Abstract
Context: The plant Cordia dichotoma Forst. f. (Boraginaceae) is commonly known as “Bhokar” in Marathi. This tree species has been of interest to researchers because traditionally its bark is reported in the treatment of ulcer and colic pain.
Objective: The present work was undertaken to validate its folk use in the treatment of ulcerative colitis (UC) by using scientific methods.
Materials and methods: Dried bark powder was extracted with methanol and this crude methanol extract was fractionated using various solvents. These fractions were tested for effectiveness against UC. Macroscopical study and histopathology of the colon, level of myeloperoxidase (MPO) and malondialdehyde (MDA) in colon and blood were studied for the assessment of the activity. Antioxidant activity of these fractions was screened by using various methods.
Results: Animals treated with the methanol fraction of the crude methanol extract showed lower pathological scores and good healing. This fraction reduced MPO and MDA levels significantly in blood and tissue. It showed antioxidant potential [in DPPH (1,1-diphenyl-2-picrylhydrazyl) assay IC50 value is 26.25; trolox equivalent (TE) antioxidant capacity µg/ml TE/g of plant material on dry basis in ABTS (2,2′-azinobis[3-ethylbenzthiazoline]-6-sulfonic acid) and FRAP (ferric reducing antioxidant potential) assay is 2.03 and 2.45, respectively]. The fraction contains a high level of phenolics.
Discussion and conclusion: The methanol fraction of crude methanol extract of C. dichotoma bark is effective in the treatment of UC.
Introduction
Ulcerative colitis (UC) is a subcategory of inflammatory bowel disease (IBD). Colitis affects the colon and rectum and typically involves only the inner most lining or mucosa, manifesting as a continuous area of inflammation and ulceration with no segments of normal tissue (CitationKathleen et al., 2003). Hypochlorous acid, produced by the action of myeloperoxidase (MPO) on hydrogen peroxide in the presence of chloride ions is importantly involved in the inflammatory reaction in colitis (CitationKirsner & Shorter, 1982). The pathological findings associated with UC are an increase in certain inflammatory mediators, signs of oxidative stress, a deranged of the mucosa, abnormal glycosaminoglycan content of the mucosa, decreased oxidation of short chain fatty acids (SCFAs), increased intestinal permeability, increased sulfide production, and decreased methylation (CitationKrawisz et al., 1984).
Cordia dichotoma Forst. f. (Boraginaceae), commonly known as “Bhokar” in Marathi, is a small to moderate-sized deciduous tree with a short bole and spreading crown (CitationKirtikar & Basu, 1956). It is an important drug for indigenous systems of medicine and has been attributed with many medicinal properties in ayurveda. The plant has been claimed to cure diseases of the kidney, liver, spleen, heart, and blood. Various parts of the plants are used as an antipyretic, for anti-anemic effect, as a remedy for impotency, and to treat gastric pain, asthma, mouth ulcers, bronchitis, diarrhea, rheumatism, and dental caries (CitationNadkarni, 1991; CitationAnonymous, 2004). Traditionally bark of the plant is reported for the treatment of UC (CitationKirtikar & Basu, 1956; CitationNadkarni, 1991). So the present work was undertaken to validate its use in the treatment of UC by using suitable scientific methods.
Materials and methods
Plant material
The bark of C. dichotoma was collected from Ahmednagar district (Maharashtra, India) in August 2008, and authenticated by Mr. P.G. Diwakar, Deputy Director, Botanical Survey of India, Pune (Voucher specimen number ABG-1/BSI/WC/Tech/2008/712).
Extraction
Dried powdered plant bark (100.0 g) was extracted using methanol in a Soxhlet extractor. Vacuum dried methanol extract (14.0 g) was converted to hydroalcoholic extract by dispersing it into water (1:1). This hydroalcoholic extract was fractioned into n-hexane, ethyl acetate and methanol fractions. The fractions were vacuum dried to get n-hexane (2.048 g), ethyl acetate (3.205 g) and methanol (5.822 g) fraction. These fractions were administered to mice at a dose of 50 mg/kg, p.o., and crude methanol extract was administered at a dose of 500 mg/kg, p.o.
Animals
Male Swiss mice (20–25 g) were used. The animals were housed under standard laboratory conditions and fed with rodent diet and water. The experimental protocol was approved by the institutional animal ethical committee (Approval No. CPCSEA/C/448/08-0-/16).
Pharmacological screening
Induction of experimental colitis
Animals were divided into six groups (n = 6). The control group of mice received vehicle (0.2 ml of 5% Tween 80 in distilled water). The standard group received prednisolone at a dose of 5 mg/kg, i.p. Animals from group III to VI received, respectively, n-hexane, ethyl acetate and methanol fractions (50 mg/kg, p.o., each) and crude methanol extract (500 mg/kg, p.o.) of the bark of C. dichotoma. Colitis was induced by intrarectal administration of 150 µl, 5% acetic acid (pH 2.5), 3 cm from the anal margin (CitationItoh et al., 2000).
Assessment of colitis severity
Mice were killed by cervical dislocation 1-day after administration of acetic acid. the entire colons were isolated, opened longitudinally, and rinsed with phosphate-buffer saline (PBS). Histological scoring of the colon damage was performed. For each mouse, the ulcer area was determined by summing the sizes of lesions measured macroscopically. The total area of damage was expressed as the relative percentage of the total surface area of the colon (CitationMahgoub et al., 2003).
Determination of colonic MPO activity
After the macroscopic measurements, the excised colons (100–150 mg) were homogenized with PBS (pH 7.4) and centrifuged at 1000 rpm for 20 min at 4°C. MPO activity of supernatants was then assayed by mixing the supernatant with citric phosphate buffer (pH 5.0) containing 0.4 mg/ml O-phenylene diamine and 0.015% hydrogen peroxide. The change in absorbance at 492 nm was measured spectrophotometrically and compared with the standard dilution with horseradish peroxidase (CitationEvans et al., 2000).
Determination of MDA
The reaction mixture containing 0.1 ml tissue sample, 0.2 ml 8.1% sodium dodecyl sulphate, 1.5 ml 2% acetic acid and 1.5 ml 0.8% aqueous solution of thiobarbituric acid. The mixture pH was adjusted to 3.5 and the volume was finally made upto 4 ml with distilled water and 5 ml of mixture of n-butanol and pyridine (15%) was added. The mixture was shaken vigorously. After centrifugation at 4000 rpm for 10 min, the absorbance of organic layer was measured at 532 nm. Malondialdehyde (MDA) was expressed as n mol/mg of protein (CitationBuege & Aust, 1978).
Histopathological study
Tissue was fixed with 10% formalin for 24–36 h and then trimmed and washed under running tap water for 2 h. Then the tissue was dehydrated with increasing grades of alcohol (50% alcohol overnight, 70% alcohol for 2 h, 80% alcohol for 2 h, 90% alcohol for 2 h and absolute alcohol for 2 h). Then the tissue was cleared with xylene for 1 h and embedded with paraffin wax at 60°C. Blocks were prepared and stored in a freezer for 45 days. Slices of tissue were cut at 5 mm thickness. Slices were taken on clean grease free glass slides smeared with egg albumin in a water bath at 60°C. Tissue was deparafinnated partially with heat and followed by immersing in the xylene for 3 min (3 changes of 3 min each). Sections were rehydrated with decreasing grades of the alcohol (100, 90, 80 and 50% (3 min in each)). Slides were kept in distilled water (5 min) and in hematoxyline (10 min). One dip was given in 1% ammonia water and immediately washed under running tap water (5 min); 2–3 drops were given in alcoholic eosin and shades again dehydrated with increasing grades of alcohol (70, 80, 90 and 100% (3 min in each)). Slices were cleared with xylene (3 min and 3 changes), mounted with DPX mountant and observed under suitable magnification.
Antioxidant activity
DPPH antioxidant assay
Free radical scavenging activity was performed according to CitationBrand-Williams et al. (1995). Antiradical activity was measured by decrease in absorbance at 576 nm of methanol solution of colored DPPH (1,1-diphenyl-2-picrylhydrazyl) brought about by sample. A stock solution of DPPH (1.3 mg/l methanol) was prepared such that 75 µl it in 3 ml methanol gives an initial absorbance of 0.9. This stock solution was used to measure the antiradical activity. The decrease in absorbance in the presence of various fractions of methanol extract and crude methanol extract at different concentration (75–100 µg/ml) was noted after 15 min. IC50 was calculated from % inhibition.
ABTS antioxidant assay
Total antioxidant status of the extract and fractions was measured using the 2,2′-azinobis[3-ethylbenzthiazoline]-6-sulfonic acid (ABTS) assay (CitationRe et al., 1999). ABTS˙+ radical cation is generated by reacting 7 mM ABTS and 2.45 mM potassium peroxodisulphate via incubation at room temperature (23°C) in the dark for 12–16 h. ABTS solution was diluted with 80% HPLC grade ethanol to an absorbance of 0.7 ± 0.04 at 734 nm and equilibrated at 30°C. Various fractions and extracts were diluted with 80% methanol such that after introduction of 30 µl aliquot of each dilution in the assay, it produced 20–80% inhibition of the blank absorbance. To 3 ml of diluted ABTS, 30 µl of plant extract/fraction were added and mixed. The mixture was allowed to stand at room temperature for 6 min and absorbance was recorded at 734 nm. Trolox standard solution in 80% methanol was prepared and assayed by using the same conditions. Inhibition of absorbance was calculated and plotted as a function of concentration of trolox for the standard reference data. Results were expressed in µg/ml trolox equivalents (TE)/g dry mass.
FRAP
The ferric reducing antioxidant potential (FRAP) assay of extract and fractions was measured according to a modified protocol developed by CitationBenzie and Strain (1996). An aliquot (200 µl) of extract was added to 3 ml of FRAP reagent [10 parts of 300 mM of sodium acetate buffer at pH 3.6, 1 part of 10 mM 2,4,6-tris(2′-pyridyl)-s-triazine solution and 1 part of 20 mM FeCl3.6H2O solution] and the reaction mixture was incubated in a water bath at 37°C. The increase in absorbance was measured after 30 min at 593 nm. Antioxidant capacity was reported as the ability of the fraction/extract to reduce ferric ions and was expressed in µg/ml TE/g dry mass.
TPC
Total phenolic content (TPC) was determined using the Folin–Ciocalteu method (CitationRagazzi & Veronese, 1973). An aliquot of 100 µl of extract was mixed with 2.5 ml Folin–Ciocalteu phenol reagent and allowed to react for 5 min, then 2.5 ml, saturated Na2CO3 solution was added and allowed to stand for 1 h before the absorbance of the reaction mixture was read at 725 nm. The TPC of the fractions/extract was expressed as mg gallic acid equivalent per g of plant material on dry basis.
Statistical analysis
The data was analyzed by one-way analysis of variance followed by Tukey–Kramar multiple comparison test. p < 0.05 was considered statistically significant.
Results and discussion
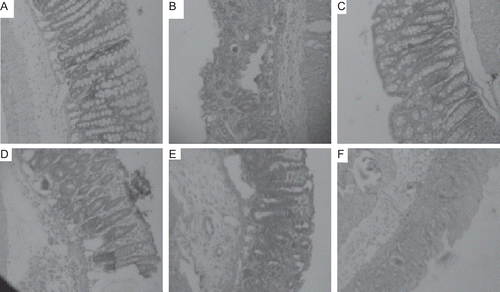
Results showed that UC was induced completely as positive control tissue that showed classical lesions of ulcer i.e., the destruction of epithelia, hemorrhage, infiltration of neutrophils and macrophage. Groups treated with various fractions and standard group showed significant healing. Groups treated with n-hexane and ethyl acetate fractions showed mild scores of pathological changes but infiltration of neutrophils and presence of edema was observed. Groups treated with methanol extract showed less edema and infiltration compared with previous groups. Groups treated with methanol fraction of crude methanol extract showed lower pathological scores and evident by good healing and less infiltration of neutrophils and other inflammatory cells (, ). In brief, methanol fraction of crude methanol extract is found to be best in the treatment of UC. Methanol fraction of crude methanol extract of C. dichotoma bark was found to be most potent in reducing level of MPO and MDA significantly than other fractions in tissue and blood (). Results from antioxidant screening showed that the methanol fraction of methanol extract of the bark of C. dichotoma showed best antioxidant potential in all the models followed by crude methanol extract. In the DPPH assay methanol fraction showed lowest IC50 value (). It showed highest TE antioxidant capacity µg/ml TE/g dry weight of the plant in ABTS antioxidant assay and FRAP assay (). Phenolic content was found to be highest into methanol fraction which supports its antioxidant potential ().
Table 1. Histopathological observations after treatment with fractions (50 mg/kg, p.o.) and crude methanol extract (500 mg/kg, p.o.) of C. dichotoma bark.
Table 2. Effect of fractions from crude methanol extract of C. dichotoma bark on myeloperoxidase (MPO) and malondialdehyde (MDA) activity in blood and tissue.
Table 3. Antioxidant activity of fractions from crude methanol extract of the bark of C. dichotoma.
Table 4. Concentration of phenolic content of fractions from crude methanol extract of the bark of C. dichotoma by Folin–Ciocalteu method.
Figure 1. Histopathological observations of colon tissue after the treatment with fractions of crude methanol extract of C. dichotoma bark. A = Standard; B = n-Hexane fraction; C = Methanol fraction; D = Crude methanol extract; E = Ethyl acetate fraction, F = Control (5% acetic acid).
The mucosal immune system is the main effectors of intestinal inflammation and injury, with cytokines playing a central role in modulating inflammation (CitationArdizzone & Bianchi Porro, 2005; CitationNakamura et al., 2006). Increased levels of both tumor necrosis factor-α (TNF-α) and PGE2, in this work, caused epithelial cell necrosis, edema, and neutrophil infiltration, as proved by the histopathological study. TNF-α is abundantly expressed in the gut of IBD patients (CitationNakamura et al., 2006), a fact that supports our findings. Recently, CitationStucchi et al. (2006) found that LITAF (lipopolysaccharide-induced TNF-α factor), which mediates TNF-α expression in human macrophages, is significantly elevated above controls in macrophages of ileal and colonic tissues from patients with either Crohn’s disease or UC. Elevated levels of PGE2, goes in harmony with CitationOtani et al. (2006), who proved that the increased level of PGE2 is attributed to its enhanced synthesis rather than reduced catabolism, both of which are mediated by TNF-α. On the other hand, the methanol fraction of C. dichotoma bark decreased significantly the gross lesion scores, and production of both TNF-α and PGE2. The effect of the methanol fraction on TNF-α may be mediated either by blocking activation of this pro-inflammatory mediator and its transcriptional regulator (CitationFrondoza et al., 2004), and/or inhibits its production from macrophages (CitationTripathi et al., 2007). Inhibition of PGE2, on the other hand, may follow that of TNF-α (CitationOtani et al., 2006), or may result from its ability to inhibit cycloxygenase enzymes (CitationGrzanna et al., 2005). Since the intestine is in a constant state of controlled inflammation, thus amplification of the inflammatory response activates infiltration of inflammatory cells that triggers pathological responses and symptoms of IBD (CitationSartor, 1997). Our study showed that acetic acid raised the levels of colonic MPO, indicating infiltration of neutrophils and perturbation of the inflammatory system (CitationKrawisz et al., 1984). This fact is documented in both animal models (CitationAkgun et al., 2005; CitationCetinkaya et al., 2005), and patients with IBD (CitationKruidenier et al., 2003). The methanol fraction ameliorated neutrophil infiltration as evidenced by suppression of colon MPO and improvement of histological features. In IBD, oxidative stress plays a role in disease initiation and progression (CitationKruidenier & Verspaget, 2002). Reactive oxygen species (ROS) attack the cellular macromolecules, thus disrupting epithelial cell integrity and hindering mucosal recovery, especially in case of impaired endogenous defense systems (CitationBuffinton & Doe, 1995). In this work, acetic acid induced ROS formation is inhibited by the methanol fraction as it is proved as a good antioxidant. Thus, its ability to inhibit free radical generation, as was proven in this work by restoring the redox state of the colonic mucosa, offers another explanation of the anti-ulcerogenic activity of this plant. This action lends pharmacological support to folkloric, ethno-medical uses of plant in the management of inflammatory gastrointestinal tract disorders.
Conclusion
It can be concluded that the methanol fraction of the crude methanol extract of C. dichotoma bark could be beneficial as a complementary agent in UC and offers an alternative approach to modulate the inflammatory process involved in this disease.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Akgun E, Caliskan C, Celik HA, Ozutemiz AO, Tuncyurek M, Aydin HH. (2005). Effects of N-acetylcysteine treatment on oxidative stress in acetic acid-induced experimental colitis in rats. J Int Med Res, 33, 196–206.
- Anonymous. (2004). The Wealth of India, Vol. IV. Council of Scientific and Industrial Research: New Delhi, 152.
- Ardizzone S, Bianchi Porro G. (2005). Biologic therapy for inflammatory bowel disease. Drugs, 65, 2253–2286.
- Benzie IF, Strain JJ. (1996). The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem, 239, 70–76.
- Brand-Williams W, Cuvelier ME, Berset C. (1995). Use of a free radical method to evaluate antioxidant activity. Food Sci Technol, 28, 25–30.
- Buege JA, Aust SD. (1978). Microsomal lipid peroxidation. Meth Enzymol, 52, 302–310.
- Buffinton GD, Doe WF. (1995). Depleted mucosal antioxidant defences in inflammatory bowel disease. Free Radic Biol Med, 19, 911–918.
- Cetinkaya A, Bulbuloglu E, Kurutas EB, Ciralik H, Kantarceken B, Buyukbese MA. (2005). Beneficial effects of N-acetylcysteine on acetic acid-induced colitis in rats. Tohoku J Exp Med, 206, 131–139.
- Evans SM, László F, Whittle BJ. (2000). Site-specific lesion formation, inflammation and inducible nitric oxide synthase expression by indomethacin in the rat intestine. Eur J Pharmacol, 388, 281–285.
- Frondoza CG, Sohrabi A, Polotsky A, Phan PV, Hungerford DS, Lindmark L. (2004). An in vitro screening assay for inhibitors of proinflammatory mediators in herbal extracts using human synoviocyte cultures. In Vitro Cell Dev Biol Anim, 40, 95–101.
- Grzanna R, Lindmark L, Frondoza CG. (2005). Ginger–an herbal medicinal product with broad anti-inflammatory actions. J Med Food, 8, 125–132.
- Itoh H, Kataoka H, Tomita M, Hamasuna R, Nawa Y, Kitamura N, Koono M. (2000). Upregulation of HGF activator inhibitor type 1 but not type 2 along with regeneration of intestinal mucosa. Am J Physiol Gastrointest Liver Physiol, 278, G635–G643.
- Kathleen A, Julie S, Jurenka. (2003). Inflammatory bowel disease Part I: Ulcerative colitis-pathophysiology and conventional and alternative treatment options. Alternative Medicine Review 8, 247–278.
- Kirsner JB, Shorter RG. (1982). Recent developments in nonspecific inflammatory bowel disease (second of two parts). N Engl J Med, 306, 837–848.
- Kirtikar KR, Basu BD. (1956). Indian Medicinal Plant, Second Edition, Vol. II, International Book Distributors: Deharadun pp.723.
- Krawisz JE, Sharon P, Stenson WF. (1984). Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology, 87, 1344–1350.
- Kruidenier L, Kuiper I, Lamers CB, Verspaget HW. (2003). Intestinal oxidative damage in inflammatory bowel disease: semi-quantification, localization, and association with mucosal antioxidants. J Pathol, 201, 28–36.
- Kruidenier L, Verspaget HW. (2002). Review article: Oxidative stress as a pathogenic factor in inflammatory bowel disease–radicals or ridiculous? Aliment Pharmacol Ther, 16, 1997–2015.
- Mahgoub AA, El-Medany AA, Hager HH, Mustafa AA, El-Sabah DM. (2003). Evaluating the prophylactic potential of zafirlukast against the toxic effects of acetic acid on the rat colon. Toxicol Lett, 145, 79–87.
- Nadkarni AK. (1991). Indian Materia Medica, Third Edition, Vol. I, Bombay Popular Prakashan: Bombay, pp. 1197–1198.
- Nakamura K, Honda K, Mizutani T, Akiho H, Harada N. (2006). Novel strategies for the treatment of inflammatory bowel disease: Selective inhibition of cytokines and adhesion molecules. World J Gastroenterol, 12, 4628–4635.
- Otani T, Yamaguchi K, Scherl E, Du B, Tai HH, Greifer M, Petrovic L, Daikoku T, Dey SK, Subbaramaiah K, Dannenberg AJ. (2006). Levels of NAD(+)-dependent 15-hydroxyprostaglandin dehydrogenase are reduced in inflammatory bowel disease: Evidence for involvement of TNF-alpha. Am J Physiol Gastrointest Liver Physiol, 290, G361–G368.
- Ragazzi E, Veronese G. (1973). Quantitative analysis of phenolic compounds after thin-layer chromatographic separation. J Chromatogr, 77, 369–375.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med, 26, 1231–1237.
- Sartor RB. (1997). Pathogenesis and immune mechanisms of chronic inflammatory bowel diseases. Am J Gastroenterol, 92, 5S–11S.
- Stucchi A, Reed K, O’Brien M, Cerda S, Andrews C, Gower A, Bushell K, Amar S, Leeman S, Becker J. (2006). A new transcription factor that regulates TNF-alpha gene expression, LITAF, is increased in intestinal tissues from patients with CD and UC. Inflamm Bowel Dis, 12, 581–587.
- Tripathi S, Maier KG, Bruch D, Kittur DS. (2007). Effect of 6-gingerol on pro-inflammatory cytokine production and costimulatory molecule expression in murine peritoneal macrophages. J Surg Res, 138, 209–213.