Abstract
Objectives To explore clinically relevant differences in severity of vulvar and vaginal atrophy (VVA) in postmenopausal women treated with ospemifene compared with placebo.
Methods Analysis of two multicenter, randomized, double-blind, 12-week phase-III studies in postmenopausal women (40–80 years, with VVA, treated with ospemifene 60 mg/day or placebo (Study 310 and Study 821)). Severity of vaginal dryness and dyspareunia were evaluated using a four-point scoring system and clinically relevant differences between ospemifene and placebo were analyzed and are presented as improvement (reduction in ≥ 1 unit on four-point scoring system), substantial improvement (reduction in 2–3 units on four-point scoring system) and relief (severity score of mild/none after 12 weeks).
Results In Study 310, significantly more women with a most bothersome symptom of dyspareunia had improvement (68.3% vs. 54.1%; p = 0.0255) or relief (57.5% vs. 41.8%; p = 0.0205) in the severity of dyspareunia from baseline to week 12 with ospemifene compared with placebo. For those with a most bothersome symptom of vaginal dryness, significantly more experienced improvement (74.6% vs. 57.7%; p = 0.0101), substantial improvement (42.4% vs. 26.9%; p = 0.0172) and relief (66.1% vs. 49.0%; p = 0.0140) of vaginal dryness from baseline to week 12 with ospemifene compared with placebo. Proportions of women with improvement/substantial improvement/relief of symptoms of vaginal dryness or dyspareunia were similar in Study 821. Clinically relevant differences were noticeable by week 4.
Conclusions Treatment with ospemifene was consistently associated with greater improvement, substantial improvement or relief in the severity of the most bothersome symptoms of vaginal dryness or dyspareunia compared with placebo.
INTRODUCTION
Vulvar and vaginal atrophy (VVA) is a chronic and progressive medical condition that develops as a result of the decline of estrogen levelsCitation1,Citation2. The process of atrophy is universal following the decline in estrogenCitation3 with physiological changes taking place in the vulval, vaginal and urogenital epitheliaCitation2,Citation4–7. These changes underlie a broad range of genital and urinary symptoms, including dryness, burning, dyspareunia, loss of vaginal secretions, leukorrhea, vulvar pruritus, feeling of pressure, itching, urethral discomfort, frequency and urgency of urination, hematuria, urinary tract infection and dysuriaCitation8,Citation9. Up to 50% of postmenopausal women suffer from the symptoms of VVACitation1,Citation2, with the onset of these symptoms varying from one woman to anotherCitation10. The presence and severity of these symptoms are variable, ranging from mild discomfort to great impairmentCitation11 and can be influenced by age, time and type of menopauseCitation12–14 as well as sexual activity and other personal history elementsCitation14. Although VVA has a negative impact on sexual health and quality of lifeCitation11, only a minority of postmenopausal women will ever seek medical treatment for their conditionCitation6,Citation8,Citation15.
The principles of treatment in women with established vaginal atrophy are to restore urogenital physiology and to alleviate symptomsCitation8. Treatment recommendations for VVA include first-line therapy with non-hormonal lubricants with intercourse and, if indicated, regular use of long-acting vaginal moisturizersCitation10. However, these approaches seldom restore premenopausal anatomy or physiology and do not provide a long-term solutionCitation8. Local estrogen therapy may be used in postmenopausal women with symptomatic VVA who do not respond to lubricants and moisturizersCitation10,Citation16. Systemic estrogen therapy is not usually recommended when VVA is the only menopausal symptomCitation8. However, long-term safety, hormone exposure and side-effects in general have been cited as concerns that women have associated with use of prescription vaginal productsCitation17 and a potential systemic effect of local estrogens cannot totally be excludedCitation8,Citation10. Furthermore, studies have suggested that satisfaction with the available treatment options is lowCitation17, with common reasons for dissatisfaction including messiness, ritual and inconvenience of administration and interference with the spontaneity of sexCitation17. Therefore, there is a need for novel pharmacological treatment options that are easy to administer by women with symptoms of VVACitation11,Citation18.
Recent clinical trial programs for drugs to treat VVA are based on draft guidance from the US Food and Drug Administration (FDA)Citation19, which specifies that a new product must demonstrate efficacy at three co-primary endpoints, namely a change in: (1) maturation index (decrease in the percentage of parabasal vaginal cells and increase in the percentage of superficial vaginal cells), (2) vaginal pH, and (3) severity of the patient-reported most bothersome symptom (MBS). The severity of MBS is based on self-assessment of the symptoms of VVA by the patient and includes vaginal dryness, vaginal pain associated with sexual activity (dyspareunia), vaginal and/or vulvar irritation/itching, dysuria and vaginal bleeding associated with sexual activity. Patients are required to rate the severity of the first four symptoms as none, mild, moderate or severe and the presence of vaginal bleeding after intercourse as either present or absent. The severity score is usually given a numerical value (none = 0, mild = 1, moderate = 2 and severe = 3) enabling a ‘mean change from baseline’ to be calculated. The MBS must be chosen at baseline from the symptoms rated as moderate or severe and the mean change in MBS score from baseline is used to evaluate symptomatic improvement. Although this approach is an important primary measure of efficacy and is a major step forward in standardizing measurement of self-assessed changes in the severity of VVA to validate potential new treatmentsCitation20, it is not easy to interpret what the resulting ‘mean change from baseline’ means in daily clinical practice.
Ospemifene is a novel selective estrogen receptor modulator (SERM), licensed in the USA for oral treatment of moderate to severe dyspareunia, a symptom of VVA, due to menopauseCitation21, and current recommendations from the North American Menopause Society include the use of ospemifene for treatment of these symptomsCitation10. Pivotal studies with ospemifene have demonstrated the tolerability and efficacy of 12 weeks of ospemifene treatment in improving the MBS of dyspareuniaCitation22,Citation23 and vaginal drynessCitation22,Citation24. These studies of ospemifene have reported the mean change from baseline (range: ospemifene, − 1.19 to − 1.5; placebo, − 0.84 to − 1.2)Citation22–24. While these data are important to show the statistical significance of ospemifene treatment, it would be beneficial to have data that are applicable to everyday clinical practice to assist health-care professionals in understanding what can be expected from prescribed treatments. This will be useful for defining the patient's expectation of potential benefits and limitations of treatment.
In the ospemifene clinical trial program, the majority of the symptoms reported as MBS were either vaginal dryness or dyspareunia. The objective of the current analysis was to explore clinically relevant differences in the severity of vaginal dryness or dyspareunia in postmenopausal women treated with ospemifene 60 mg/day compared with placebo, using data of the primary endpoint from the two previously published studiesCitation22–24. The safety and tolerability profile of 12 weeks of ospemifene in postmenopausal women, including a risk–benefit discussion, has been reported previouslyCitation22–24 and is therefore not included in this current analysis. Furthermore, safety data on ospemifene for the treatment of VVA in postmenopausal women for 52 weeksCitation25,Citation26 and up to 64 weeks for women without a uterusCitation27 have also been reported elsewhere.
METHODS
Patients and study design
The current study analyzes the results of two previously conducted trials evaluating the safety and efficacy of oral ospemifene 60 mg/day for the treatment of the symptoms of VVA (NCT00276094/sponsor protocol no. 15–50310, referred to herein as Study 310; NCT00729469/sponsor protocol no. 15-50821, referred to herein as Study 821)Citation22,Citation23. The study designs and inclusion criteria are described in detail elsewhereCitation22,Citation23. Both studies were multicenter, randomized, double-blind, 12-week phase-III studies in postmenopausal women, aged 40–80 years, with the following criteria of VVA: (1) 5% or less superficial cells on the vaginal smear (maturation index); (2) vaginal pH > 5.0; (3) at least one moderate or severe symptom of VVA (Study 310)Citation23, or moderate to severe vaginal dryness or vaginal pain associated with sexual activity (Study 821)Citation22. A total of 826 postmenopausal women were randomized (1 : 1 : 1) and treated with either ospemifene 30 mg/day (n = 282), ospemifene 60 mg/day (n = 276) or placebo (n = 268) for up to 12 weeks in Study 310. A total of 919 postmenopausal women with either vaginal dryness or dyspareunia as their MBS were randomized (1 : 1) and treated with either ospemifene 60 mg/day (n = 463) or placebo (n = 456) for up to 12 weeks in Study 821. A four-point scoring system (none = 0; mild = 1; moderate = 2; severe = 3) was used to evaluate the severity of the co-primary endpoints of dryness and dyspareunia in these studies; the mean change from baseline, based on this scoring system for the co-primary endpoint in each study has been reported elsewhereCitation22–24 and is shown in . In both studies, the women in all treatment groups were supplied with a non-hormonal lubricant (K-Y® Jelly, McNeil-PPC, Inc., NJ, USA) and were instructed to use as needed.
Table 1 Primary efficacy analysis: change from baseline to week 12 in most bothersome symptom (ITT, LOCF)Citation22–24
A last-observation-carried-forward (LOCF) approach was used for those discontinuing prematurely for the analyses of efficacy variables. Both studies were approved by the institutional review board or ethics committee for each site, the Declaration of Helsinki was followed, and informed consent obtained from all patients.
Definition of improvement, substantial improvement and relief
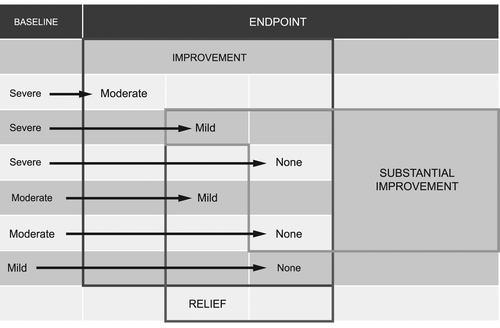
This current analysis focuses on the women who received either ospemifene 60 mg/day or placebo in both the 310 and 821 studies. Using the data from the co-primary endpoints, analysis of clinically meaningful differences in the patient's condition was assessed by the concepts of improvement, substantial improvement and relief () of symptoms in the 60 mg ospemifene group compared to placebo:
| (1) | ImprovementCitation20 was defined as a reduction in one or more units on the four-point severity scoring system (this includes patients whose baseline score changed from severe to none, mild or moderate, from moderate to mild or none, and from mild to none). | ||||
| (2) | Substantial improvement was defined as a reduction in two or three units on the four-point severity scoring system (this includes patients whose baseline score changed from severe or moderate to none, or from severe to mild). | ||||
| (3) | ReliefCitation20 was defined as having a severity score at week 12 of mild or none (i.e. does not signify a change, but records the final score). | ||||
Efficacy analysis included the change from baseline to week 4 and week 12 in the severity of symptoms. Within this model, the response variables were the change from baseline to week 4/week 12; for missing values, the last observation was used (the LOCF). The baseline value was the covariate, and treatment and study center were the fixed effects. The relative difference of the effect of ospemifene versus placebo in improvement, substantial improvement or relief in dryness or dyspareunia was determined using the following calculation per effect: (proportion of patients (%) on ospemifene 60 mg – proportion of patients (%) on placebo)/proportion of patients (%) on placebo. Change from baseline to week 4 and week 12 in the severity of symptoms was analyzed using a Cochran–Mantel–Haenszel row mean scores test, controlling for uterine status (intact uterus or post-hysterectomy) and study center. Improvement, relief and substantial improvement were analyzed using the Fisher's exact two-sided test.
RESULTS
The rate of discontinuation was low in both studies: 84.8% of women taking ospemifene 60 mg/day and 85.8% of women taking placebo completed the 12 weeks of Study 310; 89.8% of women taking ospemifene 60 mg/day and 88.4% of women taking placebo completed the 12 weeks of Study 821. After 4 weeks of treatment, the frequency of lubricant application decreased slightly in the ospemifene group with no change in the placebo group (both studies)Citation22–24, while the frequency of sexual activity remained consistent in both treatment groups (only recorded in Study 821)Citation23,Citation24.
Vaginal dryness
The clinical relevance of ospemifene treatment assessed using the definitions of improvement, substantial improvement and relief of vaginal dryness associated with VVA is shown in .
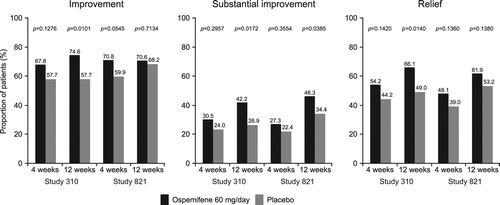
For women with a MBS of vaginal dryness in Study 310, significantly more women who received ospemifene 60 mg/day for 12 weeks reported improvement (p = 0.0101), substantial improvement (p = 0.0172) and relief (p = 0.0140) of vaginal dryness from baseline levels compared with placebo. These proportions were similar in Study 821 although the only statistical difference was with women (p = 0.0385) experiencing a substantial improvement in vaginal dryness with ospemifene 60 mg/day compared with placebo (). These differences in the proportion of women responding to treatment were noticeable after 4 weeks of treatment with ospemifene 60 mg/day. The relative differences in the proportion of women with improvement, substantial improvement or relief of their MBS of vaginal dryness following treatment with ospemifene 60 mg compared with placebo are shown in . The relative differences in the proportion of women responding to treatment with ospemifene 60 mg over placebo are also apparent after 4 weeks of treatment, with a further increase in the percentage of women showing improvement, substantial improvement or relief in dryness in Study 310 and substantial improvement in Study 821 at week 12.
Table 2 Relative differences in the proportion of women with improvement, substantial improvement or relief of vaginal dryness or dyspareunia with ospemifene 60 mg compared with placebo
Dyspareunia
The differences in the severity of dyspareunia associated with VVA, as assessed using the definitions of improvement, substantial improvement and relief are shown in .
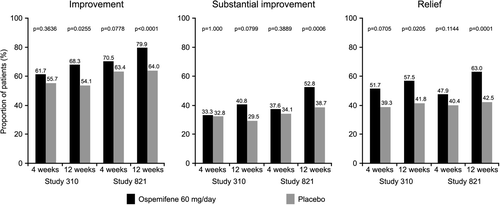
Among the women reporting dyspareunia as their MBS in Study 310, after 12 weeks of treatment with ospemifene 60 mg/day, there were significantly more women with improvement (p = 0.0255) and relief (p = 0.0205) in the severity of dyspareunia from baseline compared with placebo. There was also a trend towards significance in the proportion of women with substantial improvement (p = 0.0799) in the severity of dyspareunia from baseline. These proportions were similar in Study 821, with significantly greater improvement (p < 0.0001), substantial improvement (p = 0.0006) and relief (p = 0.0001) in the severity of symptoms after 12 weeks of treatment with ospemifene 60 mg/day compared with placebo (). The relative differences in the proportion of women with improvement, substantial improvement or relief of their MBS of dyspareunia following treatment with ospemifene 60 mg compared with placebo are shown in . The relative increases in improvement, substantial improvement or relief of treatment with ospemifene 60 mg compared with placebo are apparent after 4 weeks of treatment and continue to increase up to 12 weeks.
DISCUSSION
This current analysis demonstrates that approximately three-quarters of women treated for 12 weeks with ospemifene 60 mg/day experienced improvement of vaginal dryness and dyspareunia associated with VVA, compared with 50–60% of those who received placebo. Although previous studies have demonstrated the efficacy of ospemifene on the mean change in vaginal drynessCitation22,Citation24 from baseline (range: ospemifene, − 1.26 to − 1.3; placebo, − 0.84 to − 1.1) and dyspareuniaCitation22,Citation23 (range: ospemifene, − 1.19 to − 1.5; placebo, − 0.89 to − 1.2), this is the first analysis to show the clinical relevance of such changes. This is particularly important as numerical measures of severity are not routinely used in clinical practice and gynecologists and their patients may therefore find it difficult to apply such knowledge to understand how treatments for VVA may benefit women with symptoms attributable to the condition. The data presented here therefore provide clinically meaningful information as to the proportion of women who might expect to experience improvement or relief of their symptoms with ospemifene treatment.
A number of other studies investigating treatment of VVA in postmenopausal women have been published in recent years using the MBS as a co-primary endpointCitation28–34. However, although the concept of the clinical relevance of changes in the severity score for the MBS with treatment has been suggested through the analysis of ‘improvement’ and ‘relief’ of such symptomsCitation20, this is the first manuscript to report such changes in this way. We have also developed this further, with the inclusion of ‘substantial improvement’ as a measure of the severity of the symptoms of VVA. There are a number of reasons for the inclusion of this definition. First, a one-unit improvement in severity score is often spontaneous and can occur without intervention. For example, in the ospemifene pivotal efficacy trials, patients selected their MBS at screening and again at randomization (baseline). The period between screening and randomization was no more than 6 weeks, with no therapeutic intervention during this time. However, in Study 821, 14.9% of patients who described their MBS as severe at screening showed improvement by randomization, but only 0.2% showed substantial improvement at this point in time. This was similar in Study 310. If a substantial improvement is observed, different from placebo, it is more likely to reflect a true effect of a treatment in the patient population since a change of that magnitude in severity is rare without intervention. Furthermore, in the absence of a strict definition of the degree of severity (none, mild, moderate or severe), it is possible to question the significance of a change in severity score of one unit. For example, what may be perceived as moderate one day can be perceived as severe another day. However, there is little uncertainty about a change from moderate to none or from severe to mild or none. Using substantial improvement therefore reduces uncertainty in a subjective patient-reported outcome.
The efficacy of ospemifene in improving objective measures of VVA, i.e. increasing the maturation index and decreasing the vaginal pH, has been shown in Studies 310 and 821Citation22,Citation23. In both pivotal studies, 12 weeks of treatment with ospemifene 60 mg/day increased the percentage of superficial cells by approximately 10% compared with placebo (∼2%) and decreased the percentage of parabasal cells by 30–40% compared with placebo (0–4%)Citation22,Citation23. Notably, in both studies, improvements in maturation index were observed after 4 weeks of treatment. The clinically relevant changes in vaginal dryness and dyspareunia with ospemifene 60 mg/day reported in this current analysis, also observed after 4 weeks of treatment, suggest that symptomatic relief follows the objective signs of improvement reported previouslyCitation22,Citation23. Taken together, these data indicate that ospemifene is treating the cause of vaginal atrophy, not just ameliorating the associated symptoms. Furthermore, the onset of these benefits may be experienced within 4 weeks of treatment.
In the current analysis, there is a substantial placebo effect, with improvement of dyspareunia and vaginal dryness associated with VVA in approximately 50% of women who received placebo. However, it is important to note that the design of the two studies reported here allowed all participants to use a non-hormonal vaginal lubricant as needed and therefore the placebo effect also includes the effect of the lubricant. Indeed, in the current analysis, the placebo effect was apparent after 4 weeks of the study – this early onset of the placebo effect has also been reported previouslyCitation35. This early placebo effect usually wears off, as demonstrated by the continuing improvement in the ospemifene 60 mg/day group compared with placebo. Not only did ospemifene 60 mg/day show efficacy over and above lubricant, but lubricant use was reduced in the ospemifene treatment group towards the end of the studyCitation22–24,Citation36. Furthermore, in clinical practice, placebo is not prescribed.
There is a need for more stringent definitions for the degree of severity for the symptoms of VVA, particularly when conducting clinical trials and for comparisons of the efficacy of different products. Furthermore, while such subjective measurements are essential to ensure the clinical effectiveness of new treatments that address both the underlying physiology and symptoms experienced by postmenopausal women to improve and maintain their quality of life in older age, it would also be beneficial to include a formal assessment of quality of life in the assessment of new treatments to understand fully the impact of the symptoms of VVA in women. In the absence of specific VVA quality-of-life rating scales, sexual quality-of-life scales are currently used as surrogates to assess the effect of VVA symptoms. Establishing a practical, validated questionnaire, which takes into account the impact of VVA symptoms on personal, social and professional aspects of quality of life, should now be prioritized to assist in assessing the effectiveness of new treatment options for VVACitation18.
CONCLUSION
This analysis provides a clinically meaningful analysis of the efficacy of ospemifene 60 mg/day on vaginal dryness and dyspareunia as a result of VVA. Approximately three-quarters of women treated for 12 weeks with ospemifene 60 mg/day experienced improvement of dyspareunia and vaginal dryness associated with VVA, compared with 50–60% who received placebo. This compares favorably with previous analyses of ospemifene 60 mg/day which demonstrate significant improvements relative to placebo in the objective measurements of VVA as well as the severity of vaginal dryness and dyspareunia reported as the most bothersome symptomCitation22–24.
In conclusion, this analysis provides informative and clinically relevant data that will enable gynecologists and their patients to assess the effectiveness that patients can expect to achieve when prescribed ospemifene for the improvement of their symptoms.
Conflict of interest During the past 2 years R.E.N. has had financial relationships (lecturer, member of advisory boards and/or consultant) with Bayer-Schering Pharma, Ely Lilly, Gedeon-Richter, HRA Pharma, Merck Sharpe & Dohme, Novo Nordisk, Pfizer Inc., Shionogi Ltd and Teva/Theramex.
N.P. has received honoraria for consultancy work and lectures from a number of pharmaceutical companies including Bayer, Besins, Novo Nordisk, Pfizer, Se Cur and Shionogi Ltd.
N.B. is a consultant working for Shionogi Ltd.
During the past 2 years C.C-B. has had financial relationships (lecturer, member of advisory boards and/or consultant) with Amgen, Pierre-Fabre, Servier, Merck Sharpe & Dohme, Isdin, Pfizer Inc., Shionogi Ltd.
During the past 2 years T.De V. has had financial relationships (lecturer, member of advisory boards and/or consultant) with Bayer-Schering Pharma, Merck Sharpe & Dohme, Pfizer Inc., Amgen and Abbott.
J.A.S. has served (within the last year) or is currently serving as a consultant to or on the advisory boards of AbbVie, Inc. (North Chicago, IL), Actavis, PLC. (Dublin, Ireland), Amgen Inc. (Thousand Oaks, CA), Amneal Pharmaceuticals (Bridgewater, NJ), Apotex, Inc. (Toronto, Canada), Ascend Therapeutics (Herndon, VA), Depomed, Inc. (Menlo Park, CA), Everett Laboratories, Inc. (West Orange, NJ), Lupin Pharmaceuticals (Baltimore, MD), Meda Pharmaceuticals Inc. (Somerset, NJ), Merck & Co., Inc. (Whitehouse Station, NJ), Novartis Pharmaceuticals Corporation (East Hanover, NJ), Noven Pharmaceuticals, Inc. (New York, NY), Novo Nordisk (Bagsvrerd, Denmark), Pfizer Inc. (New York, NY), Shionogi Inc. (Florham Park, NJ), Shippan Point Advisors LLC (Upper Saddle River, NJ), Sprout Pharmaceuticals (Raleigh, NC), TherapeuticsMD (Boca Raton, FL),Teva Pharmaceutical Industries Ltd (Jerusalem, Israel). In the last year he has received or is currently receiving grant/research support from AbbVie, Inc. (North Chicago, IL), Actavis, PLC. (Dublin, Ireland), Bayer Healthcare LLC. (Tarrytown, NY), EndoCeutics Inc. (Quebec, Quebec), Novo Nordisk (Bagsvrerd, Denmark), Novogyne (East Hanover, NJ), Palatin Technologies (Cranbury, NJ), and Teva Pharmaceutical Industries Ltd (Jerusalem, Israel). He has also served or is currently serving on the speakers bureaux of Amgen Inc. (Thousand Oaks, CA), Eisai, Inc. (Woodcliff Lake, NJ), Merck (Whitehouse Station, NJ), Noven Pharmaceuticals, Inc. (New York, NY), Shionogi Inc. (Florham Park, NJ), and Teva Pharmaceutical Industries Ltd (Jerusalem, Israel). J.A.S. was the Chief Medical Officer for Sprout Pharmaceuticals (Raleigh, NC) until 4 January 2013.
Source of funding The preparation of this manuscript was supported by Shionogi Ltd. All authors take full responsibility for the content of the article. Editorial assistance was provided by Dr Marion James of apothecom scopemedical (supported by Shionogi).
References
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health 2013; 5:437–47
- Mac Bride MB, Rhodes DJ, Shuster LT. Vulvovaginal atrophy. Mayo Clin Proc 2010;85:87–94
- Castelo-Branco C, Cancelo MJ, Villero J, Nohales F, Juliá MD. Management of post-menopausal vaginal atrophy and atrophic vaginitis. Maturitas 2005;52(Suppl 1):S46–52
- Bachmann GA, Nevadunsky NS. Diagnosis and treatment of atrophic vaginitis. Am Fam Physician 2000;61:3090–6
- Goldstein I, Alexander JL. Practical aspects in the management of vaginal atrophy and sexual dysfunction in perimenopausal and postmenopausal women. J Sex Med 2005;2(Suppl 3):154–65
- Goldstein I. Recognizing and treating urogenital atrophy in postmenopausal women. J Womens Health (Larchmt) 2010;19: 425–32
- Goldstein I, Dicks B, Kim NN, Hartzell R. Multidisciplinary overview of vaginal atrophy and associated genitourinary symptoms in postmenopausal women. Sex Med 2013;1:44–53
- Sturdee DW, Panay N. Recommendations for the management of postmenopausal vaginal atrophy. Climacteric 2010;13:509–22
- Robinson D, Cardozo L. The menopause and HRT. Urogenital effects of hormone therapy. Best Pract Res Clin Endocrinol Metab 2003;17:91–104
- Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause 2013;20:888–902
- Nappi RE, Palacios S. Impact of vulvovaginal atrophy on sexual health and quality of life at postmenopause. Climacteric 2014;17:3–9
- Nappi RE, Lachowsky M. Menopause and sexuality: prevalence of symptoms and impact on quality of life. Maturitas 2009;63: 138–41
- Palacios S. Managing urogenital atrophy. Maturitas 2009;63: 315–18
- Bachmann GA, Leiblum SR. Sexuality in sexagenarian women. Maturitas 1991;13:43–50
- The role of local vaginal estrogen for treatment of vaginal atrophy in postmenopausal women: 2007 position statement of The North American Menopause Society. Menopause 2007; 14:355–69
- Rees M, Pérez-López FR, Ceasu I, et al. EMAS clinical guide: low-dose vaginal estrogens for postmenopausal vaginal atrophy. Maturitas 2012;73:171–4
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med 2013;10:1790–9
- Panay N, Fenton A. Vulvovaginal atrophy – a tale of neglect. Climacteric 2014;17:1–2
- Guidance for Industry: Estrogen and Estrogen/Progestin Drug Products to Treat Vasomotor Symptoms and Vulvar and Vaginal Atrophy Symptoms – Recommendations for Clinical Evaluation. Draft guidance, Food and Drug Administration. January 2003. At http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm071643.pdf, accessed 4 July 2014
- Ettinger B, Hait H, Reape KZ, Shu H. Measuring symptom relief in studies of vaginal and vulvar atrophy: the most bothersome symptom approach. Menopause 2008;15:885–9
- Ospemifene prescribing information. Shionogi, 2013
- Bachmann GA, Komi JO. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause 2010;17:480–6
- Portman DJ, Bachmann GA, Simon JA. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause 2013;20:623–30
- Portman D, Palacios S, Nappi RE, Mueck AO. Ospemifene, a non-oestrogen selective oestrogen receptor regulator for the treatment of vaginal dryness associated with postmenopausal vulvar and vaginal atrophy: a randomised, placebo-controlled, phase III trial. Maturitas 2014;78:91–8
- Goldstein SR, Bachmann GA, Koninckx PR, Lin VH, Portman DJ, Ylikorkala O. Ospemifene 12-month safety and efficacy in postmenopausal women with vulvar and vaginal atrophy. Climacteric 2014;17:173–82
- Simon JA, Lin VH, Radovich C, Bachmann GA. One-year long-term safety extension study of ospemifene for the treatment of vulvar and vaginal atrophy in postmenopausal women. Menopause 2012;20:418–27
- Simon JA, Portman D, Mabey RG Jr; Ospemifene Study Group Long-term safety of ospemifene (52-week extension) in the treatment of vulvar and vaginal atrophy in hysterectomized postmenopausal women. Maturitas 2014;77:274–81
- Simon JA, Reape KZ, Wininger S, Hait H. Randomized, multicenter, double-blind, placebo-controlled trial to evaluate the efficacy and safety of synthetic conjugated estrogens B for the treatment of vulvovaginal atrophy in healthy postmenopausal women. Fertil Steril 2008;90:1132–8
- Bachmann G, Bouchard C, Hoppe D, et al. Efficacy and safety of low-dose regimens of conjugated estrogens cream administered vaginally. Menopause 2009;16:719–27
- Freedman M, Kaunitz AM, Reape KZ, Hait H, Shu H. Twice-weekly synthetic conjugated estrogens vaginal cream for the treatment of vaginal atrophy. Menopause 2009;16: 735–41
- Simon J, Nachtigall L, Gut R, Lang E, Archer DF, Utian W. Effective treatment of vaginal atrophy with an ultra-low-dose estradiol vaginal tablet. Obstet Gynecol 2008;112:1053–60
- Griesser H, Skonietzki S, Fischer T, Fielder K, Suesskind M. Low dose estriol pessaries for the treatment of vaginal atrophy: a double-blind placebo-controlled trial investigating the efficacy of pessaries containing 0.2 mg and 0.03 mg estriol. Maturitas 2012;71:360–8
- Kagan R, Williams RS, Pan K, Mirkin S, Pickar JH. A randomized, placebo- and active-controlled trial of bazedoxifene/conjugated estrogens for treatment of moderate to severe vulvar/vaginal atrophy in postmenopausal women. Menopause 2010; 17:281–9
- Labrie F, Archer DF, Bouchard C, et al. Intravaginal dehydroepiandrosterone (prasterone), a highly efficient treatment of dyspareunia. Climacteric 2011;14:282–8
- Bradford A. Listening to placebo in clinical trials for female sexual dysfunction. J Sex Med 2013;10:451–9
- Constantine G, Graham S, Koltun WD, Kingsberg SA. Assessment of ospemifene or lubricants on clinical signs of VVA. J Sex Med 2014;11:1033–41
