery system using human alveolar epithelial cell line (A549) and murine precision-cut lung slices (PCLS). Incubation of these cell lines with SLN20 (20% phospholipids in the lipid matrix of the particles) and SLN50 (50% phospholipids in the lipid matrix of the particles) has demonstrated release of LDH that proved the suitability of SLNs-20 as drug delivery systems for the lung [Citation26]. Further, experiments have shown the toxicity of SLNs. SLNs (200 microg/ml) induced no significant signs of inflammation [Citation27].
Dexamethasone acetate (DXM) loaded SLNs have been administered by intravenous route for lung-targeting. Studies indicated that SLNs could have been used for lung-targeting for lipophillic drugs such as DXM [Citation28]. However, pulmonary/aerosol delivery of particulate carriers has been the most promising, non-invasive approach for pulmonary delivery of the drugs. SLNs have been utilized for pulmonary delivery of insulin and demonstrated the influence of sodium cholate and soybean phosphatidylcholine on the deposition of the nanoparticles into the lungs. Results indicated that SLNs act as a potential carrier for pulmonary delivery of insulin by improving both in vitro and in vivo stability as well as prolonging hypoglycemic effect [Citation29]. Similarly, SLNs has also been prepared by ultra-rapid freezing technique using itraconazole (ITZ): mannitol: lecithin with the excipients. ITZ-loaded SLNs colloidal dispersion was suitable for nebulization with aerodynamic properties for deep lung delivery and high lung and systemic levels. Studies revealed that bioavailability of ITZ-loaded SLNs was enhanced by decrease in particle size that accelerated dissolution and permeation at the site of administration [Citation30]. Further, several modifications have been made in SLNs to improve the stability, selective uptake, and bioavailability of encapsulated drugs. Pegylation of SLN can modify surface properties very easily and exhibited enhanced bioavailability. A mannan-based PE-grafted ligand has been used for the surface modification of DNA-loaded cationic SLN, which improved cell targeting ability. Developed ligand anchored cationic SLNs have efficiently bound DNA directly via ionic interaction and mediate site-specific gene transfection on alveolar macrophages. Studies demonstrated that non-modified SLN-DNA and Lipofectamine 2000-DNA produced fewer gene expressions than ligand-anchored SLNs [Citation31]. Microparticles containing SLNs have been used as carriers for the lung delivery of the drugs. Microparticles containing SLNs were prepared by a co-spray-drying technique using thymopentin-loaded SLNs with bulking agents for pulmonary drug delivery. Developed hybrid microparticles were spherical and hollow microparticles with high aerosolization efficiency. The biodistribution studies of pulmonary administered microparticles have confirmed strengthened bioavailability and therapeutic efficacy compared to i.v. TP5 solution [Citation32].
Liposomes
Liposomes are vesicular carriers having one or more closed, concentric lipid bilayers alternating with aqueous compartments. Drugs encapsulated within the liposome provide an extended therapeutic response due to the depot action. They have an ability to incorporate both water-soluble and lipid-soluble molecules in their aqueous and lipid phases, respectively. However, hydrophobic compound-loaded liposomes have shown better retention and deposition, and are effective for aerosol delivery to the lungs [Citation33]. Although soluble drugs rapidly clear from the lungs, 50 to 60% of liposomes are retained in the lungs for up to 24 hrs after the inhalation. The residence time of liposomal drug preparation depends on location of the lung deposition [Citation34]. Moreover, inhalation of drug- liposomal delivery enhances intracellular delivery including nucleic acids for gene delivery. Liposomes for inhalation serve as a solubilization matrix for poorly soluble drugs and thus prevent local therapeutic drug levels, generating high intracellular drug concentration. These features make liposomes an ideal carrier for cytosolic drug delivery, intended in viral infection and gene delivery. Some of the examples of liposomal formulations used in delivery to the lungs include insulin, catalase, interleukin 2, budesonide, 9 NC, rifampicin, polyethleneimine-p 53 DNA, etc. [Citation35].
Rifampicin-loaded liposomes have been prepared by soy lecithin for passive targeting to alveolar macrophages, as nebulized liposomes effectively target antibacterial agents to macrophages. The obtained results showed that rifampicin could be loaded effectively in soy lecithin vesicles only when cholesterol was absent in the film preparation. The study indicated that nebulization properties and viscosity of formulations were affected by using low-transition-temperature phospholipids. All formulations showed a good stability during nebulization and the ability to retain more than 65% of the incorporated drug, and could be suitable for aerosol delivery of drugs [Citation36]. Similarly, liposomal cyclosporine solution, administered by inhalation in the doses of 10 and 20 mg with the inhaler by 12 stable lung transplant recipients, and lung deposition were evaluated by gamma scintigraphy. Lung deposition and peripheral lung deposition increased significantly with time. The maximum cyclosporine blood concentration and the area under the time course of blood concentration have been correlated with peripheral lung deposition. The treatment showed good tolerance and no drug-related side effects [Citation37]. Investigation of the inhaled liposomes of amikacin on pulmonary deposition, clearance, and safety following nebulization found that deposition to be 32.3 +/−3.4%, consistent clearance and well tolerated safety [Citation38]. The concentration of Amphotericin B in the respiratory tract and serum of lung transplant patients post-inhalation of liposomal preparation has been assessed. No significant systemic absorption of amphotericin B was detected and no effect was observed on respiratory function [Citation39].
The inhalable liposomal systems comprised of paclitaxel and 2,5,7,8-Tetramethyl-2R-(4R, 8R-12-trimethyltridecyl) chroman-6-yloxyacetic acid (alpha-TEA) analog of vitamin E has been prepared to increase anti-tumor efficacy and minimize paclitaxel toxicity. Combination treatment reduced lung and lymph node micrometastatic foci when compared to control and individual treatment groups (P < 0.05). The sequential inhalation delivery of liposomal-formulated paclitaxel and alpha-TEA produced a better anti-tumor outcome than single treatments [Citation40]. Similarly, fullerene-paclitaxel conjugate has been encapsulated in aerosolic liposomes that sustained the release of drugs for lung cancer therapy [Citation41]. Cationic liposomal formulations of low molecular weight heparin, prepared by the hydration method using 1,2-dioleoyl-3-trimethylammonium-propane (chloride salt), cholesterol and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethyleneglycol)- 2000]. Studies revealed that half-life of the cationic liposomal formulation was 2.2-fold increased compared to low molecular weight heparin formulated in saline. Cationic liposomal formulations administered via the pulmonary route 6h prior to embolization in the lungs resulted in thrombolytic effect. Thus, pegylated cationic liposomes can carry inhalable formulations of low molecular weight heprin, an anionic drug [Citation42].
POLYMER-BASED NANOPARTICULATE DELIVERY SYSTEM
Polymeric nanoparticles are colloidal carriers ranging in size from 10-1000 nm. The smaller size helps in targeting and maintaining the encapsulated particles. It represents an attractive means of delivering the proteins. The polymer material tends to transform the physicochemical characteristics such as the drug release properties, zeta potential, and hydrophobicity. In the case of vaccines, the formulation of the particulate antigen delivery system is the choice of the polymer which must be safe, biocompatible, and biodegradable for use. The biodegradability is necessary for the release of the antigen and to avoid the surgical step for the recovery of the depleted system. Various types of the polymers used for the formulation of the nanoparticles are chitosan, alginates, PLGA, and PLA. The natural polymers like chitosan, gelatin, albumin and sodium alginate avoid the toxicological problems associated with the use of the synthetic polymers [Citation43].
Poly(Lactide-co-glycolic Acid)
PLGA has been used in the preparation of sustained release products due to its excellent biocompatibility, biodegradability, and non-toxicity. Inhalation of the nanoparticles containing PLGA/Rifampicin with mannitol has shown good aerosol performance. Developed nanoparticles are easily recognized by the alveolar macrophages and exhibited increased uptake at alveolar region of lungs [Citation44]. The PLGA and Poly (vinysulfonate–co vinyl alcohol) encapsulated salbutamol was prepared by modified solvent displacement technique with the particle size of 120 nm. The nebulized salbutamol PLGA nanoparticles showed significant increase in the mean particle size and the polydispersibility [Citation45]. Rifampicin-loaded PLGA microspheres for lung delivery via an aerosol using premix membrane homogenization have also been demonstrated [Citation46]. The preparation of simultaneously manufactured Nano-In-Micro (SIMANIM) particles for the pulmonary delivery of antibodies has been demonstated by the spray-drying of a double-emulsion containing human IgG (as a model antibody), lactose, poly(lactide-co-glycolide) (PLGA), and dipalmitoylphosphatidylcholine (DPPC) [Citation47]. Antibody release was observed for 35 days in a release medium (pH 2.5). Released antibody was found to be stable and active, which was confirmed by gel electrophoresis, field-flow fractionation, and enzyme-linked immunosorbent assay.
The combined delivery of antitubercular drugs (ATDs), i.e. isoniazid, rifampicin, pyrazinamide, in PLGA nanoparticles has shown potential for pulmonary delivery of drugs. The absolute bioavailability of PLGA nanoparticles encapsulated ATDs was increased by 6.5 for rifampicin, 19.1 for isoniazid and pyrazinamide. Enhanced lung residence and deposition have been demonstrated via large porous particles (approximately 10-15 micron) due to large diameter and reduced clearance by macrophages in comparison to small microparticles (approximately 1-5 microm) [Citation48].
Large, porous, hollow PLGA particles have also been prepared using oils as extractable porogens. Developed large porous PLGA particles exhibited a low density and a web-like or hollow interior depending on porogen concentration and type, respectively. Further, encapsulation of ciprofloxacin nanoparticles in large porous PLGA particles has shown a steady release of ciprofloxacin that was extended for larger particle diameters and for the solid particle morphology in comparison to large porous particles [Citation49]. Similarly, insulin-loaded large porous PLGA particles were prepared for pulmonary delivery. Large porous PLGA particles were prepared by a double emulsion method using hydroxypropyl-beta-cyclodextrin (HPbetaCD). In vivo data has shown that large porous PLGA particles reached alveoli, released insulin, which was then absorbed in its bioactive form [Citation50].
Surface-modified PLGA nanospheres with chitosan have been prepared for the pulmonary delivery of peptides, i.e., elcatonin. Chitosan-modified PLGA nanospheres showed slow elimination from the lungs than unmodified PLGA nanospheres and reduced blood calcium levels to 80% of the initial calcium concentration and prolonged the pharmacological action up to 24 hrs. These results were attributed to the retention of nanospheres due to their adherence to the bronchial mucus and lung tissue and sustained drug release at the adherence site [Citation51]. T. Niwa et al. developed PLGA nanospheres with nafarelin acetate (NA) and investigated improved aerosolization of nanospheres for pulmonary delivery of the peptide-drug [Citation52]. Copolymer of chitosan with PLGA has also been synthesized and utilized for pulmonary delivery of polymeric nanocarriers (NCs) pressurized-metered dose inhalers (pMDIs). Nanoparticles were prepared by modified emulsification-diffusion methodology, consisting of core-shell particles of a biodegradable hydrophilic fluoroalkane (HFA) copolymer of chitosan and poly(lactic acid), and a core of poly(d,l-lactide-co-glycolide) (PLGA). The report suggested that the methodology delivers polymeric nanoparticles to the respiratory tract using the inexpensive pMDIs, and targets and delivers drugs to treat chlamydial-related infections [Citation53].
Cynoacrylate
PCL is a biodegradable and nontoxic polyester synthesized by ring-opening polymerization. It has been used clinically as a degradable staple for wound closure and as a one-year drug delivery system for contraceptives. The preparation of effervescent dry powder containing poly butyl cyanoacrylate nanoparticles and ciprofloxacin for pulmonary delivery has been reported by spray drying [Citation54]. The evolution of gas bubbles occurs with aerodynamic diameter of 2.17μ +/−0.42, fine particle fraction of 46.47% +/−15, and GSD being 2 +/−0.06. The effervescent carrier showed the release of 56% +/−8% ciprofloxacin as compared to 32 +/−3% of lactose carrier [Citation54]. An aerosolic application of cyanoacrylate adhesive has been demonstrated to treat corneal perforations and stromal melting disorders [Citation55]. The preparation of insulin nanoparticles of poly(n-butyl cyanoacrylate) and dextran as stabilizing agent with particles sized at 255nm by an emulsion polymerization process have also been done [Citation56]. The activation of alveolar macrophages by polycyanoaacrylate on H460 lung cancer cells has also been seen [Citation57]. The experiment showed alveolar macrophages exposed to blank or DOX-loaded nanoparticles that showed cytotoxicity against cells after 8 and 24 hours.
Polylactic Acid
Poly(lactic acid) or polylactide (PLA) is a thermoplastic aliphatic polyester derived from renewable resources, such as corn starch (in the United States), tapioca products (roots, chips, or starch, mostly in Asia), or sugarcanes (in the rest of world). It can biodegrade under certain conditions, such as in the presence of oxygen, and is difficult to recycle. PLA is currently used in a number of biomedical applications, such as sutures, stents, dialysis media, and drug delivery devices.
Safety and tolerability studies of different charged nanoparticles indicated that the cationic stearylamine-based PEG-PLA nanoparticles showed increased local and systemic toxicity. The observations suggested that anionic PEG-PLA nanoparticles produce low toxicity and proved to be better drug carriers for therapeutics to lungs [Citation58].
A spray-dried formulation of isoniazid, rifabutin, and PLA has been reported [Citation59]. Microparticles with drug contact of 50% w/w and particle size of 5 microns demonstrated satisfactory aerosol characteristics. The solution upon inhalation was found to have 20 times higher drug concentration in macrophages than others. Naproxen-polylactic acid particles were formed by pulsed rapid expansion of supercritical CO2 solutions (RESS). It was demonstrated by scanning electron microscopy and powder diffraction that the coating of polylactic acid stabilized the particles of naproxen against agglomeration and coagulation [Citation60].
POLYSACCHARIDE-BASED NANOPARTICULATE DELIVERY SYSTEM
Chitosan
Chitosan is the mucoadhesive cationic polysaccharide that is the copolymer of the alpha glucosamine and N-acetyl-d-glucosamine. It is the most abundant biopolymer after cellulose, is made up of deacetylation of chitin, and is cheap, non-toxic, biodegradable, and biocompatible. It is generally used for the mucosal delivery of biomolecules due to its property of bioadhesiveness and high protein-binding efficiency. The use of chitosan for the administration of proteins to the lungs by the aerosol system has been observed and evaluated in many of the studies. The densities of non-crosslinked and glutraldehyde-crosslinked chitosan microspheres closely matched to the HFA propellant and found non-crosslinked and glutraldehyde chitosan to be potential carriers of the protein molecules to the lungs [Citation61]. The production of chitosan nanoparticles has been demonstrated having a size range from 50-300 nm with small size dispersion [Citation62]. The nanoparticle size is dependent on the concentration of the dissolved polymer and on the size of the aerosol droplets. Chitosan was included in the formulation as a potential protective agent for the delivery of lactase dehydrogenase to the lungs using a nebulizer and thus chitosan was considered to be an essential excipient in the preparation of the stable formulations of the proteins for jet nebulization [Citation63]. Chitosan/tripolyphosphate nanoparticles have promoted peptide absorption across mucosal surfaces to microencapsulate protein. Chitosan nanoparticles using mannitol and lactose have demonstrated better protein loading capacity (65-80%) and instant release of 75-80% insulin within 15 min [Citation64]. A study has demonstrated that the whole inactivated influenza virus (WIV) coated with N,N,N-trimethyl chitosan (TMC) improved the delivery and immunogenicity of the aerosol of WIV and enabled effective vaccination against influenza [Citation65]. Recently, imidazole ring-containing urocanic acid-modified chitosan (UAC) has been designed for the delivery of genetic material into the lungs. The chitosan-based aerosols of UAC-PTEN (chromosome 10) were delivered by inhalation systems into K-ras(LA1) lung cancer model mice for 4 weeks (twice in a week). The results suggested that aerosol delivery of UAC-PTEN was compatible with noninvasive in vivo gene therapy [Citation66].
Development of respirable micellar carriers for delivery of amphotericin B by jet nebulization has been reported using chitosan-stearic acid conjugated nanomicelles for encapsulation of amphotericin B. Developed formulations have demonstrated increased antifungal activity of the drug and nebulization efficiency up to 56 % [Citation67]. Similarly, highly respirable spray-dried chitosan powders have been produced using aqueous ethanol (30% v/v), chitosan, and leucine that displayed sustained release of two chemically distinct therapeutic agents that contained hydrophilic (terbutaline sulphate) and hydrophobic (beclometasone dipropionate) model drugs. Sustained drug release profiles were observed in dissolution tests for both agents: increased chitosan molecular weight associated with increased duration of drug release. The controlled co-delivery of hydrophilic and hydrophobic entities underlined the capability of spray drying to produce respirable particles with sustained release to the lung [Citation68]. The efficacy of Budesonide microparticles prepared by spray-drying technology was evaluated using a drug to chitosan ratio of 1:2. Results from IVIVC studies were comparable with conventional Budesonide DPI formulations [Citation69].
Alginates
Alginates are mucoadhesive non-immunogenic natural polymers. They consist of 1,4 linked-D-mannuronic acid, α-L-guluronic acid, and the alternating blocks. They gel in the presence of counter ions such as Ca ++, which is applied for cell immobilization, delivering drugs and antigens due to non-toxicity and biodegradability.
Aliginate nanoparticles have been prepared for combined delivery of antitubercular drugs such as isoniazid (INH), rifampicin (RIF), pyrazinamide (PZA). Developed systems have demonstrated high drug encapsulation efficiency within the respirable range. The relative bioavailabilities of the drugs in alginate were significantly higher compared with oral free drugs. Studies demonstrated that efficacy of 3 doses of alginates loaded nanoparticles nebulizers in 15 days is comparable to 45 daily doses of oral free drugs considering inhalable alginate nanoparticles to serve as an ideal carrier for controlled release of anti-tuberculosis drugs [Citation70] .
Paclitaxel-loaded alginate microparticles have been developed for site-specific pulmonary delivery of drugs to mucosal tissues. Results show that exposure of cells to pure paclitaxel and paclitaxel-loaded microparticles effectively inhibited the growth of A549 and Calu-6 cells similarly in a concentration- and time-dependent manner [Citation71]. Recently, self-assembled N-phthaloyl chitosan nanoparticles encapsulated in respirable/swellable sodium alginate semi-IPN microspheres have been developed for controlled release of drugs at mucosal sites. The developed microspheres have suitable aerodynamic diameters and an excellent fine particle fraction and also showed initial fast swelling and enzymatic degradation. Moreover, the microspheres entrapped up to 90% of the model drug and exhibited sustained in vitro sustained profiles of the entrapped drugs as compared to the control formulation [Citation72].
Hyaluronic Acid
Hyaluronic acid (HA) is a polyanionic nonsulfated polysaccharide that consists of N-acetyl-D-glucosamine and beta-glucoronic acid. HA is distributed widely in vertebrates and presents as a component of the cell coat of many strains of bacteria. One of the chief components of the extracellular matrix, hyaluronan, contributes significantly to cell proliferation and migration, and may also be involved in the progression of some malignant tumors. Due to its high biocompatibility, mucoadhesitivity, and its common presence in the extracellular matrix of tissues, hyaluronan is gaining popularity in drug delivery systems. Studies have demonstrated that hyaluronic acid with the lowest molecular weight (202 kD) exhibited better penetration enhancement properties compared with chitosan hydrochloride. In particular, a number of research groups have found hyaluronan's properties useful for cosmetic, medical, and pharmaceutical purposes [Citation73,Citation74].
Ofloxacin-loaded hyaluronic acid microspheres have been prepared by spray-drying techniques for delivery of ofloxacin to the lung and alveolar macrophages. Studies have suggested that the most efficient delivery of ofloxacin to the lung is feasible via hyaluronic acid. Moreover, in vitro uptake of ofloxacin from hyaluronic acid microspheres by air-surface cultured alveolar macrophages (RAW 264.7) was aprox. 2-fold higher than other formulations [Citation75]. Spray-dried particles of hyaluronic acid (HA) and recombinant human insulin have also been developed for pulmonary delivery. Further, release kinetics was modified by addition of excess zinc ions or hydroxypropyl cellulose. Studies demonstrate the potential of HA-based dry powder drug delivery systems in the pulmonary controlled release of insulin [Citation76]. Similarly, Morimoto et al., 2001, have demonstrated the potential of sodium hyaluronate (solution) for pulmonary delivery of rh-insulin. Hyaluronate (2140 kDa) solutions (0.1% and 0.2% w/v) at pH 7.0 significantly enhance the pharmacological availability of insulin compared to the aqueous solution of insulin at pH 7.0 [Citation77].
Novel hybrid nanoparticles comprised of hyaluronic acid (HA) and iron oxide have been developed. The iron oxide (Fe2O3) particles were electrostatically complexed with hyaluronic acid and formed a corral-like structure. Results show that iron complex hyaluronic acid particles can be efficiently utilized for delivery of peptides [Citation78]. Low-density porous microparticles of hyaluronate were prepared using positively charged lysozyme, negative-charged hyaluronate, and cyclodextrin derivative as a porogen. The interaction of lysozyme and hyaluronate not only increased protein encapsulation efficiency but also stabilized lysozyme against a denaturing organic solvent. Results suggested that porous microparticles may be applied in long-term pulmonary administration of protein or peptide drugs, especially in deeper lung epithelium [Citation79].
Carboxymethylcellulose
Carboxy Methyl Cellulose (CMC) is an anionic, biodegradable, and linear polymer cellulose ether. Carboxymethyl cellulose is a cellulose derivative with carboxymethyl groups (-CH2-COOH) bound to some of the hydroxyl groups of the glucopyranose monomers that make up the cellulose backbone. It is one of the most versatile of water-soluble hydrocolloids and has a number of important properties including solubility, rheology, adsorption on surfaces, etc. CMC is pseudoplastic by nature and can show thixotropic and essentially non-thixotropic rheology. Besides controlling the rheology, CMC is also known to be an excellent excipient for spray-dried formulation. Similarly, many studies demonstrated that addition of CMC in the aerosol particulate formulations can increase the permeation, transfection, and internalization of therapeutics within the mucosal cells and retard the release of loaded drugs/biomolecules. Sodium carboxymethylcellulose has been used as a spray-drying excipient in the preparation of inhalable formulations of proteins. Studies demonstrated that co-spray-drying proteins and peptides with NaCMC may therefore offer an alternative method for the preparation of stable and respirable pMDI formulations for pulmonary delivery [Citation80]. Further, studies have also demonstrated the role of methylcellulose in the inhibition of mucocilliary clearance that can increase the lipid mediated gene transfer. The effect of CMC in lung gene transfer was assessed by nebulization of 0.5% CMC 1h immediately before, or simultaneous with, the plasmid encode gene (GL67A/pCIKLux). Results indicated that the former did not increase gene transfer, whereas co-administration significantly increased gene transfer by 4-fold. Thus CMC could be utilized for better uptake and expression of the desired gene via pulmonary route within the lungs [Citation81]. The effect of CMC in the physicochemical parameters of branched cationic polyester, [diethylaminopropyl amine-poly(vinyl alcohol)-grafted-poly(lactide-co-glycolide) (DEAPA-PVAL-g-PLGA)] nanoparticles has been demonstrated. Studies indicated that CMC-based anionic nanoparticles showed the highest stability during nebulization and retard the degradation rate of the formulation. However, cell interaction studies indicated that cationic nanoparticles demonstrated higher association and low internalization while anion nanoparticles show lower association and higher internalization by A549 cells [Citation82]. Recently, the protein stabilization property of CMC has been evaluated. Studies indicated that co-spray-drying a model protein with sodium carboxymethylcellulose protects protein integrity during spray-drying, and that the resultant spray-dried powders can be utilized for developing an aerosol formulation that exhibits high stability of encapsulated protein and respirable fractions [Citation83].
Cyclodextrin
Cyclodextrins are cyclic polymers of alpha-D-glucopyranose made up of sugar molecules bound together in a ring (cyclic oligosaccharides). Cyclodextrins are able to form host-guest complexes with hydrophobic molecules given the unique nature imparted by their structure. Cyclodextrins have an ability to alter physical, chemical, and biological properties of guest molecules through the formation of inclusion complexes. Cyclodextrin complexes can be used to enhance drug delivery through biological barriers without affecting their barrier function, a property which makes cyclodextrins ideal penetration enhancers for intranasal drug delivery. Cyclodextrins can also act as solubilizers for lipophilic water-insoluble drugs, making it possible to formulate such drugs in aerosol formulations. Furthermore, cyclodextrin complexation can stabilize drugs that are chemically unstable in aqueous solutions, and decrease drug irritation after pulmonary application.
Recently, an aerosol formulation of 2-hydroxypropyl-beta-cyclodextrin (HPbetaCD) solubilized itraconazole (ITZ) solution was prepared for pulmonary delivery. Further, pharmacokinetics of inhaled nebulized aerosols of HPbetaCD-ITZ were compared with colloidal dispersion of ITZ nanoparticulate formulation. Studies demonstrated faster systemic absorption of ITZ via beta-cyclodextrin-based formulation across the lung epithelium compared to nanoparticulate formulation of ITZ [Citation84]. Cyclodextrin has also complexed with formoterol for improvement of solubility and development of a nebulized solution of formoterol [Citation85]. Similarly, an aqueous solution of voriconazole solubilized with sulfobutyl ether-beta-cyclodextrin has been prepared for targeted drug delivery to the lungs via nebulization. Studies revealed that voriconazole solubilization with sulfobutyl ether-beta-cyclodextrin has given rapid and high drug concentrations in plasma following inhalation [Citation86]. Hydroxypropyl-beta-cyclodextrin has also been utilized for fabrication of large porous particles of PLGA-loaded insulin. The developed insulin-loaded large porous particles of PLGA have shown significant higher and longer hyperglycaemic as compared to an insulin solution. Studies revealed that cyclodextrin-based large porous particles are able to deliver insulin into alveoli, which is absorbed in the bioactive form [Citation50]. An inhalable dry powder of recombinant human growth hormone has been prepared using dimethyl-beta-cyclodextrin for systemic delivery of the protein. Studies show better aerosol performance of the spray-dried powders and systemic absorption following administration through the rat lung [Citation87].
Carbopol
Carbopol are polymers of acrylic acid cross-linked with polyalkenyl ethers or divinyl glycol. Carbopols have been used mainly in liquid or semi-solid pharmaceutical formulations, such as gels, suspensions, and emulsions, as a thickening and viscosity agent, in order to modify the flow characteristics. Recently, they have also been used for their mucoadhesive properties and a relevant amount of work has been done on the bioadhesive potential of carbopol polymers. Formulations include ophthalmic, rectal, buccal, nasal, intestinal, vaginal, and topical preparations [Citation88].
OTHER PARTICULATE DELIVERY SYSTEMS
Emulsion
The water-in-oil solvent evaporation method was investigated to improve productivity of the PLGA microspheres for rifampicin delivery as dry aerosols. Using ethyl acetate as an organic solvent, a coarse oil-in-water emulsion (or premix) was prepared under magnetic stirring and homogenization. Microspheres obtained had MMAD of 2.63 microm and were found to be suitable for aerosol administration and delivery into the rat lungs by intratracheal insufflations [Citation89]. Alpha-tocopherol acetate (ATA) nanoparticles were prepared to target the lungs as aerosols in order to prevent cigarette smoke toxicity and the influence of the preparative method was studied. Poly-(lactide) nanoparticles prepared using nanoprecipitation and solvent evaporation techniques produced too-small and too-large nanoparticles, respectively, to be aerosolized. The emulsification-diffusion method produced two months of stable nanoparticles with a size between 500-700 nm [Citation90]. Water-soluble compounds were investigated to incorporate into metered-dose inhalers (MDIs) by using water-in-propellant lecithin microemulsions, in which dimethyl ether (DME) and propane acted as both continuous phase and propellant. Storage of microemulsion samples for up to three weeks did not affect the MMAD, GSD, or FPF. This approach enabled the pulmonary delivery of water-soluble therapeutic agents via MDIs [Citation91].
Pulmonary administration of drugs has demonstrated numerous advantages in the treatment of pulmonary diseases due to direct targeting to the respiratory tract. It enables avoiding the first pass effect, reduces the amount of drugs administered, targets drugs to specific sites, and reduces their side-effects. Reverse water-in-fluorocarbon (FC) emulsions are significant drug delivery systems for pulmonary administration of the drugs using pressurized metered-dose inhalers (pMDI). The external phase and the internal phase of these emulsions consist of perfluorooctyl bromide (PFOB, perflubron) and drugs solubilized or dispersed in water, respectively. These emulsions were found to be stabilized by a perfluoroalkylated dimorpholinophosphate (F8H11DMP), i.e. a fluorinated surfactant [Citation92]. Lecithin inverse microemulsions prepared by using dimethyl ether (DME) and propaneas propellants were found to effectively deliver polar drugs to the lungs [Citation93].
The effects of diluents like phosphate buffered saline and 0.9% NaCl were demonstrated on the stability of the nanoemulsion-based vaccine using a nasal spray device. It was reported that the stability and potency of PBS-diluted obalbumin nanoemulsion mixtures remained for eight months and NaCl diluted mixtures up to six months. Nanoemulsion-based vaccines did not physically or chemically alter following with nasal spray devices [Citation94]. Simultaneous manufactured nano in micro (SIMANIM) particles of antibodies delivered by the pulmonary route have been prepared by the spray drying of double emulsion containing human Ig G, lactose, PLGA and dipalmitoylphosphotidylcholine (DPPC).
Reverse phase microemulsion was demonstrated to be used as a template for production of nanoparticles in which two miroemulsions were obtained having water/sodium bis/2-ethyl hexyl sulfosuccinate (AOT)/isooctane and water /lecithin/propan-2-ol/isooctane. With this novel step, low-energy, non-toxic, and without chemically reactive crosslinking agents deposited the nanoparticles within lungs with deposition being mainly alveolar [Citation95].
Suspension
The applicability of a novel particle-based technology has been seen for the development of suspensions of small polar drugs and biomolecules in hydrofluoroalkane (HFA) propellants for pressurized metered-dose inhalers (pMDIs). The results found that dispersion showed excellent stability and aerosol characteristics in HFA-based pMDIs [Citation96].
Nanoparticle suspensions formulated from the branched polyester, diethylaminopropyl amine-poly(vinyl alcohol)-grafted-poly(lactide-co-glycolide) (DEAPA-PVAL-g-PLGA) alone, with increasing amounts of carboxymethyl cellulose (CMC). Particle size, zeta potential, turbidity, and morphology (atomic force microscopy) were evaluated. Three formulations chosen are cationic nanoparticles without CMC, cationic nanoparticles with CMC, and anionic nanoparticles with an excess of CMC. Nanoparticle degradation was characterized, as well as stability during nebulization. Nanoparticle-cell interactions have been quantified using confocal laser scanning microscopy and fluorescence spectrometry. Nanoparticles ranged in size from 70-250 nm and displayed zeta potentials of + 58.9 to -46.6 mV. Anionic nanoparticles showed the highest stability during nebulization. The degradation rate of each nanoparticle formulation decreased with increasing amounts of CMC. Cell association was highest among cationic nanoparticles (57% and 30%, respectively), although these were not internalized. Despite a lower rate of cell association (3%), anionic nanoparticles were internalized by A549 cells. Surfactant-free nanoparticles from DEAPA-PVAL-g-PLGA were found to be versatile drug delivery systems; however, only the anionic formulations investigated were proven suitable for aerosol therapy [Citation82].
The microparticulate suspension was assessed in vitro containing doxorubicin for aerosol delivery to the lungs in order to treat locally occurring tumors. A drug release study exhibited that the doxorubicin microparticulate suspension showed greater therapeutic effect than the drug alone [Citation97].
METAL-BASED NANOPARTICLE DELIVERY
The various metals which can be used for the nanoparticlate delivery are shown in .
Iron
Iron particles are of considerable interest for application in nanotechnology-related fields. Iron nanoparticles are sub-micrometer particles of iron metal. They are widely used in medical, diagnostic, and laboratory applications. They are highly reactive because of their large surface area. In the presence of oxygen and water, they rapidly oxidize to form free iron ions. Iron is a highly redox-active transition metal, thus the safety of iron nanomaterials needs to be studied. To access toxicity of iron particles, cationic magnetic nanoparticles have been prepared and toxticity was determined in A-549 cells (human lung adenocarcinoma). Studies demonstrated that the presence of nanoparticles did not affect cell viability or the morphologic parameters of cell lines and also shows the potential internalization mechanism of nanoparticles into cells through a macropinocytosis process [Citation98]. The ferric oxide nanoparticle on exposure could induce oxidative stress in the lung. Several inflammatory reactions occur which causes inflammatory and immune cells to increase. Clinical pathological changes like follicular hyperplasia, protein effusion, pulmonary capillary vessel hyperaemia and alveolar lipoproteinosis in lungs has been observed [Citation99].
Superparamagnetic iron oxide nanoparticles showed potential in magnetic targeting of inhaled aerosols within the lung. These particles are also used as contrast agents in magnetic resonance imaging (MRI). The nebulized droplets in the inhalation chamber had mass median aerodynamic diameter (MMAD) of 5.6 +/−0.8 micron. MRI studies have shown the in vivo distribution superparamagnetic iron oxide nanoparticles into lungs [Citation100]. The targeted aerosol delivery to the affected lung tissue may improve therapeutic efficiency and minimize unwanted side-effects. By computer-aided simulation, targeted aerosol delivery to the lung can be achieved with aerosol droplets comprising superparamagnetic iron oxide nanoparticles, so-called nanomagnetosols, in combination with a target-directed magnetic gradient field. Nanomagnetosols may be useful for treating localized lung disease, by targeting foci of bacterial infection or tumor nodules [Citation101].
Recently, oleic acid-coated superparamagnetic particles have been prepared to the potential of site-specific respiratory drug delivery. Studies show the enhanced deposition of developed iron nanoparticles in the mouse lung but not in the trachea, consistent with the analysis of the aerodynamic time allowed for deposition and required magnetic deposition time [Citation102]. Alpha(v)beta(3)-specific peptide (cyclic RGDfK) anchored fluorescent superparamagnetic polymeric micelles have been prepared for in vivo imaging of tumor angiogenesis. In-vivo studies show the alpha(v)beta(3)-specific superparamagnetic polymeric micelles accumulation in human lung cancer subcutaneous tumor xenografts [Citation103]. Similarly, multifunctional micelle systems have also been prepared for MR imaging and therapeutic delivery using lung cancer-targeting peptide, superparamagnetic iron oxide, and doxorubicin. Studies indicated that the lung cancer-targeting peptide functionalization of the micelle surface increased uptake of the developed system by more than three-fold compared to the scrambled peptide control [Citation104].
Gold
Colloidal gold is a suspension (or colloid) of sub- micrometer-sized particles of gold in a fluid, usually water. Gold nanoparticles have unique optical, electronic, and molecular-recognition properties, and are widely used in the areas of science, engineering, or medicine. Gold NPs have properties such as chemical stability, high electron density, and affinity to biomolecules, making them drug carriers and a tool for diagnosis. Different physicochemical properties of nano-sized gold particles have raised substantial concerns about the safety of gold nanoparticles in the body. During the past decades, various aspects of the interaction between gold nanoparticles and pulmonary/lungs structures have been investigated. Preliminary cytotoxic studies have shown that suspensions of well-dispersed 50 nm and 250 nm particles, as well as their agglomerates, produced very mild pulmonary inflammation at the same mass-based dose. Studies revealed that single 50 nm gold particles do not pose a greater acute hazard than their agglomerates or slightly larger gold particles when using pulmonary inflammation as a marker for toxicity [Citation105].
Besides toxicity tests, pharmacokinetic studies are an important part of investigations to evaluate a safe and sustainable use of gold nanoparticles. The fate of gold nanoparticles has been studied by intratracheal administration to adult female mice. The results reported that the instilled nanoparticles were found in lung macrophages already 1 h after a single instillation. Thus, it was demonstrated that the inert gold nanoparticles, administered intratracheally, are phagocytosed by lung macrophages. Only a tiny fraction of the gold particles is translocated into systemic circulation and the translocation rate was greatest with the 2 nm gold particles [Citation106]. In-vivo distribution of poly(ethylene glycol) (PEG) modified gold nanoparticles and plain gold nanoparticles was determined after intravenous and intratracheal applications of gold nanoparticles. After intratracheal application, the majority of gold nanoparticles stayed in the lungs: the total translocation towards the circulation did not differ considerably after PEGylation of the gold nanoparticles. However, a prolonged retention time in the circulation was detected for the small fraction of translocated 10 kDa PEG gold nanoparticles [Citation107].
Recently, oxaliplatin-conjugated gold nanoparticles have been prepared to target cancers passively or actively. The platinum-anchored gold nanoparticles demonstrated significantly better cytotoxicity than oxaliplatin alone in all of the cell lines and an unusual ability to penetrate the nucleus in the lung cancer cells [Citation108].
Zinc
Studies have been done in order to develop in vitro screening assays to determine lung hazard potential of nanomaterials involving three major objectives: a) to compare lung toxicity impacts of nanoscale (NZnO) vs fine zinc oxide (FZnO) particulates; b) assess predictability of in vitro cell culture systems; and c) compare effects of instillation vs inhalation exposures in rats. Rats were exposed in vivo either by intratracheal instillation to 1 or 5 mg/kg of nanoscale or fine size zinc oxide particle types or by inhalation to aerosols of 25 or 50 mg/m3 for 1 or 3 h. Lung inflammation, cytotoxicity, and histopathological endpoints were assessed at several time points postexposure. Cultures of (1) rat lung epithelial cells, (2) primary alveolar macrophages, and (3) alveolar macrophages-L2 lung epithelial cell cocultures were incubated with fine or nano ZnO particles and evaluated for cytotoxicity biomarkers (LDH) and proinflammatory cytokines (MIP-2 and TNF-alpha). In vitro exposures to fine or nanoscale ZnO particles produced minor cytotoxic responses at 4 and 24 h, only in co-cultures and at the highest (particle overload) dose with little detectable proinflammatory cytokine generation (MIP-2, and TNF-alpha) [Citation109]. Tong et al. investigated FeSO4, ZnSO4 effects on acute lung injury, by instilling six solutions contained PM(2.5) aerosol particles, FeSO4, ZnSO4 and their mixtures intratracheally into mouse lungs for experiment. Hemorrhages in the lung were observed more from those mice instilled by FeSO4 [Citation110]. Bronchial epithelial hyperplasia can be observed in ZnSO4 contained solution-instilled groups from histopathologic analysis. The lung injury of mice caused by solution of PM(2.5) + FeSO4 + ZnSO4 was more serious than other toxin solutions.
Inhalation toxicity of an aerosol of propineb, a zinc bisdithiocarbamate homopolymer fungicide, to Wistar rats was studied. Groups of 10 rats/sex were exposed nose-only to mean concentrations of 3.97, 11.24, and 21.95 mg propineb/m(3) using an exposure regimen of 6 h/day, 5 days/wk for 4 wk. The experiments showed that the etiopathologic cause of neuromuscular changes. The investigation suggested that the toxicity of inhaled propineb was characterized by two independent effects, namely responses occurring at the alveolar level and muscular weakness [Citation111]. Intratracheal instillation of recombinant human Cu/Zn superoxide dismutase (rhSOD) shows a lessening of lung injury produced by 48 h of hyperoxia and mechanical ventilation in neonatal piglets. The intratracheal instillation of rhSOD results in nonuniform pulmonary distribution; aerosolization enhances rhSOD distribution and alveolar deposition. Clinical trials of rhSOD prevent acute and chronic lung injury in premature neonates [Citation112]. Amdur .and Chen exposed guinea pigs to ultrafine aerosols (less than 0.1 micron) of zinc oxide with a surface layer of sulfuric acid and studied its pulmonary effects [Citation113].
Approaches to Deliver Nanoparticles to the Lungs
When particle dimensions are reduced to the order of several nanometers, their physical and chemical properties deviate significantly from the bulk properties of such materials. Due to their extremely small sizes, the particles suffer from many problems related to their surface and thermal stability, shape preservation, handling, assembly in devices, etc. It is therefore an important challenge to solve these problems by developing slightly larger particles (e.g. on the submicrometer scale) in which the properties generated by the nanoscale material are preserved. One approach to this is to trap nanoparticles in a micrometer-sized inert matrix. Nanoparticles provide sustained release, reduction in dosage, and compliance. The polymeric nanoparticles are prepared; this can be done by solvent evaporation, nanoprecipitation, or by the means of multiple emulsion.
A novel particulate form incorporating nanoparticles into micron-scale structures has been engineered to overcome the issues of storing and delivering nanoparticles to the lungs; it is referred to as “porous nanoparticle-aggregate particles” (PNAPs).
PNAPs can be formed by spray-drying nanoparticles with the addition of pharmaceutical excipients. The formation of the porous particles during the drying process can be characterized by the Peclet number (Pe), a dimensionless mass transport number that compares the times for droplet drying and nanoparticle diffusion. Another type of carrier particle for drug delivery is large porous particles. This type of particle is characterized by geometrical sizes larger than 5 μm and mass densities of 0.1 g/cm3 or less. LPPs have recently become popular for use as carriers for drugs that need to be delivered to the lungs for local and systemic applications. A principal advantage of LPPs over conventional inhaled therapeutic aerosol particles is their aerosolization efficiency. In addition, LPPs possess the potential to avoid alveolar macrophage clearance, enabling the sustained releases of a drug.
Another approach for the pulmonary delivery of nanoparticles as a dry powder by spray drying or spray freeze drying for inhalation needs to address three critical issues: (1) the need to increase biological efficacy that confers better local mucosal as well as systemic immunity (2) the need to increase safety during administration by eliminating the potential contamination risks associated with needles; (3) the need to increase stability during administration and transport; and (4) the need to improve cost-effectiveness.
Spray drying produces dry powder from the liquid or slurry by drying and is the preferred method for thermally sensitive materials. The spray freeze drying method is processing steps to freeze dry and spray dry where the protein drug is dissolved, and the solution can be nebulized into a cryogenic medium (liq Nitrogen) that generates a dispersion of shock-frozen droplets and the dispersion is dried in a lyophilizer.
New approaches include vaccines, drug, genetic material embedded in porous particles or droplets with optimal targeting which can be done by controlled air particle stream based on optimal particle diameter, density, inhalation waveform, particle release position. It is also possible to microencapsulate the nanoparticles in a biodegradable polymer leading to controlled drug delivery. shows the research details of the aerosol-based nanoparticles.
Nanotoxicology
Nanomaterial research is focused on the medical applications of nanotechnology, whereas side-effects with nanotechnology use are not taken into consideration during the engineering process. The available methods, such as physiologically based pharmacokinetic modeling or predictive structure-activity relationships, assess the toxicity and risk associated with specific nanomaterials. This can be achieved through an innovative combination of toxicology, risk assessment modeling, and tools developed in the field of multicriteria decision analysis (MCDA) [Citation114].
Recent applications of nano science include the use of nano-scale materials in electronics, catalysis, and biomedical research. Among these applications, biological processes such as blood coagulation control and multimodal bioimaging are also included, which has brought about a new and exciting research field called nanobiotechnology. Biotechnology involves the manipulation of macroscopic biological systems such as cells and mice in order to understand why and how molecular-level mechanisms affect specific biological functions, e.g., the role of APP (amyloid precursor protein) in Alzheimer's disease (AD) [Citation115].
Nanoparticles have been developed for many applications, including nanomedicines, cosmetics, food additives, and water purification. Because of thehe potential for exposure of humans and the environment to nanoparticles, it is important to assess their safety, especially if nanotechnology is to reach its full potential.
Nanoparticles vary in terms of physicochemical characteristics such as size, surface area, charge, shape, surface chemistry, and contaminants. The current focus of nanotoxicology appears to be assessment of factors that drive toxicity, therefore providing information for risk assessment, hazard prevention, and future product design. Size effects particle uptake by cells and penetration across cell barriers, while surface area is related to the potential of particles to generate lung inflammation. Shape, especially fiber shape, appears to promote inflammation, fibrosis, and granuloma formation in vivo. Positively charged particles are more toxic than negative particles, and some particles appear to leach soluble toxic components [Citation116]. It is therefore unlikely that one factor is responsible for nanoparticle-induced hazard, and instead a combination of factors will contribute. The accumulation of such information, together with good physicochemical characterization, will provide the basis of future predictive toxicity assessment, therefore reducing the need to assess the toxicity of nanoparticles [Citation117].
Nanoparticle behavior in the lungs has been investigated in environmental health fields with a focus on “ultrafine” particles (UFP < 100nm aerodynamic diameter). The small size of UFPs, which makes therapeutic nanoparticles so attractive, might be the main factor contributing to their toxicity and negative effect on human health. The large surface area per mass of nanoparticle causes them to be more. Physicochemical particle properties such as size, surface charge, and hydrophilicity all play a role in particle fate in vivo.
Regulations
There are many organizations and much research supporting the development of nanotechnology. Some of them have also participated in proposing regulations to improve the protection of human health and the environment. These organizations or research centers are mainly supported by government sources and play an essential role in performing or supporting nanotechnology research, including the basic research on nanotechnology, the applications of nanotechnology, safety assessment of nanomaterials, and the development of regulatory control [Citation118]. These organizations provide long-term coherence and platforms for interdisciplinary people or experts in order to promote nanotechnology to the public. The various initiatives, centers, institutes, or government organizations are:
From the USA: National Nanotech Initiative (NNI), Food and Drug Administration (FDA), Environmental Protection Administration (EPA), Center for Nanotechnology, National Science Foundation, Project on Emerging Nanotechnologies.
From Europe: Community Research & Development Information Service (CORDIS), European Nanotechnology Gateway, European Nanobusiness Association (ENA).
From the UK: Institute of Nanotechnology (IoN), The Royal Society and Royal Academy of Engineering, Health and Safety Executive (HSE), Institute of Food Science and Technology (IFST).
Clinical Trial
Nanotherapeutics, Inc. has been awarded a $30.9 million, five-year contract from the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH), to develop an inhaled version of the injectable antiviral drug Cidofovir for non-invasive, post-exposure prophylaxis and treatment of smallpox (Variola major). The transmission of smallpox occurs through inhalation of the airborne variola virus, usually droplets expressed from the oral, nasal, or pharyngeal mucosa of an infected person; non-invasive anti-viral treatment alternatives with proven agents (cidofovir) are needed. Development of inhaled cidofovir will also provide an alternative for those who have contraindications to the currently approved smallpox vaccinationm such as severe exfoliative skin diseases, immunosuppression from many sources, and pregnancy. Inhaled cidofovir could decrease the proportion of the population that would remain susceptible to smallpox due to their inability to be vaccinated, and has been shown in multiple studies to be highly efficacious against various pox models compared to injectable administration, which results in lower pulmonary levels, possible nephrotoxicity, and requires a health-care worker to implement treatment. The works on gold nanoshells have also begun in human patients. An overview of various clinical trials underway around the world is shown in .
Table 2. Various clinical trials underway around the world.
CONCLUSION
Nanoparticulate systems show great potential to deliver biologically active substances. The core of these systems encloses a variety of drugs, enzymes, and genes. Lipid, protein, polysaccharide-based nanoparticles are efficient modes for pulmonary delivery. Sophisticated electromechanical techniques overcome some common difficulties and ensure reproducibility of dose and delivery to the lungs. The new inhalation systems are certainly better than classic nebulizers or MDIs. Drug delivery to the lungs by aerosol inhalation can be accomplished in high dose by repetitive inhalation. Novel technologies provide significant clinical advantages in order to increase delivery efficiency and targeting of specific regions.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Sung, J.C., Pulliam, B.L. and Edwards, D.A. (2007). Nanoparticles for drug delivery to the lungs. TRENDS in Biotechnology, 25: 563–570.
- Finlay, W.H. (2005). The Mechanics of Inhaled Pharmaceutical Aerosols: An Introduction. London: Academic Press.
- Chaudhuri, S.R. and Lukacova, V. (2010). Simulating Delivery of Pulmonary (and Intranasal) Aerosolised Drugs. Lanchester: Simulations Plus, Inc., 26–28.
- Desai, A. (2007). Gibaldi's Drug Delivery Systems in Pharmaceutical Care. Bethesda, MD: American Society of Health System.
- Labiris, N.R. and Dolovich, M.B. (2003). Pulmonary drug delivery. Part I: Physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol., 56: 588–599.
- Vyas, S.P. and Khar, R.K. (2002). Targeted & Controlled Drug Delivery, Novel Carrier Systems. New Delhi: CBS Publishers and Distributors.
- Anderson, M.W., Orton, T.C., Pickett, R.D. and Eling, T.E. (1974). Accumulation of amines in the isolated perfused rabbit lung. J Pharmacol Exp. Ther189: 456–466.
- Dollery, C.T. and Junod, A.F. (1976). Concentration of (±) proponol in isolated, perfused rabbit lung. J. Pharmacol, 57: 67–71.
- Jorfeldt, L., Lewis, D.H., Lofstrom, J.B. and Post, C. (1979). Lung uptake of lidocaine in healthy volunteers. Acta Anaesth Scand, 23: 567–574.
- Roerig, D.L., Kotrly, K.J., Dawson, C.A., Ahlf, S.B., Gualtieri, J.F. and Kampine, J.P. (1989). First pass uptake of verapamil, diazepam and thiopental in the human lung. Anaesth Analg, 69: 461–466.
- Suhara, T., Sudo, Y., Yoshida, K., Okubo, Y., Fukuda, H., Obata, T., Yoshikawa, K., Suzuki, K. and Sasaki, Y., (1998). The limiting role of mucus in drug absorption: Drug permeation through mucus solution. Int J Pharm, 126: 179–187.
- Rubin, B.K. (2010). Air and soul: The science and application of aerosol therapy. Respir Care., 55: 911–21.
- Kalantarian, P., Najafabadi, A.R., Haririan, I., Vatanara, A., Yamini, Y., Darabi, M. and Gilani, K. (2010). Preparation of 5-fluorouracil nanoparticles by supercritical antisolvents for pulmonary delivery. Int J Nanomedicine, 5: 763–70.
- Zeman, K.L., Wu, J. and Bennett, W.D. (2010). Targeting aerosolized drugs to the conducting airways using very large particles and extremely slow inhalations. J Aerosol Med Pulm Drug Deliv, 23: 363–9.
- Zhang, Y., Wang, X., Lin, X., Liu, X., Tian, B. and Tang, X. (2010). High azithromycin loading powders for inhalation and their in vivo evaluation in rats. Int J Pharm, 395: 205–14.
- Tronde, A., Norden, B., Marchner, H., Wendel, A.K., Lennernas, H., Bengtsson, U.H. (2003). Pulmonary absorption rate and bioavailability of drugs in vivo in rats: structure-absorption relationships and physicochemical profiling of inhaled drugs. J Pharm Sci, 92: 1216–1233.
- Patton, J.S. (1996). Mechanisms of macromolecular absorption by the lungs. Adv Drug Deliv Rev, 19: 3–36.
- Bhat, P.G., Flanagan, D.R. and Donavan, M.D. (1995). The limiting role of mucus in drug absorption: Drug permeation through mucus solution. Int J Pharm, 126: 179–187.
- Rubin, B.K. (1996). Therapeutic aerosols and airway secretions. J. Aerosol Med, 9: 123–130.
- Lipworth, B.J. and Clark, D.J. (1997). Effects of airway calibre on lung delivery of nebulised salbutamol. Thorax, 52: 1036–9.
- Tronde, A., Bosquillon, C. and Forbes, B. (2008). The isolated perfused lungs for drug absorption studies. Ehrhardt, C., Kwang-Jin, K., Drug Absorption Studies: In Situ, In Vitro and In Silico Models. New York: Springer Sciences, 135–154.
- Dandekar, P., Venkataraman, C. and Mehra, A. (2010). Pulmonary targeting of nanoparticle drug matrices. J Aerosol Med Pulm Drug Deliv, 23: 343–53.
- Noymer, P.D., Myers, D.J., Cassella, J.V. and Timmons, R. (2011). Assessing the temperature of thermally generated inhalation aerosols. J. Aerosol Med. Pulm. Drug Del., 24: 11–15.
- Shaikh, S., Nazim, S., Khan, T., Shaikh, A., Zameeruddin, M. and Quazi, A. (2010). Recent advances in pulmonary drug delivery system: A review. Int J of AppPharm, 2: 27–31.
- Byron, P.R. (2004). Drug delivery devices: Issues in drug development. The Proceedings of the American Thoracic Society, 1: 321–328.
- Nassimi, M., Schleh, C., Lauenstein, H.D., Hussein, R., Lübbers, K., Pohlmann, G., Switalla, S., Sewald, K., Müller, M., Krug, N., Müller-Goymann, C.C. and Braun, A. (2009). Low cytotoxicity of solid lipid nanoparticles in in vitro and ex vivo lung models. Inhal Toxicol, 1: 104–9.
- Nassimi, M., Schleh, C., Lauenstein, H.D., Hussein, R., Hoymann, H.G., Koch, W., Pohlmann, G., Krug, N., Sewald, K., Rittinghausen, S., Braun, A., Müller-Goymann, C. (2010). A toxicological evaluation of inhaled solid lipid nanoparticles used as a potential drug delivery system for the lung. Eur J Pharm Biopharm, 75: 107–16.
- Xiang, Q.Y., Wang, M.T., Chen, F., Gong, T., Jian, Y.L., Zhang, Z.R. and Huang, Y. (2007). Lung-targeting delivery of dexamethasone acetate loaded solid lipid nanoparticles. Arch Pharm Res, 30: 519–25.
- Liu, J., Gong, T., Fu, H., Wang, C., Wang, X., Chen, Q., Zhang, Q., He, Q. and Zhang, Z. (2008). Solid lipid nanoparticles for pulmonary delivery of insulin. Int J Pharm, 356: 333–44.
- Yang, W., Tam, J., Miller, D.A., Zhou, J., McConville, J.T., Johnston, K.P. and Williams, R.O., 3rd (2008). High bioavailability from nebulized itraconazole nanoparticle dispersions with biocompatible stabilizers. Int J Pharm, 361: 177–88.
- Yu, W., Liu, C., Liu, Y., Zhang, N. and Xu, W. (2010). Mannan-modified solid lipid nanoparticles for targeted gene delivery to alveolar macrophages. Pharm Res, 27: 1584–96.
- Li, Y.Z., Sun, X., Gong, T., Liu, J., Zuo, J. and Zhang, Z.R. (2010). Inhalable microparticles as carriers for pulmonary delivery of thymopentin-loaded solid lipid nanoparticles. Pharm Res, 27: 1977–86.
- Arora, D., Goyal, A.K., Paliwal, S.R., Khurana, B. and Vyas, S.P. (2010). Oral mucosal immunization: Recent advancement and future prospects. Current Immunology Reviews, 6: 234–259.
- Dhand, R. (2004). New frontiers in aerosol delivery drug mechanical ventilation. Respiratory Care, 49: 666–677.
- Imran, S. and Hugh, S. (2006). Carriers in pulmonary dry powder drug delivery. Future Drug Delivery, 34–36.
- Zaru, M., Sinico, C., De Logu, A., Caddeo, C., Lai, F., Manca, M.L. and Fadda, A.M. (2009). Rifampicin-loaded liposomes for the passive targeting to alveolar macrophages: In vitro and in vivo evaluation. J Liposome Res, 19: 68–76.
- Behr, J., Zimmermann, G., Baumgartner, R., Leuchte, H., Neurohr, C., Brand, P., Herpich, C., Sommerer, K., Seitz, J., Menges, G., Tillmanns, S., Keller, M. and Munich Lung Transplant Group (2009). Lung deposition of a liposomal cyclosporine A inhalation solution in patients after lung transplantation. J Aerosol Med Pulm Drug Deliv, 22: 121–30.
- Tronde, A., Norden, B., Marchner, H., Wendel, A.K., Lennernas, H., Bengtsson, U.H. (2003). Pulmonary absorption rate and bioavailability of drugs in vivo in rats: Structure-absorption relationships and physicochemical profiling of inhaled drugs. J Pharm Sci, 92: 1216–1233.
- Monforte, V., Ussetti, P., López, R., Gavaldà, J., Bravo, C., de Pablo, A., Pou, L., Pahissa, A., Morell, F. and Román, A. (2009). Nebulized liposomal amphotericin B prophylaxis for Aspergillus infection in lung transplantation: Pharmacokinetics and safety. J Heart Lung Transplant, 28: 170–5.
- Latimer, P., Menchaca, M., Synder, R.M., Yu, W., Gilbert, B.E., Sanders, B.G. and Kline, K. (2009). Aerosol delivery of liposomal formulated paclitaxel and vitamin E analog reduces murine mammary tumor burden and metastases. Exp Biol Med (Maywood), 234: 1244–52.
- Zakharian, T.Y., Seryshev, A., Sitharaman, B., Gilbert, B.E. and Knight, V. (2004). A fullerene- paclitaxel chemotherapeutic: Synthesis, characterization and study of biological activity in tissue culture. J Am Chem Soc, 127: 12508–9.
- Bai, S., Gupta, V. and Ahsan, F. (2009). Cationic liposomes as carriers for aerosolized formulations of an anionic drug safety. Eur J Pharm Sci, 38: 165–71.
- Pitt, C.G. (1990). Poly-ε caprolactone and its copolymers. Langer, R. Biodegradable Polymers as Drug Delivery Systems. New York: Marcel Dekker, 71.
- Ohashi, K., Kabasawa, T., Ozeki, T. and Okada, H. (2009). One-step preparation of rifampicin/poly(lactic-co-glycolic acid) nanoparticle-containing mannitol microspheres using a four-fluid nozzle spray drier for inhalation therapy of tuberculosis. J Control Release, 135: 19.
- Beck-Broichsitter, M., Gauss, J., Gessler, T., Seeger, W., Kissel, T. and Schmehl, T. (2010). Pulmonary targeting with biodegradable salbutamol-loaded nanoparticles. J Aerosol Med Pulm Drug Deliv, 23: 47–57.
- Doan, T.V. and Oliver, J.C. (2009). Preparation of rifampicin-loaded PLGA microspheres for lung delivery as aerosol by premix membrane homogenization. Int J Pharm, 382: 61–6.
- Kaye, R.S., Purewal, T.S. and Alpar, H.O. (2009). Simultaneously manufactured nano-in-micro (SIMANIM) particles for dry-powder modified-release delivery of antibodies. J Pharm Sci, 98: 4055–68.
- Pandey, R., Sharma, A., Zahoor, A., Sharma, S., Khuller, G.K. and Prasad, B. (2003). Poly (DL-lactide-co-glycolide) nanoparticle-based inhalable sustained drug delivery system for experimental tuberculosis. J Antimicrob Chemother, 52: 981–6.
- Arnold, M.M., Gorman, E.M., Schieber, L.J., Munson, E.J. and Berkland, C. (2007). NanoCipro encapsulation in monodisperse large porous PLGA microparticles. J Control Release, 121: 100–9.
- Ungaro, F., d'Emmanuele di Villa Bianca, R., Giovino, C., Miro, A., Sorrentino, R., Quaglia, F. and La Rotonda, M.I. (2009). Insulin-loaded PLGA/cyclodextrin large porous particles with improved aerosolization properties: In vivo deposition and hypoglycaemic activity after delivery to rat lungs. J Control Release, 135: 25–34.
- Yamamoto, H., Kuno, Y., Sugimoto, S., Takeuchi, H. and Kawashima, Y. (2005). Surface-modified PLGA nanosphere with chitosan improved pulmonary delivery of calcitonin by mucoadhesion and opening of the intercellular tight junctions. J Control Release, 102: 373–81.
- Niwa, T., Takeuchi, H., Hino, T. and Kawashima, Y. (1995). Aerosolization of lactide/glycolide copolymer (PLGA) nanospheres for pulmonary delivery of peptide-drugs. Yakugaku Zasshi., 115: 732–41
- Bharatwaj, B., Wu, L., Whittum-Hudson, J.A. and da Rocha, S.R. (2010). The potential for the noninvasive delivery of polymeric nanocarriers using propellant-based inhalers in the treatment of Chlamydial respiratory infections. Biomaterials, 31: 7376–85.
- Ely, L., Roa, W., Finlay, W.H. and Löbenberg, R. (2007). Effervescent dry powder for respiratory drug delivery. Eur J Pharm Biopharm, 65: 346–53.
- Quillen, D.A. and Rosenwasser, G.O. (1994). Aerosol application of cyanoacrylate adhesive. J Refract Corneal Surg, 10: 149–50.
- Zhang, Q., Shen, N.T. (2001). Prolonged hypoglycaemic effect of insulin loaded polybutyl cyanoacrylate nanoparticles after pulmonary administration to normal rats. Int. J. Pharm, 218: 78–80.
- Kamal, M.H., Shirzad, A., Anwar, A., Wilson, H. and Raimar, L. (2010). Secondary cytotoxicity mediated by alveolar macrophages: A contribution to the total efficacy of nanoparticles in lung cancer therapy? European Journal of Pharmaceutics and Biopharmaceutics, 76: 112–119.
- Harush-Frenkel, O., Bivas-Benita, M., Nassar, T., Springer, C., Sherman, Y., Avital, A., Altschuler, Y., Borlak, J. and Benita, S. (2010). A safety and tolerability study of differently-charged nanoparticles for local pulmonary drug delivery. Toxicol Appl Pharmacol, 246: 83–90.
- Muttil, P., J. Kaur, K. Kumar, A.B. Yadav, R. Sharma, and A. Misra. (2007). Inhalable microparticles containing large payload of anti-tuberculosis drugs. Eur. J. Pharm. Sci., 32: 140–150.
- Gadermann, M., Kular, S., Al-Marzouqi, A.H. and Signorell, R. (2009). Formation of naproxen-polylactic acid nanoparticles from supercritical solutions and their characterization in the aerosol phase. Phys Chem Chem Phys, 11: 7861–8.
- Shoyele, S.A. and Slowey, A. (2006). Prospects of formulating proteins/peptides as aerosols for pulmonary drug delivery. Int J.of Pharmaceutics, 314: 1–8.
- Wright, I.K., Higginbotham, A., Baker, S.M. and Donnelly, T.D. (2010). Generation of nanoparticles of controlled size using ultrasonic piezoelectric oscillators in solution. ACS Appl Mater Interfaces, 2: 2360–4.
- Albasarah, Y.Y., Somavarapu, S. and Taylor, K.M. (2010). Stabilizing protein formulations during air-jet nebulization. Int J Pharm, 402: 140–5.
- Grenha, A., Seijo, B. and Remuñán-López, C. (2005). Microencapsulated chitosan nanoparticles for lung protein delivery. Eur J Pharm Sci, 25: 427–37.
- Hagenaars, N., Mastrobattista, E., Verheul, R.J., Mooren, I., Glansbeek, H.L., Heldens, J.G., van den Bosch, H., Jiskoot, W. (2009). Physicochemical and immunological characterization of N,N,N-trimethyl chitosan-coated whole inactivated influenza virus vaccine for intranasal administration. Pharm Res, 26: 1353–64.
- Jin, H., Xu, C.X., Kim, H.W., Chung, Y.S., Shin, J.Y., Chang, S.H., Park, S.J., Lee, E.S., Hwang, S.K., Kwon, J.T., Minai-Tehrani, A., Woo, M., Noh, M.S., Youn, H.J., Kim, D.Y., Yoon, B.I., Lee, K.H., Kim, T.H., Cho, C.S. and Cho, M.H. (2008). Urocanic acid-modified chitosan-mediated PTEN delivery via aerosol suppressed lung tumorigenesis in K-ras(LA1) mice. Cancer Gene Ther, 15: 275–83.
- Gilani, K., Moazeni, E., Ramezanli, T., Amini, M., Fazeli, M.R. and Jamalifar, H. (2011). Development of respirable nanomicelle carriers for delivery of amphotericin B by jet nebulization. J Pharm. Sci, 100: 252–9.
- Learoyd, T.P., Burrows, J.L., French, E., Seville, P.C. (2009). Sustained delivery by leucine-modified chitosan spray-dried respirable powders. Int J Pharm., 372: 97–104.
- Naikwade, S.R., Bajaj, A.N., Gurav, P., Gatne, M.M. and Singh, P. (2009). Development of budesonide microparticles using spray-drying technology for pulmonary administration: design, characterization, in vitro evaluation, and in vivo efficacy study. AAPS Pharm Sci Tech, 10: 993–1012.
- Ahmad, Z. and Khuller, G.K. (2008). Alginate-based sustained release drug delivery systems for tuberculosis. Expert Opin Drug Deliv, 5: 1323–34.
- Alipour, S., Montaseri, H. and Tafaghodi, M. (2010). Preparation and characterization of biodegradable paclitaxel loaded alginate microparticles for pulmonary delivery. Colloids Surf B Biointerfaces, 81: 521–9.
- El-Sherbiny, I.M. and Smyth, H.D. (2010). Biodegradable nano-micro carrier systems for sustained pulmonary drug delivery: (I) self-assembled nanoparticles encapsulated in respirable/swellable semi-IPN microspheres. Int J Pharm, 395: 132–41.
- Frasher, J.R.E., Laurent, T.C., Laurent, U.B.G. (1997). Hyaluronan: Its nature, distribution, functions and turnover. Journal of Internal Medicine, 242: 27–33.
- Liao, Y.H., Jones, S.A., Forbes, B., Martin, G.P. and Brown, M.B. (2005). Hyaluronan pharmaceutical characterization and drug delivery. Drug Deliv, 12: 327–42.
- Hwang, S.M., Kim, D.D., Chung, S.J. and Shim, C.K. (2008). Delivery of ofloxacin to the lung and alveolar macrophages via hyaluronan microspheres for the treatment of tuberculosis. J Control Release, 129: 100–6.
- Surendrakumar, K., Martyn, G.P., Hodgers, E.C., Jansen, M. and Blair. J.A. (2003). Sustained release of insulin from sodium hyaluronate based dry powder formulations after pulmonary delivery to beagle dogs. J Control Release, 91: 385–94.
- Morimoto, K., Metsugi, K., Katsumata, H., Iwanaga, K. and Kakemi, M. (2001). Effects of low-viscosity sodium hyaluronate preparation on the pulmonary absorption of rh-insulin in rats. Drug Dev Ind Pharm, 27: 365–71.
- Kumar, A., Sahoo, B., Montpetit, A., Behera, S., Lockey, R.F. and Mohapatra, S.S. (2007). Development of hyaluronic acid-Fe2O3 hybrid magnetic nanoparticles for targeted delivery of peptides. Nanomedicine, 3: 132–7.
- Lee, E.S., Kwon, M.J., Na, K. and Bae, J.H. (2007). Protein release behavior from porous microparticle with lysozyme/hyaluronate ionic complex. Colloids Surf B Biointerfaces, 55: 125–30.
- Li, H.Y., Song, X. and Seville, P.C. (2010). The use of sodium carboxymethylcellulose in the preparation of spray-dried proteins for pulmonary drug delivery. Eur J Pharm Sci, 40:56–61.
- Griesenbach, U., Meng, C., Farley, R., Wasowicz, M.Y., Munkonge, F.M., Chan, M., Stoneham, C., Sumner-Jones, S.G., Pringle, I.A., Gill, D.R., Hyde, S.C., Stevenson, B., Holder, E., Ban, H., Hasegawa, M., Cheng, S.H., Scheule, R.K., Sinn, P.L., McCray, P.B. Jr. and Alton, E.W. (2010). The use of carboxymethylcellulose gel to increase non-viral gene transfer in mouse airways. Biomaterials, 31: 2665–72.
- Dailey, L.A., Kleemann, E., Wittmar, M., Gessler, T., Schmehl, T., Roberts, C., Seeger, W., Kissel, T. (2003). Surfactant-free, biodegradable nanoparticles for aerosol therapy based on the branched polyesters, DEAPA-PVAL-g-PLGA. Pharm Res, 20: 2011–20.
- Li, H.Y. and Seville, P.C. (2009). Novel pMDI formulations for pulmonary delivery of proteins. Int J Pharm, 385: 73–8.
- Yang, W., Chow, K.T., Lang, B., Wiederhold, N.P., Johnston, K.P. and Williams, R.O. (2010). In vitro characterization and pharmacokinetics in mice following pulmonary delivery of itraconazole as cyclodextrin solubilized solution. Eur J Pharm Sci, 39: 336–47.
- Thi, T.H., Azaroual, N., Flament and M.P. (2009). Characterization and in vitro evaluation of the formoterol/cyclodextrin complex for pulmonary administration by nebulization. Eur J Pharm Biopharm, 72: 214–8.
- Tolman, J.A., Nelson, N.A., Son, Y.J., Bosselmann, S., Wiederhold, N.P., Peters, J.I., McConville, J.T. and Williams, R.O. (2009). Characterization and pharmacokinetic analysis of aerosolized aqueous voriconazole solution. Eur J Pharm Biopharm, 72: 199–205.
- Jalalipour, M., Najafabadi, A.R., Gilani, K., Esmaily, H. and Tajerzadeh, H. (2008). Effect of dimethyl- beta-cyclodextrin concentrations on the pulmonary delivery of recombinant human growth hormone dry powder in rats. J Pharm Sci, 97: 5176–85.
- Bonacucina, G., Martelli, S. and Palmieri, G.F. (2004). Rheological, mucoadhesive and release properties of Carbopol gels in hydrophilic cosolvents. Int. J. Pharm, 282: 115–130.
- Doan, T.V. and Oliver, J.C. (2009). Preparation of rifampicin-loaded PLGA microspheres for lung delivery as aerosol by premix membrane homogenization. Int J Pharm, 382: 61–6.
- Anais, J.P., Razzouq, N., Carvalho, M., Fernandez, C., Astier, A., Paul, M., Astier, A., Fessi, H. and Lorino, A.M. (2009). Development of alpha-tocopherol acetate nanoparticles: Influence of preparative processes. Drug Dev Ind Pharm, 35: 216–23.
- Sommerville, M.L. and Hickey, A.J. (2003). Aerosol generation by metered-dose inhalers containing dimethyl ether/propane inverse microemulsions. AAPS PharmSciTech, 4: E58.
- Butz, N., Porté, C., Courrier, H., Krafft, M.P. and Vandamme, T.F. (2002). Reverse water-in-fluorocarbon emulsions for use in pressurized metered-dose inhalers containing hydrofluoroalkane propellants. Int J Pharm, 238: 257–69.
- Sommerville, M.L., Cain, J.B., Johnson, C.S. Jr. and Hickey A.J. (2000). Lecithin inverse microemulsions for the pulmonary delivery of polar compounds utilizing dimethylether and propane as propellants. Pharm Dev Technol, 5: 219–30.
- Makidon, P.E., Nigavekar, S.S., Bielinska, A.U., Mank, N., Shetty, A.M., Suman, J., Knowlton, J., Myc, A., Rook, T. and Baker, J.R., Jr. (2010). Characterization of stability and nasal delivery systems for immunization with nanoemulsion-based vaccines. J Aerosol Med Pulm Drug Deliv, 23: 77–89.
- Dickinson, P.A., Howells, S.W. and Kellaway, I.W. (2001). Novel nanoparticles for pulmonary drug administration. J Drug Target, 9: 295–302.
- Wu, L., Bharatwaj, B., Panyam, J. and da Rocha, S.R. (2008). Core-shell particles for the dispersion of small polar drugs and biomolecules in hydrofluoroalkane propellants. Pharm Res, 25: 289–301.
- Tian, Y., Klegerman, M.E. and Hickey, A.J. (2004). Evaluation of microparticles containing doxorubicin suitable for aerosol delivery to the lungs. PDA J Pharm Sci Technol, 58: 266–75.
- Cañete, M., Soriano, J., Villanueva, A., Roca, A.G., Veintemillas, S., Serna, C.J., Miranda, R. and Del Puerto Morales, M. (2010). The endocytic penetration mechanism of iron oxide magnetic nanoparticles with positively charged cover: A morphological approach. Int J Mol Med, 26: 533–9.
- Zhu, M.T., Feng, W.Y., Wang, B., Wang, T.C., Gu, Y.Q., Wang, M., Wang, Y., Ouyang, H., Zhao, Y.L., Chai, Z.F. (2008). Comparative study of pulmonary responses to nano- and submicron-sized ferric oxide in rats. Toxicology, 247: 102–11.
- Martin, A.R., Thompson, R.B. and Finlay, W.H. (2008). MRI measurement of regional lung deposition in mice exposed nose-only to nebulized superparamagnetic iron oxide nanoparticles. J Aerosol Med Pulm Drug Deliv, 21: 335–42.
- Dames, P., Gleich, B., Flemmer, A., Hajek, K., Seidl, N., Wiekhorst, F., Eberbeck, D., Bittmann, I., Bergemann, C., Weyh, T., Trahms, L., Rosenecker, J. and Rudolph, C. (2007). Targeted delivery of magnetic aerosol droplets to the lung. Nat Nanotechnol, 2: 495–9.
- Xie, Y., Longest, P.W., Xu, Y.H., Wang, J.P. and Wiedmann, T.S. (2010). In vitro and in vivo lung deposition of coated magnetic aerosol particles. J Pharm Sci, 99: 4658–68.
- Kessinger, C.W., Khemtong, C., Togao, O., Takahashi, M., Sumer, B.D. and Gao, J. (2010). In vivo angiogenesis imaging of solid tumors by alpha(v)beta(3)-targeted, dual- modality micellar nanoprobes. Exp Biol Med (Maywood), 235: 957–65.
- Guthi, J.S., Yang, S.G., Huang, G., Li, S., Khemtong, C., Kessinger, C.W., Peyton, M., Minna, J.D., Brown, K.C. and Gao, J. (2010). MRI-visible micellar nanomedicine for targeted drug delivery to lung cancer cells. Mol Pharm, 7: 32–40.
- Gosens, I., Post, J.A., de la Fonteyne, L.J., Jansen, E.H., Geus, J.W., Cassee, F.R. and de Jong, W.H. (2010). Impact of agglomeration state of nano- and submicron sized gold particles on pulmonary inflammation. Part Fibre Toxicol, 7: 37.
- Sadauskas, E., Jacobsen, N.R., Danscher, G., Stoltenberg, M., Vogel, U., Larsen, A., Kreyling, W. and Wallin, H. (2009). Biodistribution of gold nanoparticles in mouse lung following intratracheal instillation. Chem Cent J, 3:16.
- Lipka, J., Semmler-Behnke, M., Sperling, R.A., Wenk, A., Takenaka, S., Schleh, C., Kissel, T., Parak, W.J. and Kreyling, W.G. Biodistribution of PEG-modified gold nanoparticles following intratracheal instillation and intravenous injection. Biomaterials, 31: 6574–81.
- Brown, S.D., Nativo, P., Smith, J.A., Stirling, D., Edwards, P.R., Venugopal, B., Flint, D.J., Plumb, J.A., Graham, D. and Wheate, N.J. (2010). Gold nanoparticles for the improved anticancer drug delivery of the active component of oxaliplatin. J Am Chem Soc, 132: 4678–84.
- Warheit, D.B., Sayes, C.M. and Reed, K.L. (2009). Nanoscale and fine zinc oxide particles: Can in vitro assays accurately forecast lung hazards following inhalation exposures? Environ Sci Technol, 43: 7939–45.
- Tong, Y., Zhang, G., Li, Y., Tan, M., Wang, W., Chen, J., Hwu, Y., Hsu, P.C., Je, J.H., Margaritondo, G., Song, W., Jiang, R. and Jiang, Z. (2006). Synchrotron microradiography study on acute lung injury of mouse caused by PM (2.5) aerosols. Eur J Radiol58: 266–72.
- Pauluhn, J. and Rosenbruch, M. (2003). Inhalation toxicity of propineb. Part I: Results of subacute inhalation exposure studies in rats. Inhal Toxicol, 15: 411–34.
- Langenback, E.G., Davis, J.M., Robbins, C., Sahgal, N., Perry, R.J. and Simon, S.R. (1999). Improved pulmonary distribution of recombinant human Cu/Zn superoxide dismutase, using a modified ultrasonic nebulizer. Pediatr Pulmonol, 27: 124–9.
- Amdur, M.O and Chen, L.C. (1989). Furnace-generated acid aerosols: Speciation and pulmonary effects. Environ Health Perspect, 79: 147–50.
- Igor, L., Satterstrom, F. and Corey, L. (2008). Nanotoxicology and nanomedicine: Making hard decisions. Nanomedicine: Nanotechnology, Biology and Medicine, 4: 167–171.
- Suh, W.H., Suslick, K.S., Stucky, G.D. and Suh, Y.H. (2009). Nanotechnology, nanotoxicology, and neuroscience. Prog Neurobiol, 87: 133–70.
- Shvedova, A., Valerian, E.K. and Bengt, F. (2010). Close encounters of the small kind: Adverse effects of man made material interfacing with the nano-cosmos of biological systems. Annu Rev Pharmacol Toxicol, 50: 63–88.
- Stone, V., Johnston, H. and Clift, M.J. (2007). Air pollution, ultrafine and nanoparticle toxicology: Cellular and molecular interactions. IEEE Trans Nanobioscience, 6: 331–40.
- Tsytsikova, L. (2009). Global List of Organizations and Efforts Related to Nanotechnology, Nanoscience, Nanomaterials, and Food and Agriculture Products. Available at: http://www.ilsi.org/NorthAmerica/Documents/FOOD%20CHEMICAL%20SAFETY/Global%20List%20of%20Organizations%20and%20Efforts%20Related%20to%20Nanotechnology.pdf. Accessed on 18 March 2011.