Abstract
Context: Verbascum phlomoides L. (Scrophulariaceae) (mullein) used in the European folk medicine due to its anti-inflammatory and soothing action on the respiratory tract is thoroughly documented in handbooks and scientific literature. Nevertheless, information regarding the influence of the polyphenol content on pharmacological activity is scarce.
Objective: This study explored the antioxidant and anti-inflammatory potential of V. phlomoides polyphenol-rich extract.
Materials and methods: Dried mullein flowers (200 g) were subjected to water extraction (60 °C, 2 h, herb/solvent ratio = 1/10 m/v) and further to n-butanol partition. Total phenolics were spectrophotometrically determined and specific compounds were evaluated by HPLC. The antioxidant activity was assessed by the 2,2-di(4-tert-octylphenyl)-1-picrylhydrazyl (DPPH) assay. The anti-inflammatory potential of the extract (50–200 μg/mL) was evaluated in vitro by ELISA measurement of ICAM-1 expression in TNF-α-stimulated endothelial cells and in vivo by the rat paw edema assay.
Results: The mullein extract contained 4.18% total polyphenols expressed as gallic acid. The main components identified by HPLC were: rosmarinic acid (14.93 mg/g), caffeic acid (39.96 mg/g), ferulic acid (29.61 mg/g) and quercetin (17.29 mg/g). Acteoside was not detected; aucubin was detected in traces (0.028 mg/g). Depending on concentration, the extract exerted scavenging activity on DPPH radical (EC50 7.09 mg/mL), significantly inhibited TNF-α-induced ICAM-1 expression by 55–58.8% on human umbilical vein endothelial cells at 100 and 200 μg/mL, but failed to reduce egg-white-induced rat paw edema.
Discussion and conclusion: Mullein polyphenols play an important role in exerting the antioxidant effect but have a weak influence on anti-inflammatory activity that is correlated, probably, to a higher content of iridoids and phenylethanoids.
Introduction
The traditional use of Verbascum phlomoides L. (Scrophulariaceae) (mullein) goes back to ancient times. Mullein flowers are highly valued herbal drugs used in the treatment of inflammation, asthma, spasmodic coughs and other respiratory tract diseases (Klimek et al., Citation2010). For some Verbascum species, the anti-inflammatory effect has been confirmed: V. pycnostachyium Boiss. & Heldr. (Tatli et al., Citation2007), V. salviifolium Boiss. (Tatli et al., Citation2008), V. latisepalum Hub.-Mor., V. mucronatum Lam., V. pterocalycinum var. mutense Hub.-Mor. (Akdemir et al., Citation2011; Suntar et al., Citation2010), V. lasianthum Boiss. ex Benth. (Kupeli et al., Citation2007). As regards the pharmacological activity of V. phlomoides, only a few studies were carried out.
According to European Medicines Agency’s (EMEA, 2009) monograph and other scientific papers (Gvazava & Kikoladze, Citation2012; Tatli & Akdemir, Citation2004), Verbascum flowers contain iridoid glycosides [0.56% including aucubin, catalpol, 6-xylosylaucubin and 6-xylosylcatalpol, 6-(4′-p-coumaroyl)-xylosylaucubin-phlomoide and another iridoid ester glycoside, specioside], flavonoids (0.57% including tamarixetin 7-rutinoside, tamarixetin 7-glucoside, apigenin and luteolin and their 7-glucosides, diosmin, chrysoeriol, eriodictyol, kaempferol, quercetin and rutin), phenylethanoid glycosides [traces of verbascoside (acteoside) and forsythoside B, triterpene saponins (verbascosaponin), polysaccharides (2–3% water-soluble acidic polysaccharides, principally a highly branched arabinogalactan with a β-1,6-linked galactan backbone and neutral polysaccharides – an arabinogalactan and a xyloglucan], phenolic acids (vanillic, p-hydroxybenzoic, p-coumaric, ferulic, protocatechuic and p-hydroxycinnamic acids), phytosterol glycosides and digiprolactone (a bicyclic monoterpene). Knowledge about the contents of phenolics in flowers is limited. Previous studies have reported that aucubin (iridoid) and verbascoside (acteoside) (phenylethanoid) are the major compounds responsible for anti-inflammatory activity (Recio et al., Citation1994; Schapoval et al., Citation1998). However, there are some assumptions that claim the anti-inflammatory and antimicrobial activities of the herb are directly correlated to the polyphenol content (Klimek et al., Citation2010).
Inflammation seems to be the root of many chronic diseases and the need to develop effective drugs has become imperative. With the current understanding of the complexity of the inflammatory process, there are hundreds of potential therapeutic targets. Generation of reactive oxygen species (ROS) is one of the most important aspects of the inflammatory process, ROS being able to override pro-inflammatory gene expression by activating transcription factors like NF-κB. The present study explored the antioxidant and anti-inflammatory effects of a selective extract of mullein, establishing a possible correlation with the polyphenols content.
Materials and methods
Cell line, chemicals and biochemicals
Folin-Ciocalteu reagent, 2,2-di(4-tert-octylphenyl)-1-picrylhydrazyl (DPPH), rosmarinic acid, caffeic acid, ferulic acid, gallic acid, quercetin and apigenin were purchased from Sigma Aldrich-Fluka (St. Louis, MO). Aucubin and acteoside were purchased from PhytoPlan (Baden-Württemberg, Germany) and acetylsalicylic acid (aspirin) from Zentiva (Bucharest, Romania). Human umbilical vein endothelial cells (HUVECs) cell line was purchased from ATCC. ELISA kit for ICAM-1 quantification was purchased from Invitrogen (Carlsbad, CA) and TNF-α from Alexis (Geneva, Switzerland). The assay was performed according to manufacturer’s instructions. All other reagents used in cell culture assay were from Sigma (St. Louis, MO).
Plant material
Verbascum phlomoides flowers were cultivated in standard conditions by a commercial grower (Hofigal Import-Export SA, Bucharest, Romania) in 2011, dried and ground as a fine powder. A voucher specimen was deposited at the manufacturer (184/2011). The taxonomic identity of the plant was confirmed by the Institute’s biologists (A. Grigore and G. Neagu).
Extraction and isolation
The method consisted of extraction of the active substances from 200 g of Verbasci flores with distilled water (vegetal material/solvent ratio = 1/10 m/v) at 60 °C for 2 h with continuous stirring, followed by filtration of the extract and centrifugation. The clear water extract was further concentrated under reduced pressure to a minimal volume of 200 mL (vegetal material/aqueous extract ratio = 1/1, m/v) and extracted with n-butanol until complete discoloration of the organic extract. All n-butanol extracts were evaporated to dryness at 60 °C and reduced pressure (72–74 mm Hg) and kept in a cool, dry place for further use (7.13 g).
Determination of total phenolics
Total phenolics content was determined according to the Folin–Ciocalteu method, using gallic acid as a standard (Javanmardi et al., Citation2003). The aliquots (500 μL) of each extract were added to the test tubes containing 2.5 mL of Folin–Ciocalteu reagent and 2 mL of 7.5% sodium carbonate. After 30 min incubation, the absorbance was measured at 740 nm with a Helios Gamma spectrophotometer. Total phenolic content was expressed as gallic acid equivalents by reference to the gallic acid standard calibration curve in milligrams per gram sample.
HPLC analysis
Two methods for quantification of polyphenols and iridoids were developed. A modular Shimadzu system (Kyoto, Japan) consisting of an LC-20AD pump, a CTO-20A column oven, a DAD-MS detector with SPD-M20A diode array and MS Shimadzu, a DGU-20As degasser and an LCMS solution software was used. Phytochemicals were separated on a Kromasil C18 analytical column (Sigma, St. Louis, MO) for polyphenols (4.6 mm × 100 mm) and a C8 column for iridoids (2.1 mm × 100 mm).
Separation of polyphenols was performed using a mobile phase consisting of an A solution (water acidified with 1% formic acid) and a B solution (methanol acidified with 1% formic acid) at an initial flow rate of 0.1 mL/min; with an injection of 15 µL. A gradient flow of mobile phase and also a gradient of mobile phase composition were applied.
Separation of aucubin was performed using a mobile phase gradient of ammonia formiate (10 mM) and acetonitrile at a flow rate of 0.1 mL/min for 70 min. All mobile phases were filtered through a 0.20 µm membrane (CHROMAFIL® O-20/25, Duren, Germany) and deaerated in an ultrasonic bath, before use.
Free radical scavenging assay
The extracts were diluted in a methanol solution at the concentrations of 5 and 1 mg/mL. Aliquots (50 μL) of the extract were mixed with 2950 μL of the methanol DPPH solution (0.025 g/L). The reduction of DPPH free radical was measured by reading the absorbance at 517 nm and related to the absorbance of the control without the herbal drug. The inhibition ratio (%) was calculated from the following equation: % inhibition = [(absorbance of control – absorbance of sample)/absorbance of control] × 100%. The concentration which decreased initial concentration of DPPH by 50% was defined as the EC50. Quercetin (0.2 mg/mL) was used as positive control.
ICAM-1 assay
HUVECs were cultivated on 96-well plates (Corning, NY) in Dulbecco’s Modified Eagle’s Medium low glucose containing 10% fetal bovine serum and 1% penicillin–streptomycin–neomycin solution. The cultures were maintained at 37 °C and 5% CO2. After reaching a subconfluent stage, the cells were treated with various concentrations of the extract (50–200 μg/mL dissolved in culture medium without serum) and 10 mM acetylsalicylic acid (Zentiva, Romania) which were maintained in culture for 2 h. After this, extracts were discarded and one experimental group received 50 ng/mL TNF-α dissolved in medium with 10% serum and another group received only medium with 10% serum. Cells cultivated in the culture medium untreated with extract but stimulated with TNF-α and cells untreated and unstimulated were used as controls. After 20 h incubation, supernatants were taken for measuring adhesion molecule expression by an ELISA kit. All samples were tested in triplicate.
Rat paw edema
Fresh egg-white-induced rat hind paw edema assay was carried out as a model of acute inflammation (Vogel, Citation2008). Wistar rats (150–200 g) of either sex were purchased from “Cantacuzino” Institute’s animal house from Bucharest, Romania. The animals were kept under controlled condition (temperature: 22 ± 2 °C, humidity: 50–60%, 12 h light–dark cycle) and had free access to food and water. The animals were acclimatized for seven days to the laboratory conditions before doing experiments and were deprived of food 12 h before doing tests. The experiment was carried out in accordance with the guidelines for the care of laboratory animals and ethical guidelines, and was approved by the ethics committee for research on laboratory-animal use of the National Institute for Chemical-Pharmaceutical Research and Development where studies were carried out. The number of animals was the minimum necessary to show consistent results.
The extracts (100, 250 and 500 mg/mL/100 g body weight) were administered by gastric gavage 1 h before inducing inflammation. The controls were given the same volume of saline as in test group. The reference group was treated with 100 mg/kg of acetylsalicylic acid (Zentiva, Romania).
Inflammation of the hind paw was induced by injection of 0.1 mL of fresh egg white into the subplantar surface of the right hind paw of the rats. The thickness (mm) of the paw was measured by a plethysmometer (Ugo-Basile, Italy) immediately before the administration of the phlogistic agent (“zero” time) and 30, 60, 120 and 180 min thereafter. The anti-inflammatory activity was calculated as percentage of edema inhibition according to the following equation:
where a is the mean paw volume of the treated group (mL); b is the mean paw volume of the untreated group (mL); x is the mean initial paw volume of the treated group (mL); y is the mean initial paw volume of the untreated group (mL).
The results were processed by CUB soft for data acquisition and storing and expressed as mean ± standard error for each measurement time. Data were analyzed using one-way analysis of variance test followed by Student’s t-test. p Values <0.05 were considered as statistically significant.
Results
Determination of total phenolics and HPLC analysis
Verbascum extract contains 4.18% total polyphenols expressed as gallic acid, as determined by the Folin-Ciocalteu method. It was also shown by HPLC that polyphenolcarboxylic acids such as ferulic, caffeic and rosmarinic are found in significant amounts. Acteoside was not detected and aucubin was present in traces (). Acteoside seems to be a minor constituent of V. phlomoides flower, as it has been already shown in other previous studies (Klimek, Citation1996).
Table 1. Major compounds of Verbascum sample quantified by HPLC.
DPPH inhibition
The DPPH antioxidant assay is based on the ability of DPPH, a stable free radical, to discolor in the presence of antioxidants. The DPPH radical contains an odd electron that is responsible for the absorbance at 540 nm and also for the visible deep purple color. When DPPH accepts an electron donated by an antioxidant compound, the DPPH is discolored, which can be quantitatively measured from the changes in absorbance.
The polyphenol content seems to be correlated with scavenging activity. DPPH inhibition was dose-dependent and the EC50 value was established at 7.09 mg/mL. Scavenger potential varied from 6.65% ± 0.09 at 0.1 mg/mL to 80.9% ± 0.5 at 10 mg/mL. The reference substance, quercetin, exhibited 86% ± 0.08 scavenging activity at 0.2 mg/mL.
ICAM-1 assay
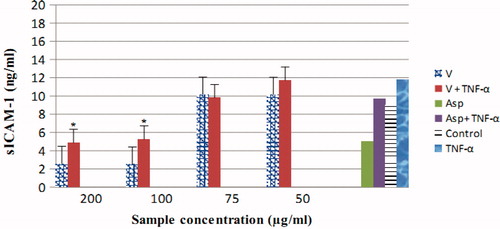
Verbascum extract is capable of inhibiting TNF-α-induced ICAM-1 expression. HUVECs were preincubated for 2 h with either control medium (Control) or medium containing Verbascum extract of various concentrations. Following the 2 h preincubation, TNF-α (50 ng/mL) was added to the cultures where indicated. After 20 h incubation, all reagents were removed and ICAM-1 expression of the cells was determined by ELISA. The results showed an obvious dose–effect correlation. The effect of extract on ICAM-1 production was comparable to reference substance, only at maximum concentrations tested (100–200 μg/mL, p < 0.05) ().
Anti-inflammatory assay
We examined the anti-inflammatory effect of Verbascum selective extract (in doses of 100, 250 and 500 mg/100 g body weight) using the fresh egg-white-induced edema model. Paw volumes of all groups are presented in . Maximal edema inhibition was observed for the highest concentration tested (500 mg/100 g body weight) at 30 min after the edema induction, but below the anti-inflammatory index of 20% (). The inhibitory effect exerted by the reference substance was observed during the entire experiment.
Table 2. Paw volumes variation in rat paw edema assay.
Table 3. Edema inhibition percentage relative to control.
Discussion and conclusion
Mullein is a well-known species which exhibits various pharmacological effects. In this study, we showed the antioxidant and anti-inflammatory effects of a selective extract enriched in polyphenols. Previously, it was shown using different methods that the compounds of Verbascum sp., such as forsythoside B, verbascoside and chlorogenic acid, exhibit antioxidant capacity. It was also stated that a high number of –OH groups would possess elevated antioxidant value (Georgiev et al., Citation2011). The extract studied in this paper contained important amounts of hydroxylated compounds (caffeic, ferulic, rosmarinic acids, quercetin, apigenin but no verbascoside) which are responsible for the dose-dependent antioxidant effect (80.9% maximum inhibition of DPPH radical at 10 mg/mL). On the other hand, the inhibition of adhesion molecules in TNF-α stimulated HUVECs was achieved only at high doses, and moreover the extract showed no anti-inflammatory effect on rat paw edema assay.
The method carried out in this study, egg-white-induced inflammation, is similar to that produced by carrageenan (Akah et al., Citation1993) and the mechanism involved in this assay implies the release of several mediators such as histamine, serotonin and bradykinin in the initial phase (0–1 h), and an increase in the production of prostaglandins through the activation of cyclooxygenase-2 and release of nitric oxide in the later phase (1–6 h) (Crunkhorn & Meacock, Citation1971). Our results supported the previous findings that verbascoside reduces the production of superoxide radicals (Speranza et al., Citation2010), exerts exquisite corticosteroid-like inhibition of pro-inflammatory chemokines (Georgiev et al., Citation2012) and is a specific inhibitor of paw edema (Akdemir et al., Citation2011) which explain its beneficial effect in the treatment of inflammatory diseases. The absence of this compound in the mullein extract is a possible hypothesis for the failure in exhibiting a certain anti-inflammatory effect.
As a conclusion, V. phlomoides is used for anti-inflammatory effect, but we could not find this activity in our study. It seems that mullein polyphenols play an important role in exerting the antioxidant effect but have a weak influence on anti-inflammatory activity that is correlated, probably, to a higher content of iridoids and phenylethanoids. Still, further investigation is required to confirm which constituent of the extract exerts this activity (because previous data showed the relationship between polyphenols and anti-inflammatory effect) and to explore its exact mechanism underlying the anti-inflammatory effect by studies related to the influence on lipid-derived eicosanoids, enzyme expression (COX-2, lipoxygenase) and cytokines.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Akah P, Okogun J, Ekpendu T. (1993). Anti-oedema analgesic actions of Diodia scandems extract in rats and in mice. Psychother Res 7:319–27
- Akdemir Z, Zahraman C, Tatli I, et al. (2011). Bioassay-guided isolation of anti-inflammatory, antinociceptive and wound healer glycosides from the flowers of Verbascum mucronatum Lam. J Ethnopharmacol 136:436–43
- Crunkhorn P, Meacock S. (1971). Mediators of the inflammation induced in the rat paw by carrageenin. Br J Pharmacol 42:392–402
- European Medicines Agency (EMEA) (2009). Assessment report on Verbascum thapsus L, V. densiflorum Bertol, V. phlomoides L. flos with traditional use, EMEA [Online]. Available from: http://www.ema.europa.eu/ema/index.jspcurl=pages/medicines/herbal/medicines/herbal_med_000002.jsp&mid=WC0b01ac058001fa1d [last accessed 11 Jun 2012]
- Georgiev M, Alipieva K, Orhan I, et al. (2011). Antioxidant and cholinesterases inhibitory activities of Verbascum xanthophoeniceum Griseb. and its phenylethanoid glycosides. Food Chem 128:100–5
- Georgiev M, Lulli D, Alipieva K, et al. (2012). Verbascum xanthophoeniceum-derived phenylethanoid glycosides are potent inhibitors of inflammatory chemokines in dormant and interferon-gamma-stimulated human keratinocytes. J Ethnopharmacol 14:754–60
- Gvazava N, Kikoladze V. (2012). Orobanchoside and flavonoids from Verbascum phlomoides and V. densiflorum. Chem Nat Comp 47:991–2
- Javanmardi J, Stushnoff C, Locke E, Vivanco JM. (2003). Antioxidant activity and total phenolic content of Iranian Ocimum accessions. Food Chem 83:547–50
- Klimek B. (1996). Hydroxycinnamoyl ester glycosides and saponins from flowers of Verbascum phlomoides. Phytochemistry 43:1281–4
- Klimek B, Olszewska MA, Tokar M. (2010). Simultaneous determination of flavonoids and phenylethanoids in the flowers of Verbascum densiflorum and V. phlomoides by high-performance liquid chromatography. Phytochem Anal 21:150–6
- Kupeli E, Tatli I, Akdemir Z, Yesilada E. (2007). Bioassay-guided isolation of anti-inflammatory and antinociceptive glycoterpenoids from the flowers of Verbascum lasianthum Boiss. ex Bentham. J Ethnopharmacol 110:444–50
- Recio M, Giner R, Máñez S, Ríos J. (1994). Structural considerations on the iridoids as anti-inflammatory agents. Planta Med 60:232–4
- Schapoval E, Winter de Vargas M, Chaves C, et al. (1998). Antiinflammatory and antinociceptive activities of extracts and isolated compounds from Stachytarpheta cayennensis. J Ethnopharmacol 60:53–9
- Speranza L, Franceschelli S, Pesce M, et al. (2010). Antiinflammatory effects in THP-1 cells treated with verbascoside. Phytother Res 24:1398–404
- Suntar I, Tatli I, Kupeli E, et al. (2010). An ethnopharmacological study on Verbascum species: From conventional wound healing use to scientific verification. J Ethnopharmacol 132:408–13
- Tatli I, Akdemir Z. (2004). Chemical constituents of Verbascum L. species. J Pharm Sci 29:93–107
- Tatli I, Akdemir Z, Yesilada E, Kupeli E. (2008). Anti-inflammatory and antinociceptive potential of major phenolics from Verbascum salviifolium Boiss. Z Naturforsch 63:196–202
- Tatli I, Schuly W, Akdemir Z. (2007). Secondary metabolites from bioactive methanolic extract of Verbascum pycnostachyum Boiss. & Helder flowers. Hacettepe Univ J Fac Pharm 27:23–32
- Vogel G. (2008). Paw edema. In: Vogel G, ed. Drug Discovery and Evaluation: Pharmacological Assays. Berlin, New York: Springer, 1103–6