Abstract
Context: The main use of stem bark infusions of Alnus acuminata ssp. arguta (Schlecht.) Furlow (Betulaceae) includes treatments for acute inflammation in Mexican traditional medicine.
Objective: n-Hexane (CHE), chloroform (CCE), and methanol (CME) extracts of the stem bark were investigated for anti-inflammatory activity and its safety.
Materials and methods: The anti-inflammatory effects of the orally administered CME, CCE, and CHE extracts, using carrageenan-induced rat hind paw edema model, and acute oral toxicity in mice, using Lorke’s method, were determined.
Results and discussion: The column chromatographic fraction (CME-3) showed a higher anti-inflammatory activity (92.2%) (IC50: 60.8 mg/mL) as compared with CME (76.9%); both were in the same order of magnitude as that of indomethacin, the positive control drug. Safety parameters for acute oral toxicity test showed that CME was not toxic (LD50: >5000). Several triterpenoids (1–7) from hexane extracts and diarylheptanoids (10–14) from methanol extracts of A. acuminata ssp. arguta were isolated and characterized.
Conclusions: These results confirm the traditional uses of A. acuminata in acute inflammatory conditions and its safety for consumption.
Introduction
Alnus acuminata ssp. arguta (Schlecht.) Furlow (Betulaceae) is native to the high mountain regions of tropical America, from Mexico to the north of Argentina, especially along rivers. Its synonyms include Alnus jorullensis H.B.K., Alnus ferruginea Kundh, Alnus mirbelli Spach, Alnus spachii and is locally called aile, aliso, ilite, in Mexico; palo de lama in Guatemala; jaúl in Costa Rica; and cerezo in Colombia. In the State of Chiapas (southern Mexico), the stem bark is widely used ethnomedically as an anti-inflammatory drug, also for the treatment of scrophula, syphilis, rheumatic conditions, and in skin infectious diseases (CitationMartínez, 1984).
Triterpenoids, diarylheptanoids, oregonine, myricetin (CitationKuroyanagi et al., 2005; CitationJin et al., 2007a), and β-sitosteryl glycoside (CitationGonzalez-Laredo et al., 1998) have been isolated and identified from other species of Alnus. Some of them have shown antioxidant (CitationKuroyanagi et al., 2005) and anti-inflammatory activities due to the inhibition of COX-2 (CitationMin-Won et al., 2000), and inhibition of the tumor necrosis factor alpha (TNF-α) production and nitric oxide synthesis (CitationJin et al., 2007b). Some of the above mentioned compounds also exhibited activity against VIH-1 reverse transcriptase (CitationYu et al., 2007). δ-Amirone and 4′,7-dimethoxy apigenin, having abortive and anti-inflammatory activities, have been isolated from A. acuminata leaf (CitationSalama et al., 1996; CitationSalama & Avendaño, 2005). Although there are many biological and chemical studies on other Alnus species, up to date, stem bark of A. acuminata has not been investigated. In this work, we evaluated the anti-inflammatory effect of its extracts as well as its chemical contents.
Methods
General experimental procedures
IR spectra were obtained using KBr disks or films on a Perkin-Elmer FT 1605 spectrophotometer. NMR spectra including COSY, NOESY, HMBC, and HSQC experiments were recorded on a Varian Unity INOVA at 300 or 400 MHz (1H) or 75 or 100 MHz (13C). EIMS were recorded on a JEOL SX 102A mass spectrometer and optical rotations were determined on a Perkin-Elmer Mod. 241 Polarimeter. For the open column chromatographies, we used Si gel 60 (70–230 mesh; Merck, Germany), Lipophilic Sephadex LH-20 (Sigma-Aldrich, St. Louis, MO), and silica gel 60 F254 (Merck) for thin-layer chromatography (TLC).
Plant material
The stem bark of A. acuminata ssp. arguta was collected from Huistan town, in the State of Chiapas, in May 2006. The identification of the plant was realized by Dr. M. Ishiki Ishihara and a voucher specimen (Ishiki-324) was deposited in the Herbarium of El Colegio de la Frontera Sur (ECOSUR), Chiapas.
Preparation and fractionation of stem bark extracts
The air-dried stem bark (1.16 kg) was ground into powder and separately macerated at room temperature with n-hexane, chloroform, and methanol (3 L each) for 3 days and filtered. This procedure was repeated twice and each extract was combined and evaporated in vacuo to give the corresponding hexane (CHE, 31.3 g), chloroform (CCE, 28.13 g), and methanol (CME, 10.9 g) syrupy residues. A bioguided fractionation procedure based on the anti-inflammatory properties of A. acuminata ssp. arguta using the carrageenan-induced rat hind paw edema model (CitationWinter et al., 1962) was employed with the extracts. Active CME (10 g) was subjected to column chromatography over Si gel 60 (220 g) and was eluted with a gradient of n-hexane–ethyl acetate (10:0, 50:50, 0:10). Fractions of 250 mL each were collected and pooled based on each system of solvents used to give three main fractions (CME-1, CME-2, and CME-3) from less to more polar solvent.
Animals
Male Wistar rats (200–230 g) and ICR male mice (25–30 g) obtained from Centro UNAM—Harlan (Harlan México, S.A. de C.V.) were used for the anti-inflammatory and toxicity studies, respectively. Procedures involving animals and their care were conducted according to the Mexican Official Norm for Animal Care and Handling (NOM-062-ZOO-1999) and in compliance with the international rules on care and use of laboratory animals.
Anti-inflammatory studies
The control groups of eight animals each received p.o. only vehicle (Tween 80 in 0.9% saline solution). Test groups were treated with extracts/fractions suspended in the vehicle. Indomethacin (100 mg/kg) was given to the positive control group.
Carrageenan-induced hind paw edema model
The rats were left for 2 days for acclimatization to animal room conditions and maintained on standard pellet diet (Teklad Global for rodents, 2018S, Harlan) with water ad libitum. Food was withdrawn 24 h prior to the experiment, but free access to water was allowed. The carrageenan-induced hind paw edema model was used for determination of anti-inflammatory activity (CitationLiso et al., 1996). The extracts (CME, CHE, and CCE) were tested at doses of 30, 100, and 300 mg/kg, whereas CME-1, CME-2, and CME-3 fractions were employed at 300 mg/kg and indomethacin at 100 mg/kg.
The right and left hind paws were marked to a point on the skin over the lateral malleolus to record the initial paw volume (Vo). After 30 min p.o. of test or vehicle samples, each animal was injected with 0.1 mL of freshly prepared carrageenan (3%) (Sigma-Aldrich) in s.s. into the subplantar tissue of the right hind paw. Saline solution (0.1 mL) was administered in the left paw. The effect of each test sample was determined by subtracting the value of the inflammatory effect between test and control paws using a plethysmometer (Plethysmometer UGO Basile Mod. 7140). The volume of the edema (mL) was measured immediately for all treatments and afterward at 1, 2, 3, 4, 5, and 6 h after carrageenan injection (CitationLiso et al., 1996).
Fasted rats (24 h) were randomly grouped, to determine the dose–response curve for anti-inflammatory effect of each extract per group. The variation of edema volume at each time (Vt) was calculated as delta volume (ΔVt = Vt − Vo) in milliliter. The inhibition of edema (% EI) was calculated as previously described (CitationLiso et al., 1996). Data were expressed as mean ± SEM of eight rats. One-way analysis of variance (ANOVA), followed by Dunnett’s test, was used to compare differences between treatments and were considered to reach statistical significance when P < 0.05.
Acute toxicity study in mice
Food was withheld 12 h before experiment, but animals had free access to drinking water. Two groups of three mice each fed orally with CME at doses of 10, 100, and 1000 mg/kg or 0, 1600, 2900, and 5000 mg/kg were daily observed for a period of 14 days for mortality, toxic effects, and/or changes in behavioral pattern, according to Lorke’s (1983) method. At the end of the experiments, the animals were sacrificed in a CO2 chamber.
Isolation and purification of triterpenoids
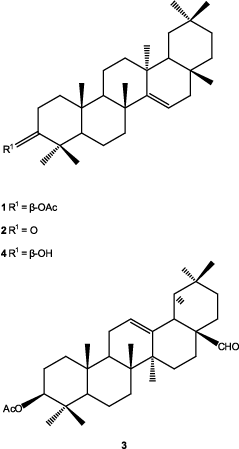
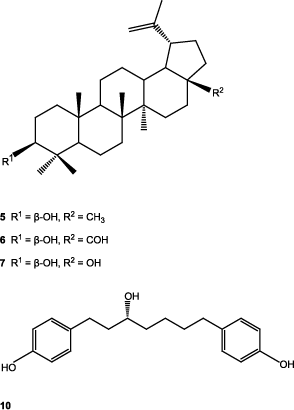
The CHE (13.1 g) was column-chromatographed (200 g, Si gel Merck 60) and eluted with a gradient of n-hexane–EtOAc (10:0/0:10). Fractions of 100 mL each were collected and pooled based on the TLC profiles to yield eight major fractions (F01–F08). All triterpenoids were obtained as white crystals. Fractions F03 and F04 eluted with hexane–EtOAc (95:5) gave taraxeryl acetate (1), 14-taraxeren-3-one (2), and 3β-acetoxy-olean-12-ene-28-al (3). Compounds 1 and 2 were separated by preparative TLC (CH2Cl2 100%) showing yields of 3.0 and 210 mg, respectively, and compound 3 was purified by recrystallization (n-hexane–AcOEt) (18 mg). Fraction F05 eluted with n-hexane–AcOEt (90:10) gave 4 mg of 3β-hydroxy-14-taraxerene (4) and 1.01 g of 3β-hydroxy-lup-20(29)-ene (5). Fraction F06 eluted with n-hexane–AcOEt (85:15) gave 35 mg of compounds 6 and 8. Successive preparative TLC of this fraction (CHCl3) led to the isolation of 3β-hydroxy-lup-20(29)-ene-28-al (6, 14 mg) and β-sitosterol (8, 20 mg). Fraction F08 (hexane–ethyl acetate, 70:30) gave 79 mg of 3β,28-dihydroxy-lup-20(29)-ene (7).
Isolation and purification of diphenylheptanoids
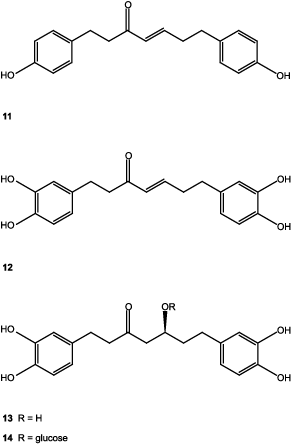
A second CME was prepared from a new batch of plant (1.2 kg) and its activity was verified as previously described. Then, the CME (10.5 g) was column-chromatographed over Sephadex LH-20 (150 g) and eluted with 100% MeOH to give six fractions (F1–F6). Fraction F3 (2.89 g) was further separated by column chromatography on Sephadex (50 g) (MeOH 100%) to furnish seven fractions (F1a–F7a). Column chromatography of fraction F5a (2.6 g) on silica gel (40 g) eluted with CH2Cl2–MeOH (10:0/0:10) gave eight fractions (F1b–F8b). Acetylation of fraction F2b (100 mg) (CitationKuroyanagi et al., 2005) gave three main products that were separated and purified by preparative TLC (CH2Cl2 100% × 3): β-sitosteryl glycoside (9, 10 mg), (−)-centrolobol (10, 30 mg), and 1,7-bis-(4-hydroxyphenyl)-4-hepten-3-one (11, 40 mg), which were identified as the corresponding acetyl derivatives. Hirsutenone (1,7-di-(3′,4′-dihydroxyphenyl)-4-hepten-3-one) (12) was present in fraction F3b (154 mg) and was identified as its tetraacetyl derivative. Fraction F4b (358 mg) was thin layer-chromatographed (CH2Cl2–acetone, 30:70), the band with retention factor of 0.31 afforded hirsutanonol (13, 200 mg) and fraction 7b yielded hirsutanonol 5-O-β-d-glucopyranoside as a major compound (14, 250 mg).
Results and discussion
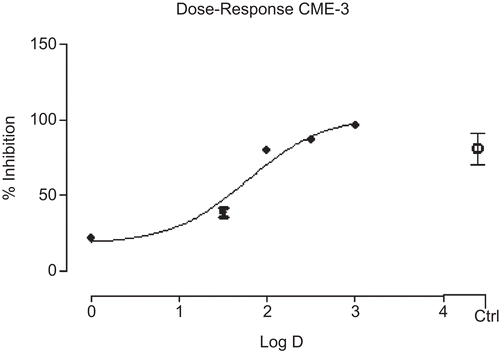
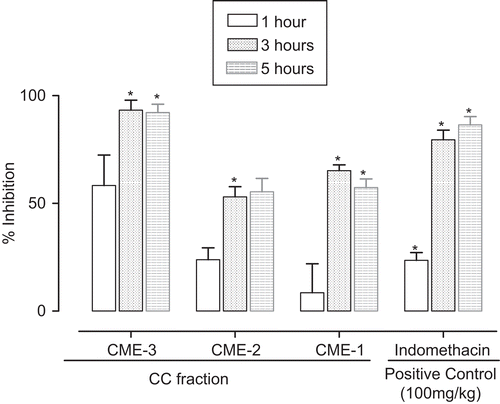
The stem bark of A. acuminata ssp. arguta, the vegetative part commonly employed in popular remedies, was selected for this anti-inflammatory study. The CHE, CCE, and CME extracts gave time-dependent reduction of inflammation at the highest dose tested (300 mg/kg). At fifth h, which was the time of the highest anti-inflammatory activity, the extracts showed 40.7, 52.0, and 76.9% of inhibition, respectively; the last value was comparable with 89.9% of indomethacin (). The CC fractions CME-1, CME-2, and CME-3 gave similar time-dependent anti-inflammatory activities. The CME-3 (300 mg/kg) gave 93.3 and 92.2% inhibition at 3 and 5 h, which were comparable with 79.5 and 86.5% inhibition of indomethacin (100 mg/kg) at the same hours (). The order of activity for CME-3 > CME-2 = CME-1 suggested that the anti-inflammatory constituents of the stem bark could be polar. The most active CME-3 fraction also gave dose-dependent anti-inflammatory activity, with IC50 of 60.77 mg/mL, response comparable with that of indomethacin at same dose (100 mg/kg) ().
Figure 1. Anti-inflammatory effect of hexane, chloroform, and methanol extracts expressed as percentage of inflammation inhibition obtained from the delta volume at 300 mg/kg, p.o., in the carrageenan rat hind paw edema model. Bars represent the mean ± SEM (n = 8). *P < 0.05 compared with the control group.
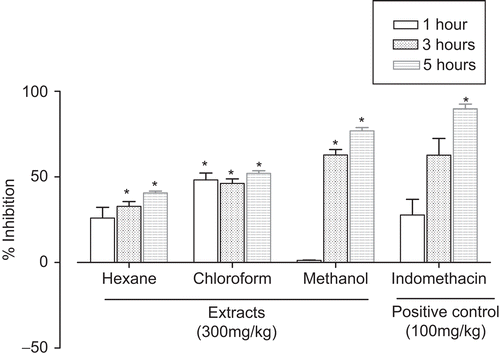
Figure 2. Anti-inflammatory effect of CME-3, CME-2, and CME-1, expressed as percentage of inflammation inhibition obtained from the delta volume with 300 mg/kg, p.o., in the carrageenan rat hind paw edema model. Bars represent the mean ± SEM (n = 8). *P < 0.05 compared with the control group.
Using the acute toxicity test and at the tested doses of 10 to 5000 mg/kg of CME, an LD50 value >5000 mg/kg was calculated using the geometric mean of the doses for which 0/3 deaths were found. Hence, the methanolic extract of A. acuminata ssp. arguta stem bark could be regarded as non-toxic (CitationDéciga-Campos et al., 2007). Changes in body weight or macroscopical morphology of heart, liver, kidney, and lung were not observed.
In this article, we described the biological studies of extracts of A. acuminata, as well as the isolation of triterpenoids 1–7 from the CHE extract, and of compounds 9–14 from the CME, belonging to the linear diarylheptanoids type compounds 10–14. Compounds 1, 2, and 4, gave a mass spectrum that showed intense peaks at m/z 300 and 204, typical for the ring of taraxerane (CitationSakurai et al., 1987). In compound 3, the oleanane ring was evident by the HMBC correlations, and by the mass fragments of m/z 203, 133, and 189. In compounds 10–14, the linear diarylheptanoid skeleton was evident in the 1H-NMR spectra, where two 3,4-dihydroxyphenyl (compounds 12–14) or two 4-hydroxyphenyl (compounds 10 and 11) groups were shown and in all cases a C7 moiety between them. In compounds 11 and 12, α,β-unsaturated carbonyl groups were present in positions 3, 4, and 5 of the heptanoid chain and in compounds 13 and 14, two benzylic and four unconjugated methylenes besides one carbinolic proton were present, and together with the optical rotation values of both compounds agreed for structures of hirsutanonol (13) (CitationOhta et al., 1984) and hirsutanonol 5-O-β-d-glucopyranoside (14) (CitationOhta et al., 1984). In compound 10, the carbinolic proton was evident in position 3 of the heptanoid chain and the optical rotation sign was the same as for (−)-centrolobol (CitationNagai et al., 1986). The structures of compounds 1–14 were confirmed by comparing their physical and spectroscopic data with previously reported results (IR, one- and two-dimensional 1H-NMR, 13C-NMR, HSQC, HMBC, NOESY correlations, and optical rotations).
The isolated compounds are as follows: taraxeryl acetate (1) (CitationMatsunaga et al., 1988), 14-taraxeren-3-one (2) (CitationValente et al., 2004), 3β-acetoxy-olean-12-ene-28-al (3) (CitationKim et al., 2004; CitationChen et al., 2006), 3β-hydroxy-14-taraxerene (4) (CitationZou et al., 2006; CitationJutiviboonsuk et al., 2007), 3β-hydroxy-lup-20(29)-ene (5) (CitationUddin & Ur-Rahman, 1994; CitationRagasa et al., 2005), 3β-hydroxy-lup-20(29)-ene-28-al (6) (CitationPohjala et al., 2009), 3β,28-dihydroxy-lup-20(29)-ene (7) (CitationPohjala et al., 2009), β-sitosterol (8), β-sitosteryl glycoside (9) (CitationDella Greca et al., 1991), (−)-centrolobol (10) (CitationCraveiro 1970; CitationNagai et al., 1986; CitationAlegrio et al., 1989; CitationPark et al., 2010), 1,7-bis(4-hydroxyphenyl)-4-hepten-3-one (11) (CitationNomura et al., 1981; CitationFuchino et al., 1996), 1,7-di-(3′,4′-dihydroxyphenyl)-4-hepten-3-one (12) (CitationKuroyanagi et al., 2005), hirsutanonol (13) (CitationOhta et al., 1984), and hirsutanonol 5-O-β-d-glucopyranoside (14) (CitationOhta et al., 1984).
Triterpenoids 1, 7, and sterol 9 have been previously isolated from other species of the genus Alnus (CitationHarborne, 1985; CitationDuke, 1992; CitationMáñez et al., 1997; CitationHuguet et al., 2000; CitationChen et al., 2006), as well as diarylheptanoids 11–14 (CitationNomura et al., 1981; CitationChen et al., 2000; CitationPark et al., 2010; CitationTung et al., 2010). CitationAkihisa et al. (2006) demonstrated that (−)-centrolobol (10) possesses marked anti-inflammatory activity against TPA-induced inflammation in mice and antitumor-promoting effect in two-stage carcinogenesis initiated by 7,12-dimethylbenz[a]anthracene. CitationMorikawa et al. (2003) found that centrolobol (10) exhibited inhibitory activity on nitric oxide production in lipopolysaccharide-activated macrophages. Compound 10 also showed good antileishmanial activity (CitationAraujo et al., 1998), and exhibited a remarkable inhibitory effect of antigen-induced degranulation in RBL-2H3 cells (CitationKim et al., 2010). Compounds 11–14 showed a hepatoprotective effect against t-butylhydroperoxide-induced toxicity in HepG2 cells (CitationPark et al., 2010). Natural products 12–14 displayed potent antioxidative activity in a superoxide radical scavenging test and a DPPH radical scavenging test (CitationKuroyanagi et al., 2005). Compound 13 inhibited the NF-κB activation and NO and TNF-α production (CitationJin et al., 2007b). Compounds 1–14 were isolated for the first time from A. acuminata ssp. arguta and (−)-centrolobol (10), a diarylheptanoid constituent of Centrolobium species, was isolated for the first time in a non-glycosylated form from Alnus species.
Conclusions
The results in this study confirmed that A. acuminata ssp. arguta has a significant anti-inflammatory activity and justifies its ethnomedical use as such. The plant is also safe for consumption as traditional remedy.
Acknowledgement
The authors wish to thank Dirección General de Asuntos del Personal Académico de la UNAM (DGAPA) for economical support (PAPIIT IN210709 and IN205008), so as to thank A. Acosta, N. Esturau, R.I. del Villar, G. Duarte, M. Guzmán, M. Gutiérrez, and M.Y. González for spectroscopic assistance.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Akihisa T, Taguchi Y, Yasukawa K, Tokuda H, Akazawa H, Suzuki T, Kimura Y. (2006). Acerogenin M, a cyclic diarylheptanoid, and other phenolic compounds from Acer nikoense and their anti-inflammatory and anti-tumor-promoting effects. Chem Pharm Bull, 54, 735–739.
- Alegrio LV, Braz-Filho R, Gottliebs OR. (1989). Diarylheptanoids and isoflavonoids from Centrolobium species. Phytochemistry, 28, 2359–2362.
- Araujo CAC, Alegrio LV, Leon LL. (1998). Antileishmanial activity of compounds extracted and characterized from Centrolobium sclerophyllum. Phytochemistry, 49, 751–754.
- Chen C, Tuh S, Chen C, Kuo C, Lee S. (2006). Triterpenoids and sterols from the stem of Alnus formosana Burk. Nat Prod Comm, 1, 299–301.
- Chen J, Gonzalez-Laredo RF, Karchesy JJ. (2000). Minor diarylheptanoid glycosides of Alnus rubra bark. Phytochemistry, 53, 971–973.
- Craveiro AA, da Costa Prado A, Gottlieb OR, Welerson de Albuquerque PC. (1970). Diarylheptanoids of Centrolobium species. Phytochemistry, 9, 1869–1875.
- Déciga-Campos M, Rivero-Cruz I, Arriaga-Alba M, Castañeda-Corral G, Angeles-López GE, Navarrete A, Mata R. (2007). Acute toxicity and mutagenic activity of Mexican plants used in traditional medicine. J Ethnopharmacol, 110, 334–342.
- Della Greca M, Molinaro A, Monaco P, Previtera L. (1991). Acylglycosyl sterols from Pistia stratiotes. Phytochemistry, 30, 2422–2424.
- Duke AJ. (1992). Handbook of Medical Herbs. Boca Raton, FL: CRC Press, pp. 145–148.
- Fuchino H, Konishi S, Satoh T, Yagi A, Saitsu K, Tatsumi T, Tanaka N. (1996). Chemical evaluation of Betula species in Japan. II. Constituents of Betula platyphylla var. japonica. Chem Pharm Bull, 44, 1033–1038.
- Gonzalez-Laredo RF, Helm RF, Chen J, Karchesy JJ. (1998). Two acylated diarylheptanoid glycosides from red alder bark. J Nat Prod, 61, 1292–1294.
- Harborne B. (1985). Dictionary of Organic Compounds. New York: Marcel Dekker, pp. 276–320.
- Huguet A, del Carmen Recio M, Máñez S, Giner R, Ríos J. (2000). Effect of triterpenoids on the inflammation induced by protein kinase C activators, neuronally acting irritants and other agents. Eur J Pharmacol, 410, 69–81.
- Jin W, Cai XF, Na M, Lee JJ, Bae K. (2007a). Triterpenoids and diarylheptanoids from Alnus hirsuta inhibit HIF-1 in AGS cells. Arch Pharm Res, 30, 412–418.
- Jin W, Cai XF, Na M, Lee JJ, Bae K. (2007b). Diarylheptanoids from Alnus hirsuta inhibit the NF-kB activation and NO and TNF-alpha production. Biol Pharm Bull, 30, 810–813.
- Jutiviboonsuk A, Zhang H, Kondratyuk T, Herunsalee A, Chaukul W, Pezzuto J, Fong H, Bunyapraphatsara N. (2007). Isolation and characterization of cancer chemopreventive compounds from Barringtonia maunwongyathiae. Pharm Biol, 45, 185–194.
- Kim MR, Lee HH, Hahm KS, Moon YH, Woo ER. (2004). Pentacyclic triterpenoids and their cytotoxicity from the stem bark of Styrax japonica S. et Z. Arch Pharm Res, 27, 283–286.
- Kim SH, Park JH, Kim TB, Lee HH, Lee KY, Kim YC, Sung SH. (2010). Inhibition of antigen-induced degranulation by aryl compounds isolated from the bark of Betula platyphylla in RBL-2H3 cells. Bioorg Med Chem Lett, 20, 2824–2827.
- Kuroyanagi M, Shimomae M, Nagashima Y, Muto N, Okuda T, Kawahara N, Nakane T, Sano T. (2005). New diarylheptanoids from Alnus japonica and their antioxidative activity. Chem Pharm Bull, 53, 1519–1523.
- Liso PA, Rebuelta M, San Román J, Gallardo A, Villar AM. (1996). Polymeric drugs derived from ibuprofen with improved antiinflammatory profile. J Biomed Mater Res, 32, 553–560.
- Lorke D. (1983). A new approach to practical acute toxicity testing. Arch Toxicol, 54, 275–287.
- Máñez S, Recio MC, Giner RM, Ríos JL. (1997). Effect of selected triterpenoids on chronic dermal inflammation. Eur J Pharmacol, 334, 103–105.
- Martínez J. (1984). La Flora de Veracruz. México: Ed. El Ateneo, Fascículo no. 20, pp. 128–136.
- Matsunaga S, Reiko T, Masao A. (1988). Triterpenoids from Euphorbia maculata. Phytochemistry, 27, 535–537.
- Min-Won L, Jung-Hwan K, Dong-Wook J, Kyoung-Hwan A, Sang-Hak T, Young-Jonn S. (2000). Inhibition of cyclooxygenase-2 expression by diarylheptanoid from the bark of Alnus hirsuta var. sibirica. Biol Pharm Bull, 23, 517–518.
- Morikawa T, Tao J, Toguchida I, Matsuda H, Yoshikawa M. (2003). Structures of new cyclic diarylheptanoids and inhibitors of nitric oxide production from Japanese folk medicine Acer nikoense. J Nat Prod, 66, 86–91.
- Nagai M, Kenmochi N, Fujita M, Furukawa N, Inoue T. (1986). Studies on the constituents of Aceraceae plants. VI. Revised stereochemistry of (−) centrolobol, and new glycosides from Acer nikoense. Chem Pharm Bull, 34, 1056–1060.
- Nomura M, Tokoroyama T, Kubota T. (1981). Biarylheptanoids and other constituents from wood of Alnus japonica. Phytochemistry, 20, 1097–1104.
- Ohta S, Aoki T, Hirata T, Suga T, (1984). The structures of four diarylheptanoid glycosides from the female flowers of Alnus serrulatoides. J Chem Soc Perkin Trans I, 1635–1642.
- Park D, Kim HJ, Jung SY, Yook CS, Jin C, Lee YS. (2010). A new diarylheptanoid glycoside from the stem bark of Alnus hirsuta and protective effects of diarylheptanoid derivatives in human HepG2 cells. Chem Pharm Bull, 58, 238–241.
- Pohjala L, Alakurtti S, Ahola T, Yli-Kauhaluoma J, Tammela P. (2009). Isolation of cytotoxic metabolites from targeted Peruvian Amazonian medicinal plants. J Nat Prod, 72, 1917–1926.
- Ragasa CY, De Luna RD, Hofilena JG. (2005). Antimicrobial terpenoids from Pterocarpus indicus. Nat Prod Res, 19, 305–309.
- Sakurai N, Yaguchi Y, Inoue T. (1987). Triterpenoids from Myrica rubra. Phytochemistry, 26, 217–219.
- Salama AM, Avendaño YI. (2005). Actividad antiinflamatoria de δ-amirona y 4′,7-dimetoxiapigenina aislados de Alnus acuminata. Rev Colomb Cienc Quím Farm, 34, 117–121.
- Salama AM, Rincón J, Torres M A, Iregui C. (1996). Efecto abortivo, aislamiento e identificación de principios activos de Alnus acuminata. Rev Colomb Cienc Quím Farm, 25, 36–40.
- Tung NH, Kwon HJ, Kim JH, Ra JC, Ding Y, Kim JA, Kim YH. (2010). Anti-influenza diarylheptanoids from the bark of Alnus japonica. Bioorg Med Chem Lett, 20, 1000–1003.
- Uddin AV, Ur-Rahman A. (1994). Handbook of Natural Products. Amsterdam: Elsevier, Vol. 2, pp. 533–534.
- Valente C, Pedro M, Duarte A, Nascimento MS, Abreu PM, Ferreira MJ. (2004). Bioactive diterpenoids, a new jatrophane and two ent-abietanes, and other constituents from Euphorbia pubescens. J Nat Prod, 67, 902–904.
- Winter CA, Risley EA, Nuss GW. (1962). Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med, 111, 544–547.
- Yu YB, Miyashiro H, Nakamura N, Hattori M, Park JC. (2007). Effects of triterpenoids and flavonoids isolated from Alnus firma on HIV-1 viral enzymes. Arch Pharm Res, 30, 820–826.
- Zou JH, Dai J, Chen X, Yuan JQ. (2006). Pentacyclic triterpenoids from leaves of Excoecaria agallocha. Chem Pharm Bull, 54, 920–921.