Abstract
Purpose: Aging in men is characterized by a moderate decrease in plasma testosterone (T) levels. However, the association between partial androgen deficiency of the aging male and clinical symptoms and the ideal screening test are controversial. In this study, we investigated the association between the androgen levels and erectile function, cognitive functions and hypogonadism symptoms in aging males.
Materials and methods: We investigated the association between total (TT), calculated free (FT) and bioavailable (BT) testosterone, and various clinical and laboratory parameters in 103 healthy males, 50–80 years old. Biochemical assessment was done after overnight fasting. Questionnaires were used to test for hypogonadism symptoms, erectile and cognitive functions.
Results: TT levels were not correlated with aging in this study. However, FT and BT were found to decrease with age due to rising sex hormone binding globulin. TT levels were strongly correlated with FT and BT levels (respectively p = 0.0001, p = 0.0001). TT, FT and BT were only correlated with cognitive functions (p = 0.012, p = 0.004, p = 0.02 respectively). There was no correlation between TT, FT and BT levels and erectile function and hypogonadism symptoms.
Conclusion: T values in our study sample did not correlate with clinical signs and symptoms of hypogonadism. Thus, according to our data, symptoms in the aging male should not be indiscriminately assigned to a decrease in TT, FT or BT levels.
Keywords::
Introduction
World population is gradually aging owing to declining fertility, improved health and longevity. The global population of +60 year-old people reached 680 million in 2009; 11% of the world’s population. According to the latest estimates, the population aged 60 and older will increase from 680 million to 2 billion by 2050 [Citation1]. Therefore, the Earth is host to a rapidly aging population [Citation2].
Age associated alterations in hormone levels in general, and androgens in particular, in men are significant and require further research and acceptance by the medical community [Citation2,Citation3]. Up to now many published studies have stated that androgen levels decrease with age in males [Citation4–7]. Recently, this clinical issue has been named late onset hypogonadism (LOH) by International Society of Andrology, International Society for the Study of the Aging Male and European Association of Urology [Citation8].
LOH is a clinical and biochemical syndrome characterized by deficiency in the levels of serum androgen with aging. This might result in reduced quality of life and many organs being adversely affected [Citation9]. Along with aging, some physiological functions start declining, and clinical findings emerge in the form of decline in sexual desire, erectile dysfunction, depression and decrease in muscle and bone density. Such changes also occur in untreated hypogonadal young males.
In this study, we characterize the erectile function, cognitive functions and hypogonadism symptoms and attempt to associate these clinical parameters with testosterone levels in middle-aged and elderly men.
Materials and methods
This study, included males between 50–80 years old having been admitted to the urology outpatient clinic with sub-urinary system symptoms or for routine checkups between July 2005 and July 2008. We informed the participants about the study and received their written consent. After receiving the sexual, psychosocial and medical history in line with the purpose of the study, we carried out systemic and urological examinations of all patients.
Subjects with diagnosed Diabetes Mellitus, on medication due to neuropsychiatric diseases, with acute diseases 6 months prior to the study, with chronic disease, major depression or psychosis history were excluded from the study. Subjects with a body mass index higher than 40 kg/m2 and who had received hormonal treatment 6 months prior were also excluded from the study. None of the participants were taking medications such as opiates, cimetidine, glucocorticoids, phenytoin or spironolactone, which might affect the testosterone (T) level.
Hormonal and biochemical assessments
Blood samples were taken following one night of fasting between 8 and 9 am. Serum total testosterone (TT) level was measured through Chemiluminiscent Immunoassay technique via Abbott i2000 Immunoassay Analyzer (Abbott Laboratories, Abbott Park, Chicago, IL), sex hormone binding globulin (SHBG) level was measured via Immulite Analyzer (Diagnostic Products Corp., Los Angeles, CA) and albumin was measured via Beckman-LX-201 (Beckman Coulter, Inc. Carlsbad, CA). The data obtained and the levels of free testosterone (FT) and bioavailable testosterone (BT) were calculated using the Vermeulen formula [Citation10].
The following levels were accepted for this study:
Questionnaires
This study benefited from the Aging Male Survey (AMS) form, the Turkish version of which has been verified in terms of reliability and validity by the Turkish Andrology Association, in order to assess LOH symptoms of males [Citation12]. Participants were requested to grade 17 questions on a 1–5 scale. While a total score of 17–26 indicated a lack of symptoms, a score of 37–49 indicated moderate symptoms and a score over 50 indicated severe symptoms [Citation13].
This test utilized the Standardized Mini Mental Test (SMMT), which has been verified in terms of reliability and validity for the diagnosis of mild dementia in Turkish society by Dr. Can Gungen et al. [Citation14] in order to assess cognitive functions. This study considered 24 points as the threshold value.
The study used the International Index of Erectile Function (IIEF) containing 15 questions, the Turkish version of which was verified in terms of reliability and validity by the Turkish Andrology Association in 1998, in order to assess the sexual functions of the subjects [Citation15]. While a total score of 26–30 indicated no erectile dysfunction, a score of 17–25 indicated mild erectile dysfunction, a score of 11–16 indicated moderate erectile dysfunction and a score of 0–10 indicated severe erectile dysfunction. Five sub-items are assessed within the IIEF:
Erectile Function (EF) (Q1, Q2, Q3, Q4, Q5 and Q15)
Orgasmic Function (OF) (Q9 and Q10)
Sexual Desire (SD) (Q11 and Q12)
Intercourse Satisfaction (IS) (Q6, Q7 and Q8)
Overall Satisfaction (OS) (Q13 and Q14)
Statistical evaluation
We benefited from SPSS 15.0 (Statistician Package of Social Science) software while evaluating the results of the study. We used independent sample t-test to compare independent groups, and bivariate correlation analysis to investigate the correlation between the parameters of the subjects. We considered a Pearson correlation coefficient (r) of 0–0.49 as poor correlation, an r of 0.5–0.74 as moderate correlation and an r of 0.75–1 as strong correlation. We considered a value of p < 0.05 as significant and a value of p < 0.01 as highly significant.
Results
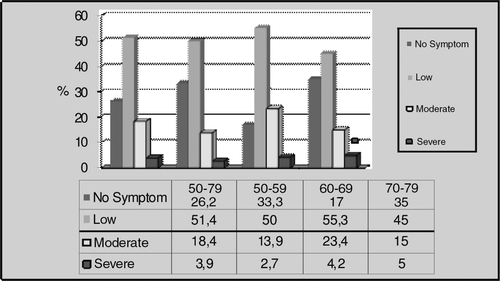
This study, included 103 subjects examined between July 2005 and July 2008. The subjects were between 51–79 years old, with an average age of 62.47 ± 6.62. We grouped the subjects into four age groups: 50-year-old–79-year-old subjects were the General Age Group; 50-year-old–59-year-old subjects as First Age Group; 60-year-old–69-year-old subjects as Second Age Group and 70-year-old–79-year-old subjects as Third Age Group. summarizes the mean and median values regarding the parameters of all age groups. We found the in-group age distribution to be homogenous in all groups.
Table I. Average values of the parameters of all age groups.
Of the subjects 34.9% (36 individuals) were in the First Age Group, 45.6% (47 individuals) were in the Second Age Group and the last 19.4% (20 individuals) were in the Third Age Group. Decline of TT, FT and BT per year were 0.4%, 1.8% and 1.7%, respectively. Decline of TT levels with age were statistically insignificant but the other levels were significant (). When the clinical parameters are specifically compared to T values, we found no significant association between the biochemical findings and results of AMS and IIEF questionnaires. But we discovered a statistically significant correlation between the SMMT scores and age, TT, FT and BT parameters. The correlation between SMMT scores and FT was stronger than the others ().
Table II. Correlation analysis of the age and T levels and other parameters in general age group.
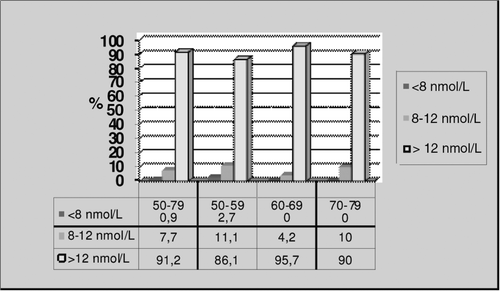
The individuals with normal TT levels (over 12 nmol/L) were 91.2% in the General Age Group and 86.1%, 95.7% and 90% in the other age groups respectively. There was only one individual with a TT level lower than 8 nmol/L, which is accepted to be the level of biochemical hypogonadism, and this individual was in the First Age Group ().
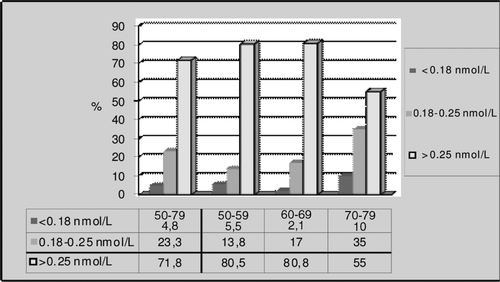
When we examined the subjects in terms of FT, we found the percentage of individuals with a value lower than 0.18 nmol/L was 4.8% in the General Age Group, and 5.5%, 2.1% and 10% in the other age groups, respectively ().
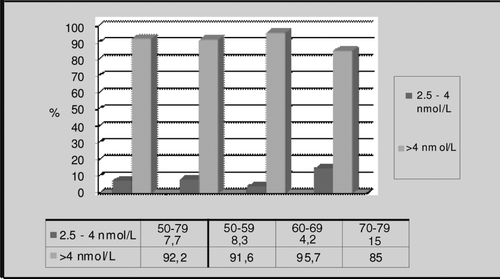
When we examined the subjects in terms of BT, we found no individual to have a value lower than 2.5 nmol/L, which indicates hypogonadism ().
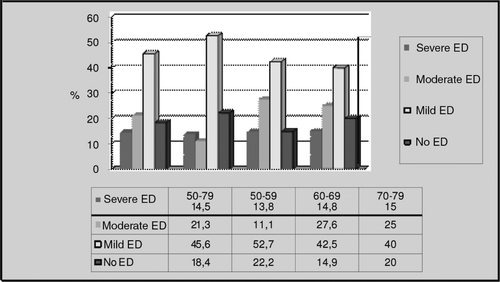
When we evaluated the subjects in terms of erectile function, we found that 81.6% of males in the General Age Group had various levels of erectile dysfunction, with 77.8%, 85.1% and 80% in the other age groups, respectively (). According to the results of the AMS questionnaire, 73.8% of General Age Group subjects had various levels of hypogonadism symptoms, with 66.7%, 83% and 65% in the other age groups, respectively (). Mean values of AMS and IIEF questionnaires were same in each group and they did not change with age.
When we assessed the subjects in terms of cognitive functions with the SMMT questionnaire, none of them received a score below the threshold value of 24.
Discussion
The role of testosterone in men’s health is still controversial [Citation16]. Exogenous testosterone or other anabolic steroids in high doses have been associated with several adverse health effects [Citation17]. However, the use of lower doses of exogenous testosterone in hypogonadal men has benefits for well-being [Citation18,Citation19].
The decrease in the testosterone level with age and its clinical consequences also remains controversial [Citation4,Citation20–22]. The indefinite numerous clinical signs that have a potential association with testosterone deficiency makes it difficult to establish a diagnosis of LOH [Citation6]. Furthermore, the prevalence of specific sexual symptoms of androgen deficiency was relatively high among men with normal testosterone levels [Citation23,Citation24]. Another controversial issue is the biochemical reference range. Until now the generally used standard for the diagnosis of biochemical LOH is the healthy young adult male range [Citation8]. Difficulties in diagnosis may also arise from uncertainty about the correct reference range to apply to various age groups, and there are reliability issues with various testosterone assays [Citation7,Citation25]. To solve this ambiguity, guidelines suggest that particular symptoms and a low level of serum testosterone are most important for the diagnosis of LOH [Citation26,Citation27].
Prevalence of LOH has been reported at different rates such as 78%, 23.3%, 12.3% and 2.1% [Citation4–7]. In our study, it was 0.9% when the lower limit for TT is accepted as 8 nmol/L but it was 8.8% if the accepted threshold of TT is 12 nmol/L. Different rates of prevalence of LOH may be attributed to measurement error in the characterization of testosterone concentrations in individuals with only one blood sample, study designs and the different used reference ranges for TT. We diagnosed biochemical hypogonadism in one subject when the lower limit for TT was accepted as 8 nmol/L, in no subjects when the threshold for BT was accepted as 2.5 nmol/L and in five subjects when the threshold for FT was accepted as 0.18 nmol/L. Wu et al. [Citation6] reported that measurement of FT when the TT level was under 8 nmol/L did not make a substantial difference with respect to the association between symptoms and testosterone level. Their findings are similar to those in previous studies [Citation28,Citation29] and supports the recommendation that TT be used as the primary biochemical criterion for the diagnosis of late-onset hypogonadism [Citation26,Citation27]. Wu et al. also reported that applying the threshold for FT may be useful in patients with multiple symptoms and a borderline TT level (8–11 nmol/L). We suggest that FT could be measured in patients who are older than 70 years and who suffer from memory loss. In our study, the average age in the third age group was 72.1, and the average TT level was 18.05 nmol/L and FT level was 0.24 ± 0.07. When the lower limit for FT was accepted as 0.25 nmol/L FT levels were in the hypogonadal range in this age group and their memory scores were lower than the other age groups.
TT levels in men decline progressively by 0.4%–2% per year from the third decade onward [Citation5,Citation30]. FT levels also decline 2.8% per year according to the Massachusetts male aging study [Citation20]. We assessed the decline in TT, FT and BT per year and found it to be 0.4%, 1.8% and 1.7%, respectively. Decline of TT levels with age was statistically insignificant but the decline in the others was significant.
There are different results on predicting biochemical hypogonadism with the AMS questionnaire. According to Moore et al. [Citation31] the AMS questionnaire has 83% sensitivity and 39% specificity to predict biochemical hypogonadism. However, a study conducted in Europe with males over 70 years old reported no significant correlation between the AMS scores and the TT, FT and BT levels [Citation32]. We found no correlation between AMS scores and the TT, FT and BT levels in this study. We diagnosed biochemical hypogonadism mostly by using FT levels. However, its rate was 4.8% of participants but the clinical hypogonadism rate determined by AMS questionnaire was 73.8%.
When we correlated clinical parameters with age, there was no significant association between age and clinical symptoms (screened with AMS and IIEF questionnaires), except for memory-related cognitive functions. Mean values of AMS and IIEF questionnaires were high and the same in each group, they did not change with age. The high and unchanging prevalence of erectile dysfunction and symptoms of hypogonadism in our study cohort may have a very different explanation: first, it depends upon social and ethical factors and second, most of the participants had been referred for urinary tract symptoms, which might adversely affect their erectile function.
Erectile dysfunction (ED) is a major health problem in aging males and it leads to impairment of quality of life [Citation33]. ED is experienced at least some of the time by most men who have reached 45 years of age, and it is projected to affect 322 million men worldwide by 2025 [Citation34]. Effects of endogenous T levels on ED in aging males is still controversial. Kratzik et al. [Citation35] reported that only severe ED was correlated with low TT or BT levels. Another study reported that 35% of males with ED had low or threshold low TT levels [Citation36]. Males whose BT levels were less than threshold value reported their ED rates as 59%; if examined according to FT threshold levels ED rates were still reported as 54% [Citation8]. In our study, ED rate of subjects was 81.6%. But interestingly this high rate was not correlated with age and there was also no association between ED symptoms and any T values.
Neuropsychological functions may decrease in males whose T levels decreased with aging. Memory loss is the most common cognitive complaint in the elderly. A longitudinal study including 407 males between 50 and 91 years old reported that the subjects whose calculated FT levels were at hypogonadal level had significantly decreased visual memory and visuospatial performance scores [Citation37]. According to the Baltimore Longitudinal Study of Aging, FT leads to positive effect on visual-verbal memory, visuospatial functions and visuomotor screening functions [Citation38]. In this study, we screened subjects with the SMMT questionnaire for assessment of cognitive functions. Nobody had an SMMT score below 24 points, which is the accepted threshold for dementia. SMMT contains 6 points related to memory functions. We identified those subjects who were unable to gain the maximum score (30 point) and we noticed that their point loss came from memory related items. In this study, we found a strong negative correlation between the SMMT scores and age, and a strong positive correlation between the SMMT scores and FT.
This study has some limitations. The results are based on cross sectional data from questionnaires and a single testosterone measurement in men from the general population. It is still controversial whether a cross sectional sample can provide enough information about the general population. The sample size of the study is limited to 103 subjects, which is relatively a small sample size. However, a power analysis was completed and found the study able to evaluate the association between the T levels and clinical and metabolic parameters.
Conclusion
In this study, we found that the TT levels were not significantly decreased with age but FT and BT levels significantly decreased owing to increased SHBG levels. The decrease in the TT level was only correlated with a decrease in cognitive functions.
We observed that memory function decreased with age and also there was a statistically significant association between the testosterone levels and memory functions. The subjects with high FT levels had better memory functions.
Sexual, metabolic and psychological changes developed with age in males are not directly associated with testosterone levels. In order to better understand the association between testosterone levels and aging symptoms multicentered, longitudinal studies including participant males younger than 50 years old must be carried out.
Declaration of interest: The authors declare no conflict of interest.
References
- David E. Bloom, David Canning, Günther Fink. The Graying of global population and its macroeconomic consequences. October 2009 PGDA working paper no. 47. Available at: http://www.hsph.harvard.edu/pgda/working.htm.
- Morales A, Heaton JP, Carson CC 3rd. Andropause: A misnomer for a true clinical entity. J Urol 2000;163:705–712.
- Ferrini RL, Barrett-Connor E. Sex hormones and age: A cross-sectional study of testosterone and estradiol and their bioavailable fractions in community-dwelling men. Am J Epidemiol 1998;147:750–754.
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Baltimore longitudinal study of aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore longitudinal study of aging. J Clin Endocrinol Metab 2001;86:724–731.
- Tajar A, Forti G, O’Neill TW, Lee DM, Silman AJ, Finn JD, Bartfai G, et al. EMAS Group. Characteristics of secondary, primary, and compensated hypogonadism in aging men: Evidence from the European Male ageing study. J Clin Endocrinol Metab 2010;95:1810–1818.
- Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, O’Neill TW, et al. EMAS Group. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 2010;363:123–135.
- Arianayagam R, Arianayagam M, McGrath S, Rashid P. Androgen deficiency in the aging man. Aust Fam Physician 2010;39:752–755.
- Wang C, Nieschlag E, Swerdloff RS, Behre H, Hellstrom WJ, Gooren LJ, Kaufman JM, et al. ISA, ISSAM, EAU, EAA and ASA recommendations: Investigation, treatment and monitoring of late-onset hypogonadism in males. Aging Male 2009;12:5–12.
- Morales A, Lunenfeld B. Standards, guidelines and recommendations of the International Society for the Study of the Aging Male (ISSAM). The Aging Male 2002;5:74–86.
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84:3666–3672.
- Lunenfeld B, Morley JE, Schulman C, Wang C, Weidner W, Wu FC. Investigation, treatment and monitoring of lateonset hypogonadism in males: ISA, ISSAM, and EAU recommendations. Int J Androl 2005;28:125–127.
- Canguven O, Gurkan L, Horuz R, Albayrak S, Kadioglu A. Yaslanan erkek semptom sorgulama formu: Turkce gecerlilik calismasi (validity study for the Turkish version of symptom investigating form in aging males). Androloji Bulteni 2005;21:93–98.
- Heinemann LA, Saad F, Heinemann K, Thai DM. Can results of the aging Males’ symptoms (AMS) scale predict those of screening scales for androgen deficiency? Aging Male 2004;7:211–218.
- Gungen C, Ertan T. Standardize mini mental test’in Turk Toplumunda Hafif Demans Tanisinda Gecerlik ve Guvenilirligi (validity and reliability study on standardized mini mental test for the diagnosis of mild dementia in the Turkish Society). Turk Psikiyatri Dergisi 2002;13:273–281.
- Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): A multidimensional scale for assessment of erectile dysfunction. Urology 1997;49:822–830.
- Khaw KT, Dowsett M, Folkerd E, Bingham S, Wareham N, Luben R, Welch A, Day N. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) prospective population study. Circulation 2007;116:2694–2701.
- Rhoden EL, Morgentaler A. Risks of testosterone-replacement therapy and recommendations for monitoring. N Engl J Med 2004;350:482–492.
- Liverman CT, Blazer DG, Eds. Testosterone and aging. Washington, DC: Institute of Medicine, National Academies Press; 2004.
- Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev 2005;26:833–876.
- Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay JB. Age trends in the level of serum testosterone and other hormones in middle-aged men: Longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab 2002;87:589–598.
- Wu FC. Commentary: Guideline for male testosterone therapy: A European perspective. J Clin Endocrinol Metab 2007;92:418–419.
- Shames D, Gassman A, Handelsman H. Commentary: Guideline for male testosterone therapy: A regulatory perspective. J Clin Endocrinol Metab 2007;92:414–415.
- Araujo AB, Esche GR, Kupelian V, O’Donnell AB, Travison TG, Williams RE, Clark RV, McKinlay JB. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab 2007;92:4241–4247.
- Travison TG, Araujo AB, Wruck LM, Kupelian V, O’Donnell AB, McKinlay JB. Assessment of screening evaluations is not straightforward. Maturitas 2006;54:305–308.
- Handelsman DJ, Liu PY. Andropause: Invention, prevention, rejuvenation. Trends Endocrinol Metab 2005;16:39–45.
- Nieschlag E, Swerdloff R, Behre HM, Gooren LJ, Kaufman JM, Legros JJ, Lunenfeld B, et al. Investigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, and EAU recommendations. J Androl 2006;27:135–137.
- Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM. Testosterone therapy in adult men with androgen deficiency syndromes: An endocrine society clinical practice guideline. J Clin Endocrinol Metab 2006;91:1995–2010.
- Zitzmann M, Faber S, Nieschlag E. Association of specific symptoms and metabolic risks with serum testosterone in older men. J Clin Endocrinol Metab 2006;91:4335–4343.
- Travison TG, Araujo AB, Kupelian V, O’Donnell AB, McKinlay JB. The relative contributions of aging, health, and lifestyle factors to serum testosterone decline in men. J Clin Endocrinol Metab 2007;92:549–555.
- Orwoll E, Lambert LC, Marshall LM, Phipps K, Blank J, Barrett-Connor E, Cauley J, et al. Testosterone and estradiol among older men. J Clin Endocrinol Metab 2006;91:1336–1344.
- Moore C, Huebler D, Zimmermann T, Heinemann LA, Saad F, Thai DM. The aging Males’ symptoms scale (AMS) as outcome measure for treatment of androgen deficiency. Eur Urol 2004;46:80–87.
- T’Sjoen G, Goemaere S, De Meyere M, Kaufman JM. Perception of males’ aging symptoms, health and well-being in elderly community-dwelling men is not related to circulating androgen levels. Psychoneuroendocrinology 2004;29:201–214.
- Kapoor D, Clarke S, Channer KS, Jones TH. Erectile dysfunction is associated with low bioactive testosterone levels and visceral adiposity in men with type 2 diabetes. Int J Androl 2007;30:500–507.
- La Vignera S, Condorelli R, Vicari E, D’Agata R, Calogero A. Physical activity and erectile dysfunction in middle-aged men: A brief review. J Androl 2011. [ePub ahead of print].
- Kratzik CW, Schatzl G, Lunglmayr G, Rücklinger E, Huber J. The impact of age, body mass index and testosterone on erectile dysfunction. J Urol 2005;174:240–243.
- Isidori AM, Giannetta E, Gianfrilli D, Greco EA, Bonifacio V, Aversa A, Isidori A, et al. Effects of testosterone on sexual function in men: Results of a meta-analysis. Clin Endocrinol (Oxf) 2005;63:381–394.
- Moffat SD, Zonderman AB, Metter EJ, Blackman MR, Harman SM, Resnick SM. Longitudinal assessment of serum free testosterone concentration predicts memory performance and cognitive status in elderly men. J Clin Endocrinol Metab 2002;87:5001–5007.
- Janowsky JS, Chavez B, Orwoll E. Sex steroids modify working memory. J Cogn Neurosci 2000;12:407–414.