Abstract
Soy- or red clover- derived products containing isoflavones have been amply studied in climacteric and postmenopausal women, and confusing contradicting results have been published. The beneficial effects on climacteric complaints, cholesterol and the development of osteoporosis are marginally at best and there are no uterine and mammary safety studies. In males, however, isoflavones may protect the prostate to make them less prone to develop cancer. Cell biological and animal experimental data support this notion. Clinical data about possible beneficial effects on cholesterol or in the bone are largely missing. Hence, soy or red clover products containing the mild estrogenic isoflavones with a slightly higher affinity to the estrogen receptor of the β in comparison to the α subtype may prove to have some beneficial effects in males.
Introduction
The sales volume of phytoestrogens-containing food additives has increased since the publication of the Women's Health initiative [Citation1] and the million women study. In these studies, hormone replacement therapy of postmenopausal women with estrogens and progestins was shown to result in an increased incidence of mammary cancers and arteriosclerotic events such as heart attacks and strokes. The basis for increased sales of phytoestrogen-containing food additives is the ‘Japanese Phenomenon’ (for review see Refs. [Citation2,Citation3]). The incidence of mammary cancer in Japanese women is lower than in Central European or Caucasian Americans. When Japanese women migrate to the United States the prevalence of mammary cancer in the daughter generation is as high as in the Caucasian American population. This indicates that environmental rather than racial differences are responsible for this phenomenon. The most radical change in lifestyle of Japanese following migration is dietary habit. In Japan, protein needs are primarily covered by soy products whereas in America more meat is being consumed. Therefore, scientists sought for soy ingredients that might be responsible for this phenomenon and discovered that isoflavones may be of critical importance for the ‘Japanese Phenomenon’. On this basis, food additives claimed that their isoflavone-containing products would reduce the incidence of mammary cancer and it was inferred that this was also true when isoflavone was started at the time of menopause. It was also claimed that the slightly higher preference of isoflavones to estrogen receptor (ER) of the beta subtype (ERβ) in comparison to the alpha subtype (ER-α) would be beneficial for the mammary glands. However, it turned out that the biological potency of the isoflavones exerted at the transcriptional level was similar for both, ER-α and ERβ [Citation4].
Experimentally it was made probable that the exposure of the developing mammary gland to isoflavones may be of critical importance for later effects [Citation5] and later Rowell et al. [Citation6] were able to demonstrate that this was due to an increased differentiation of mammary gland following isoflavone exposure during puberty. In this context, a study investigating the migration of Japanese girls prior to and after puberty became important because they unraveled the fact that in the human the peri-pubertal exposure to isoflavones may result in decreased incidence of mammary cancers some decades later as those girls who migrated prior to puberty had a higher rate of mammary cancers than those who migrated from Japan to the United states post-pubertally, i.e. at a time when the mammary gland was peri-pubertally exposed to soy products [Citation7]. In addition, it was demonstrated [Citation8] that the peri-pubertal soy (isoflavone) intake inversely correlated with the occurrence of breast cancer. In adult, ovariectomized animals isoflavones stimulate uterine and mammary gland tissue and cause proliferation of carcinogen induced mammary cancers [Citation9]. Furthermore, isoflavone containing preparations proved to have little, if any effect on climacteric complaints [Citation10].
Surprisingly, little attempts were made to ‘conquer’ males as consumers of isoflavone containing preparations although Japanese men experience significantly less prostate cancers than Caucasian US Americans. Recently it was shown that populations which had a lifelong high-soy intake had a 30% reduction in prostate cancer risk [Citation11].
Isoflavones, also called phytoestrogens are substances produced at low levels by almost all plants and they have weak estrogenic properties. High amounts of isoflavones are produced by soy beans and red clover and therefore highly purified isoflavone containing extracts of these plants dominate the commercial market. The best studied isoflavones are Genistein (Gen) and Daidzein (Daid) and lately, also Equol, which is not naturally occurring in soy or red clover but which can be formed by the gut flora of some (20–30%) but not of all human beings [Citation12,Citation13].
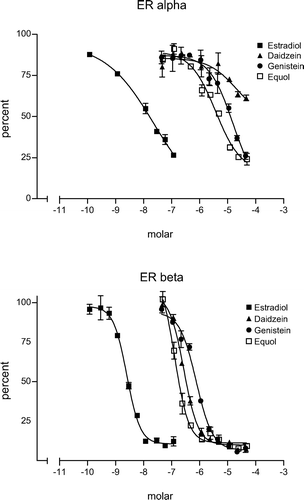
Because of their structural similarity with estradiol, 17β isoflavones bind to ER (), and we and others demonstrated that clear actions are mediated via the ER-α in vitro and in vivo such as increased uterine proliferation in ovx rodents [Citation4,Citation9,Citation10]. It was therefore surprising to see that at the recent, ‘Eighth International Symposium on the role of Soy in Health Promotion and Chronic disease Prevention and Treatments’, studies in males were underrepresented particularly in view of the potentially beneficial effects not only in the prostate but also in the bone.
Effects of isoflavones in the male
Experimental data
Highly controversial data have been published concerning the effects of isoflavones on male reproduction when animals – primarily rodents – were exposed to isoflavones at different ages. While in studies pre- and early postnatal treatment impaired function of the male reproductive system including sperm counts later in life [Citation14], other studies did not see such effects [Citation15,Citation16]. Since many baby foods contain high amounts of soy protein-containing isoflavones these data deserve particular attention. It is also important that in a study involving infertile male patients with high isoflavone intake had impaired reproductive parameters [Citation17] while in other studies such effects were not observed [Citation18].
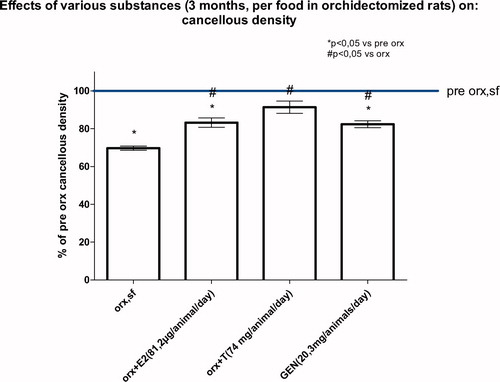
In orx rats Gen as well as daidzein appear to have relatively potent antiosteoporotic effects following castration. Rats develop severe osteoporosis (reduction of cancellous mineral density by more than 30%) and this can be highly effectively prevented by estradiol, Gen, and testosterone (). Studies on the clinical data of bone metabolism in male patients prone to develop osteoporosis are scarce and largely inconclusive.
Figure 2. Effect of a 3 months lasting treatment of orx rats with estradiol (E2), testosterone (T) or genistein (Gen) on cancellous density of the metaphysis of the tibia. Within the 3 months following orx rats lost ∼30% of their cancellous density and this was partially prevented by E2, T or Gen.
In more than 40 studies involving animals that develop either spontaneously prostate tumors or in which tumors were evoked by carcinogens (in methynitrosuria (MNU) or dimethyl-benzanthrazen (DMBA) or 2-amino-1 methyl-6-phenylimidazopyridin (PAIP]) or in transgenic mice xenografted models, etc., it was shown that isoflavones are capable to reduce incidence and size of prostate tumors. Their effects are obviously exerted at different levels of development of prostate cancers as an early event, i.e. the development of prostate intraepithelial neoplasias (PINs) as well as their development to carcinomas can be clearly effected by isoflavones.
Animal experiments gave evidence that all the three receptors for sex steroids, i.e. the androgen receptor (AR) and the two ERs, ER-α and ERβ, play important roles in maintaining the homeostasis within the prostate gland.
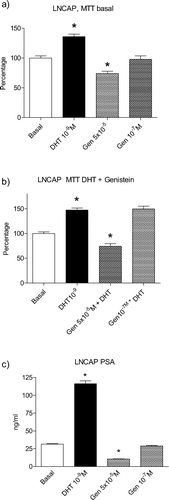
There is also unequivocal evidence that isoflavones, again Gen and Daid are the best explored, may inhibit the development of prostate cancers in a number of experimental models [Citation11,Citation19,Citation20]. The human prostate cancer-derived cell line LNCaP is widely used to test the efficacy of compounds under in vitro conditions. The proliferation of these cells under basal conditions was dose dependently inhibited by Gen (). Likewise, the proliferation of LNCaP cells, under dihydrotestosterone (DHT)-stimulation conditions was also significantly inhibited by Gen (). Prostate-specific antigen (PSA) secretion of these cells was stimulated by DHT () but as profoundly reduced by Gen. This effect was not seen under concentrations which address most likely only ERs. The higher concentrations necessary to evoke these inhibitory effects are known to inhibit also tyrosine kinase activity [Citation21,Citation22]. For Tectoreginin, another potent estrogenic isoflavone, some of the mechanisms of actions were elaborated [Citation23] and they involved reduced cell proliferation, reduced AR expression as expression of a AR co-activator and of the prostate derived Ets transcription factor (PDEF). Also, expression of the PSA gene and its protein production were reduced by Tectoreginin. Interestingly this compound increased gene expression of ERβ. Deletion of ERβ in ERβ-knockout mice resulted in the development of PIN and such PINs are believed to be precancerous events occurring also in the human prostate [Citation24]. Hence, an overexpression of ERβ or treatment with ERβ selective drugs may be beneficial for the prostate to reduce the occurrence of PINs and hence, of later occurring prostate cancers.
Figure 3. Effects of 2 concentrations of genistein (Gen) on basal proliferation () or DHT stimulated proliferation () of LNCaP cells. depicts serum PSA levels in the culture medium.
There is overwhelming evidence that testosterone-mediated mechanisms are involved in the development of the prostate and on its cancerous dedifferentiation [Citation25]. We showed recently that the potent anti-androgen Vinclocolin increased the gene expression of the AR in the prostate and the epididymidis of rats [Citation26] and consequently we demonstrated that treatment of orchidectomized rats with testosterone decreased AR gene expression. Nevertheless, the androgen treatment stimulated prostate growth and others showed that it stimulated the development and growth of prostate cancer in gene targeted mice which develop prostate cancers spontaneously [Citation27].
Potent effects on the expression of the sex steroid receptor genes were also exerted by estradiol-17β (E2). The expression of the AR and ERβ genes was increased under E2 treatment whereas ER-α gene expression remained unaffected. Treatment with moderately high doses of Gen which exerted clear antiosteoporotic effects in the orx animals had no effects on the expression of the sex steroid receptors.
The ER-α mediated effects of estrogens in the prostate appear to be adverse. Chronic E2 administration results in squamous metaplasia of the basal epithelial cells due to a proliferative effect of the steroid [Citation28,Citation29]. In addition estrogens may cause inflammation in the prostate. These adverse effects of estrogens are ER-α mediated as they cannot be elicited in ER-α knockout mice and only ER-α but not ERβ selective ligands will induce such effects [Citation29]. In normal prostates, ER-α is only expressed by stromal cells which are capable of converting androgens into estrogens due to their aromatase activity [Citation29,Citation30]. In contrast to the adverse action of estrogens mediated via the ER-α there is increasing evidence that estrogens and particularly estrogens with a high affinity to the ERβ may exert beneficial effects in the prostate [Citation28,Citation29,Citation31,Citation32]. The ERβ is primarily expressed within the glandular epithelium and following initiation of transactivation antiproliferative, anti-inflammatory, and anticarcinogenic effects appear to be mediated upon stimulation of this receptor subtype [Citation28,Citation29]. This is substantiated in ERβ-knockout mice which often develop focal lesions, so called PINs which are pre-malignancies [Citation31].
Asian populations cover their protein needs primarily through soy, i.e. isoflavone-containing food stuff and they develop less prostate cancers than populations which are primarily meat eaters. This has led to intensive investigations of the effects of isoflavones and selective ERβ agonists on the development of prostate cancer in a number of animal models. As mentioned earlier many phytoestrogens including Gen or Daid have a slightly higher affinity for the ER-β (see ) and it was therefore suggested that the ERβ may be responsible for the anticarcinogenic effects of the isoflavones.
In vivo, the effects of isoflavones have been studied in mice and rats under a variety of endocrine or gene targeted conditions.
Under very high doses, isoflavones appear to downregulate AR gene expression in the prostate of intact rats [Citation33] suggesting that such treatment may decrease the effects of androgens within the prostate. This is in line with the observation that Gen and Daid prevented the carcinogen-induced occurrence of prostate cancers in Lobund-Wistar rats in which their high testosterone levels result often in spontaneous occurrence of prostate tumor which can be augmented by MNU, a classical carcinogen [Citation34].
The classical treatment of prostate carcinomas in men is minimization or elimination of AR transactivation. Isoflavones are not ligands for the AR. Their effects appear to involve primarily activation of the ER of the β-subtype. The observations that Gen may reduce AR gene expression may be an explanation for the observed effects on PSA secretion which we and others observed [Citation22,Citation35].
The mechanisms of action of isoflavones are multifactorial. A reduction of testosterone levels in rats fed with a phytoestrogen-rich diet in mice contrasts inconsistent effects of isoflavones on hormone levels in men [Citation36]. Consequently, in men no effects on gonadotropins were observed. Molecular studies in prostate cancer cells (primarily in LNCaP cells were investigated) uncovered the involvement of proteasome activity which plays an essential role in promoting tumor cell proliferation and on angiogenesis and matrix metal proteinase expression. Both mechanisms are important for invasion and metastasis of tumor cells. Growth factor signaling pathways such as the epidermal growth factor and the insulin like growth factor as well as expression of a number of important intracellular signaling pathways have been investigated and this was recently reviewed by Steiner et al. 2008 [Citation19].
Human data
In numerous clinical studies, the effects of isoflavone-containing foods on serum lipids were determined. On the basis of 38 trials which reported low density lipoprotein (LDL) lowering effects of soy protein, the US FDA approved a claim that such preparations have protective effects against heart diseases [Citation37]. This was however, revised in 2006. The American Health Association stated that ‘the direct cardiovascular health benefit of soy protein or isoflavone supplements is minimal at best’ [Citation38]. Therefore, the Food and Drug Administration of the United States (US FDA) is currently debating this issue.
On the basis of in vitro and in vivo animal studies concern had arisen that isoflavones may inhibit thyroid peroxidase, the enzyme responsible for thyroid hormone synthesis [Citation39,Citation40]. This was however, not found in healthy males under a daily intake of a high isoflavone (62 mg/day) soy protein isolate. All thyroid parameters remained unchanged when compared to values in the same subjects treated daily with 1.6 mg isoflavones [Citation41].
Besides radical surgery, which is still the therapy of choice, in earlier days, prostate cancer was often treated with synthetic estrogen diethylstilbestrol which inhibited pituitary luteinizing hormone (LH) and thereby testosterone production. This therapy is nowadays obsolete because of severe adverse cardiovascular side effects [Citation42]. Castration or ‘unbloody’ castration by gonadotropin releasing hormone (GnRH) analogs is still widely used for the therapy of prostate cancer and lately 5α-reductase-inhibitors – the best investigated in finasteride – proved to reduce the number of prostate cancers when given prophylactically [Citation43] or therapeutically [Citation44].
Development of prostate cancer involves estrogenic effects primarily mediated via the EB-α and activation of the AR. Isoflavones have a slightly higher affinity to the ERβ and activation of ERβ-mediated intraprostatic cascades appear to have anticarcinogenic effects. This may explain the beneficial effects exerted in Far East Asian population with a high lifelong-lasting soy intake.
In a number of epidemiological studies it was a shown that the consumption of soy foods is associated with the reduction in cancer risk in men. These studies were primarily performed in Asian populations and the conclusions were drawn on the basis of either the amount of soy food intake or on the basis of serum isoflavones. In a recent review of 15 epidemiologic studies it was concluded that consumption of soy foods reduces the risk of prostate cancer but this may depend on the type and quality of the consumed soy food [Citation20]. In three recent epidemiologic studies – one performed in Japan, the two others in Europe – negative data were reported. The Japanese investigators performed a nested case–control study involving more than 14,000 men aged 40–69 with a mean observation time of 12.8 years and 251 cases of prostate cancer were observed [Citation45]. The authors reported a trend for an inverse relation between blood Gen levels and prostate cancer. Particularly localized but not advanced cases of prostate cancer correlated inversely with serum isoflavones. On that basis, the authors conclude that isoflavones may reduce the risk to develop prostate cancer. Median serum Gen and Daid concentrations in these patients were 89.3 and 37.0 ng/ml in the patients with prostate cancers. The two European studies were also nested in a larger investigation. In one report [Citation46] no correlation was found between seven phytoestrogens (Gen, Daid, glycitein, ortho-desmethylangolensin [o-DMA], equol, enterodiol, enterolactone) in the serum and urine when 193 cases of prostate cancer were compared with 828 controls. In the other study [Citation47], a larger European population was included and 950 cases of prostate cancers were compared with 1042 healthy controls. Overall, in patients no significant correlation between Gen, Daid equol enterolactone or enterodiol could be established. Higher plasma levels of Gen (<7ng/ml) however, correlated inversely with the occurrence of prostate cancer. In the two European studies much lower serum phytoestrogens levels were reported ranging all below 10 ng/ml. It can be assumed that not only in all Asian patients but also in western patients with a high-isoflavone intake the isoflavone exposition was of a long duration. Therefore, the generally assumed opinion that high-serum isoflavone concentrations protect against cancer may be true but is not very solid. There are few intervention studies available in which results were inconsistent. In a study involving 34 men with elevated serum PSA levels, the daily intake of 42 mg Gen and 27 mg Daid for 6 weeks did not significantly affect the levels of this tumormarker [Citation48,Citation49]. Also when effects of a 1 year lasting daily administration of 83 mg isoflavones were compared to placebo treatment no significant changes in serum PSA levels in apparently healthy men (mean age 64 years) [Citation49] were seen whereas in one small study [Citation50] a minimal reducing effect was shown to be exerted by a low-fat, high-soy diet. In another study [Citation51] 100 mg of soy isoflavones given orally to patients with prostate carcinoma over a median period of 2–5 months yielded similar data. This treatment did not result in decreased PSA levels but a stabilization of PSA occurred in 83% of hormone sensitive and in 35% of hormone refractory patients and it did not affect serum testosterone levels. The authors of this study suggested that soy isoflavones may be beneficial for some patients with prostate cancer.
In a most recent phase II intervention study 20 patients with prostate cancer with rising PSA levels received three times daily 47 mg isoflavones over a period of 10 weeks. Slope of PSA rise was decreased in six patients although their overall levels still increased. In two patients the slope increased, in the rest no change in slope was observed [Citation52].
Take home message
Taken together, these results suggest that a lifelong isoflavone exposure may result in epigenetic phenomena which may reduce the occurrence of prostate cancer in aged men. Whether isoflavones may have such beneficial effects when taken up by aged men at a time prior to clinical occurrence of prostate cancer or even when prostate cancer had occurred may be possible and is thus an interesting hypothesis but clearly more intervention studies need to be done to solve this question.
Declaration of interest: The authors report no conflict of interest. Authors alone are responsible for the content and writing of the paper.
References
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA 2002;288:321–333.
- Adlercreutz H. Phyto-oestrogens and cancer. Lancet Oncol 2002;3:364–373.
- Munro IC, Harwood M, Hlywka JJ, Stephen AM, Doull J, Flamm WG, Adlercreutz H. Soy isoflavones: a safety review. Nutr Rev 2003;61:1–33.
- Mueller SO, Simon S, Chae K, Metzler M, Korach KS. Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor alpha (ERalpha) and ERbeta in human cells. Toxicol Sci 2004;80:14–25.
- Lamartiniere CA. Timing of exposure and mammary cancer risk. J Mammary Gland Biol Neoplasia 2002;7:67–76.
- Rowell C, Carpenter DM, Lamartiniere CA. Chemoprevention of breast cancer, proteomic discovery of genistein action in the rat mammary gland. J Nutr 2005;135:2953S–2959S.
- Ziegler RG, Hoover RN, Pike MC, Hildesheim A, Nomura AM, West DW, Wu-Williams AH, Kolonel LN, Horn-Ross PL, Rosenthal JF, Hyer MB. Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst 1993;85:1819–1827.
- Shu XO, Jin F, Dai Q, Wen W, Potter JD, Kushi LH, Ruan Z, Gao YT, Zheng W. Soyfood intake during adolescence and subsequent risk of breast cancer among Chinese women. Cancer Epidemiol Biomarkers Prev 2001;10:483–488.
- Rimoldi G, Christoffel J, Seidlova-Wuttke D, Jarry H, Wuttke W. Effects of chronic genistein treatment in mammary gland, uterus, and vagina. Environ Health Perspect 2007;115 (Suppl 1):62–68.
- Thelen P, Burfeind P, Schweyer S, Scharf JG, Wuttke W, Ringert RH. Molecular principles of alternative treatment approaches for hormone-refractory prostate cancer. Urologe A 2007;46:1271–1274.
- Yan L, Spitznagel EL. Meta-analysis of soy food and risk of prostate cancer in men. Int J Cancer 2005;117:667–669.
- Vatanparast H, Chilibeck PD. Does the effect of soy phytoestrogens on bone in postmenopausal women depend on the equol-producing phenotype. Nutr Rev 2007;65:294–299.
- Vafeiadou K, Hall WL, Williams CM. Does genotype and equol-production status affect response to isoflavones? Data from a pan-European study on the effects of isoflavones on cardiovascular risk markers in post-menopausal women. Proc Nutr Soc 2006;65:106–115.
- Wisniewski AB, Cernetich A, Gearhart JP, Klein SL. Perinatal exposure to genistein alters reproductive development and aggressive behavior in male mice. Physiol Behav 2005;84:327–334.
- Jung EY, Lee BJ, Yun YW, Kang JK, Baek IJ, Jurg MY, Lee YB, Sohn HS, Lee JY, Kim KS, Yu WJ, Do JC, Kim YC, Nam SY. Effects of exposure to genistein and estradiol on reproductive development in immature male mice weaned from dams adapted to a soy-based commercial diet. J Vet Med Sci 2004;66:1347–1354.
- Roberts D, Veeramachaneni DN, Schlaff WD, Awoniyi CA. Effects of chronic dietary exposure to genistein, a phytoestrogen, during various stages of development on reproductive hormones and spermatogenesis in rats. Endocrine 2000;13:281–286.
- Chavarro JE, Toth TL, Sadio SM, Hauser R. Soy food and isoflavone intake in relation to semen quality parameters among men from an infertility clinic. Hum Reprod 2008;23:2584–2590.
- Hamilton-Reeves JM, Vazquez G, Duval SJ, Phipps WR, Kurzer MS, Messina MJ. Clinical studies show no effects of soy protein or isoflavones on reproductive hormones in men: results of a meta-analysis. Fertil Steril 2009. [Epub ahead of print].
- Steiner C, Arnould S, Scalbert A, Manach C. Isoflavones and the prevention of breast and prostate cancer: new perspectives opened by nutrigenomics. Br J Nutr 2008;99E (Suppl 1):ES78–ES108.
- Yan L, Spitznagel EL. Soy consumption and prostate cancer risk in men: a revisit of a meta-analysis. Am J Clin Nutr 2009;89:1155–1163.
- Ravindranath MH, Muthugounder S, Presser N, Viswanathan S. Anticancer therapeutic potential of soy isoflavone, genistein. Adv Exp Med Biol 2004;546:121–165.
- Bektic J, Berger AP, Pfeil K, Dobler G, Bartsch G, Klocker H. Androgen receptor regulation by physiological concentrations of the isoflavonoid genistein in androgen-dependent LNCaP cells is mediated by estrogen receptor beta. Eur Urol 2004;45:245–251; discussion 251.
- Thelen P, Peter T, Hunermund A, Kaulfuss S, Seidlova-Wuttke D, Wuttke W, Ringert RH, Seseke F. Phytoestrogens from Belamcanda chinensis regulate the expression of steroid receptors and related cofactors in LNCaP prostate cancer cells. BJU Int 2007;100:199–203.
- Harkonen PL, Makela SI. Role of estrogens in development of prostate cancer. J Steroid Biochem Mol Biol 2004;92:297–305.
- Imamoto T, Suzuki H, Yano M, Kawamura K, Kamiya N, Araki K, Komiya A, Nihei N, Naya Y, Ichikawa T. The role of testosterone in the pathogenesis of prostate cancer. Int J Urol 2008;15:472–480.
- Loutchanwoot P, Wuttke W, Jarry H. Effects of a 5-day treatment with vinclozolin on the hypothalamo-pituitary-gonadal axis in male rats. Toxicology 2008;243:105–115.
- Eng MH, Charles LG, Ross BD, Chrisp CE, Pienta KJ, Greenberg NM, Hsu CX, Sanda MG. Early castration reduces prostatic carcinogenesis in transgenic mice. Urology 1999;54:1112–1119.
- Ellem SJ, Risbridger GP. The dual, opposing roles of estrogen in the prostate. Ann N Y Acad Sci 2009;1155:174–186.
- Risbridger GP, Ellem SJ, McPherson SJ. Estrogen action on the prostate gland: a critical mix of endocrine and paracrine signaling. J Mol Endocrinol 2007;39:183–188.
- Ho CK, Nanda J, Chapman KE, Habib FK. Oestrogen and benign prostatic hyperplasia: effects on stromal cell proliferation and local formation from androgen. J Endocrinol 2008;197:483–491.
- Weihua Z, Makela S, Andersson LC, Salmi S, Saji S, Webster JI, Jensen EV, Nilsson S, Warner M, Gustafsson JA. A role for estrogen receptor beta in the regulation of growth of the ventral prostate. Proc Natl Acad Sci USA 2001;98:6330–6335.
- Carruba G. Estrogen and prostate cancer: an eclipsed truth in an androgen-dominated scenario. J Cell Biochem 2007;102:899–911.
- Legg RL, Tolman JR, Lovinger CT, Lephart ED, Setchell KD, Christensen MJ. Diets high in selenium and isoflavones decrease androgen-regulated gene expression in healthy rat dorsolateral prostate. Reprod Biol Endocrinol 2008;6:57.
- Pollard M, Suckow MA. Dietary prevention of hormone refractory prostate cancer in Lobund-Wistar rats: a review of studies in a relevant animal model. Comp Med 2006;56:461–467.
- Davis JN, Muqim N, Bhuiyan M, Kucuk O, Pienta KJ, Sarkar FH. Inhibition of prostate specific antigen expression by genistein in prostate cancer cells. Int J Oncol 2000;16:1091–1097.
- Dillingham BL, McVeigh BL, Lampe JW, Duncan AM. Soy protein isolates of varying isoflavone content exert minor effects on serum reproductive hormones in healthy young men. J Nutr 2005;135:584–591.
- Food labeling: health claims; soy protein and coronary heart disease. Food and Drug Administration, HHS. Final rule. Fed Regist 1999;64:57700–57733.
- Cutler GJ, Nettleton JA, Ross JA, Harnack LJ, Jacobs DR Jr., Scrafford CG, Barraj LM, Mink PJ, Robien K. Dietary flavonoid intake and risk of cancer in postmenopausal women: the Iowa Women's Health Study. Int J Cancer 2008;123:664–671.
- Doerge DR, Sheehan DM. Goitrogenic and estrogenic activity of soy isoflavones. Environ Health Perspect 2002;110 (Suppl 3):349–353.
- Schmutzler C, Gotthardt I, Hofmann PJ, Radovic B, Kovacs G, Stemmler L, Nobis I, Bacinski A, Mentrup B, Ambrugger P, Gruters A, Malendowicz LK, Christoffel J, Jarry H, Seidlova-Wuttke D, Wuttke W, Kohrle J. Endocrine disruptors and the thyroid gland – a combined in vitro and in vivo analysis of potential new biomarkers. Environ Health Perspect 2007;115 (Suppl 1):77–83.
- Dillingham BL, McVeigh BL, Lampe JW, Duncan AM. Soy protein isolates of varied isoflavone content do not influence serum thyroid hormones in healthy young men. Thyroid 2007;17:131–137.
- Malkowicz SB. The role of diethylstilbestrol in the treatment of prostate cancer. Urology 2001;58:108–113.
- Thompson IM, Tangen CM, Goodman PJ, Lucia MS, Klein EA. Chemoprevention of prostate cancer. J Urol 2009;182:499–507; discussion 508.
- Vis AN, Schroder FH. Key targets of hormonal treatment of prostate cancer. Part 2: the androgen receptor and 5alpha-reductase. BJU Int 2009;104:1191–1197.
- Kurahashi N, Iwasaki M, Inoue M, Sasazuki S, Tsugane S. Plasma isoflavones and subsequent risk of prostate cancer in a nested case–control study: the Japan Public Health Center. J Clin Oncol 2008;26:5923–5929.
- Ward H, Chapelais G, Kuhnle GG, Luben R, Khaw KT, Bingham S. Lack of prospective associations between plasma and urinary phytoestrogens and risk of prostate or colorectal cancer in the European Prospective into Cancer-Norfolk study. Cancer Epidemiol Biomarkers Prev 2008;17:2891–2894.
- Travis RC, Spencer EA, Allen NE, Appleby PN, Roddam AW, Overvad K, Johnsen NF, Olsen A, Kaaks R, Linseisen J, Boeing H, Nothlings U, Bueno-de-Mesquita HB, Ros MM, Sacerdote C, Palli D, Tumino R, Berrino F, Trichopoulou A, Dilis V, Trichopoulos D, Chirlaque MD, Ardanaz E, Larranaga N, Gonzalez C, Suarez LR, Sanchez MJ, Bingham S, Khaw KT, Hallmans G, Stattin P, Rinaldi S, Slimani N, Jenab M, Riboli E, Key TJ. Plasma phyto-oestrogens and prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Br J Cancer 2009;100:1817–1823.
- Urban D, Irwin W, Kirk M, Markiewicz MA, Myers R, Smith M, Weiss H, Grizzle WE, Barnes S. The effect of isolated soy protein on plasma biomarkers in elderly men with elevated serum prostate specific antigen. J Urol 2001;165:294–300.
- Adams KF, Chen C, Newton KM, Potter JD, Lampe JW. Soy isoflavones do not modulate prostate-specific antigen concentrations in older men in a randomized controlled trial. Cancer Epidemiol Biomarkers Prev 2004;13:644–648.
- Spentzos D, Mantzoros C, Regan MM, Morrissey ME, Duggan S, Flickner-Garvey S, McCormick H, DeWolf W, Balk S, Bubley GJ. Minimal effect of a low-fat/high soy diet for asymptomatic, hormonally naive prostate cancer patients. Clin Cancer Res 2003;9:3282–3287.
- Hussain M, Banerjee M, Sarkar FH, Djuric Z, Pollak MN, Doerge D, Fontana J, Chinni S, Davis J, Forman J, Wood DP, Kucuk O. Soy isoflavones in the treatment of prostate cancer. Nutr Cancer 2003;47:111–117.
- Pendleton JM, Tan WW, Anai S, Chang M, Hou W, Shiverick KT, Rosser CJ. Phase II trial of isoflavone in prostate-specific antigen recurrent prostate cancer after previous local therapy. BMC Cancer 2008;8:132.