Abstract
Context: Anticancer treatments such as anthracyclines are effective; however, they induce cardiotoxicity by releasing radical oxygen species (ROS). Saffron (Crocus sativus; Iridaceae) is a widely used spice with antioxidant properties and numerous health benefits that may provide cardioprotection.
Objective: To assess the effect of saffron against acute myocardium damage by anthracyclines compared with electrolysis as a free radical generating system.
Materials and methods: According to the Langendorff method, we used the model of an isolated rabbit heart perfused in retrograde. In one set of experiments, ROS was generated by electrolysis of the perfused heart solution (3 mA for 30 min) in the presence and absence of saffron extracts at the optimal dose (10 μg/ml). In another set, we perfused the heart with anthracycline, i.e. 30 μM doxorubicin (Doxo) in the presence and absence of 10 μg/ml saffron extracts. We evaluated cardiodynamics, as well as biochemical and pathological parameters, to emphasize the effectiveness of the treatment with saffron extract using the optimal dose of catalase (150 IU) as a positive control.
Results: ROS generated, respectively, by electrolysis and by Doxo significantly (p < 0.05) affects cardiovascular function; it decreased ventricular pressure (45.02 and 40.41%), heart rate (36.31 and 22.39%) and coronary flow (50.98 and 36.67%). Increased lipid peroxidation of the myocardium was also observed (118.22 and 56.58%), while superoxide dismutase activity decreased (48.33 and 38.70%). The myocardial architecture was altered and the intercellular spaces increased.
Conclusion: Saffron perfused during electrolysis helps trap ROS and significantly improves myocardial function; however, saffron was less effective against Doxo, thus suggesting that mechanisms other than oxidative stress underlie Doxo cardiotoxicity.
Introduction
Many drugs used in chemotherapy have serious side effects on the heart. For example, anthracyclines, a class of anticancer drugs that is widely used in the treatment of adults and children with certain leukemias and in the treatment of patients with breast cancer, unfortunately induce short- and long-term heart failure and arrhythmias (Jensen et al., Citation2002; Volkova & Russell, Citation2011). This side effect can result in death for some patients in remission of cancer (Swain et al., Citation2003). It is known that the cardiotoxicity of these drugs is related to the fact that they induce the formation of toxic compounds, which are derived from oxygen, known as free radicals, which in turn induce the inhibition of the mitochondrial respiratory chain, dissipation of membrane potential and mitochondrial calcium overload, thus resulting in dysfunction of heart cells (Sarvazyan, Citation1996; Wang et al., Citation2004; Zhou et al., Citation2001).
Polyphenols are metabolites that widely occur in plant food and possess antioxidant and free radical scavenging properties (Visioli et al., Citation2011). While the cytoprotective effect of the commonly used antioxidant vitamins (ascorbic acid and vitamin E) remains controversial (Quiles et al., Citation2002; Shimpo et al., Citation1991), several naturally occurring antioxidants (propolis, butterfield, spinach, arabic gum and proanthocyanidin) have demonstrated protective roles against doxorubicin (Doxo) cardiotoxicity (Abd-Allah et al., Citation2002; Bagchi et al., Citation2003; Breitbart et al., Citation2001; Chopra et al., Citation1995; DeAtley et al., Citation1999). All these antioxidants, although efficient in cellular or acute animal experiments, have failed to alleviate anthracycline cardiotoxicity in clinically relevant chronic animal models or in clinical trials (Simůnek et al., Citation2009).
Currently, there is no compound that can efficiently protect the heart against the toxic effects of anthracyclines (Hu, Citation2011). Therefore, we elected to study, in animals, the possible protective effect of saffron, a natural plant known for its antioxidant effect (Fatehi et al., Citation2003; Wattanapitayakul et al., Citation2005). In this context, research was undertaken in our laboratory on the antioxidant properties of Lebanese saffron; our results showed that the extracts of this plant have a powerful scavenger potential against free radicals generated in vitro by electrolysis (Makhlouf et al., Citation2011).
Based on these results, we first aimed to compare the deleterious effects caused by electrolysis as an ROS-generating system with those produced after Doxo perfusion. On the other hand, we tested whether saffron extract could potentially have a protective effect against Doxo acute cardiotoxicity. Isolated perfused rabbit heart was taken as an experimental model subjected either to electrolysis or to Doxo injection in the presence or absence of saffron extract. Cardiodynamic measures, biochemical assays and anatomopathological tests were used to assess the degree of cardiac damage and possibly the beneficial effect of antioxidant treatment, which modulate the level of oxidative stress. This work may eventually lead to clinical studies that will better prevent anthracycline cardiotoxicity in chemotherapy-treated patients.
Materials and methods
Saffron extraction
Lebanese saffron (Crocus sativus), cultivated in the Bekaa valley, was identified by Dr. Makhlouf, and voucher specimens were deposited at the Ministry of Agriculture. Dried stigmas from the plant were suspended in a mixture of methanol and water (50:50, v/v) and magnetically stirred for 24 h at 4 °C in a dark room. Thereafter, the solution was filtered before evaporating the methanol – via a rotavapor at low temperature (40 °C). The obtained solution was subjected to refrigeration for 3 d before lyophilization. The dried extract achieved a 40.6% yield. This dried extract was reconstituted to prepare a solution of 1 mg/mL in distilled water just before the start of the experiments. The extracts were quantified for crocin, picrocrocin and safranal using a high-performance liquid chromatography method because the pharmacological effect of saffron is dependent on the quantity of its main components. This shows that Lebanese saffron contains these ingredients at standard levels; the carotenoid compound crocin being the highest one (data not shown). In addition, we calculated total polyphenols using the Folin–Ciocalteu method and obtained 16 mg gallic acid equivalent/g dry weight.
Isolated heart preparation
We used common rabbits (Oryctolagus cuniculus) (1.5–2.5 kg) obtained from a local rabbit breeder. The animals were maintained at ambient temperature (22 ± 1 °C) with 12:12 h light–dark cycles and free access to water and food. All procedures were approved by the ethical committee of our university, and the experiments were performed according to international accepted guidelines for the use of animals. The rabbits were injected intravenously with heparin and anesthetized with ketamine and xylazine. After the heart was quickly removed, the ascending aorta was immediately cannulated and perfused with a tyrode solution at a constant pressure (60 cm H2O) at 37 °C and continuously bubbled with a mixture of 95% O2/5% CO2 according to the Langendorff technique. The Tyrode solution consists of 137 mM NaCl, 2.7 mM KCl, 1 mM MgCl26H2O, 1.5 mM CaCl22H2O, 0.4 mM NaH2PO42H2O, 12 mM NaHCO3 and 5 mM glucose. Left ventricular pressure (LVP), left ventricular end diastolic pressure (LVEDP), coronary flow (CF), heart rate (HR) and the incidence of arrhythmias were monitored with a 4-channel physiograph (Harvard Apparatus, Holliston, MA) using the appropriate devices. A catheter with a latex balloon was introduced in the left ventricle via the atrium and served to measure LVP and LVEDP. CF was measured by collecting exuded heart perfusate in a 25-ml graduated cylinder. Epicardial electrocardiogram and HR were recorded via two electrodes; one electrode was positioned on the aorta and the other on the apex of the heart (Nemr et al., Citation2003).
ROS generation by electrolysis
The electrolysis method to generate ROS in the rabbit’s heart was used and was modified from the method for the rat’s heart (Lecour et al., Citation1998). Electrolysis was performed using two platinum wire electrodes placed above the heart into the inflow cannula. The anode was placed 12 cm from the left atrium and the cathode 15 cm away. A constant 3 mA direct current generated by a stimulator was applied for 30 min. A glass bubble trap, placed above the aorta, prevented the formation of gas bubbles. We used a sensitive galvanometer to ensure that no electrical current was reaching the heart during electrolysis.
Experimental protocol
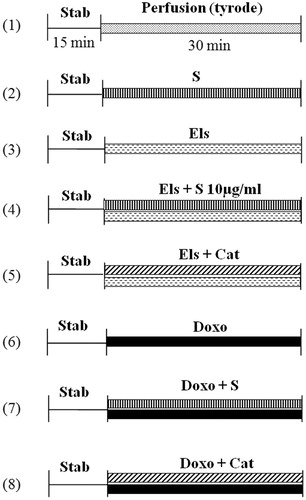
Langendorff hearts were allowed to equilibrate for 15 min; hearts presenting any irregularities in function were discarded. The volume of the balloon inserted into the left ventricle was adjusted to maintain a LVEDP of 10 mm Hg. All the heart performance parameters were monitored throughout the experiment. The rabbits were randomly assigned to one of the following eight groups (n = 5–10 for each group, ):
– (C): Control hearts perfused only with standard tyrode solution
– (S): Saffron administrated in the tyrode solution during 30 min at different concentrations
– (Els): Electrolysis of the standard tyrode solution perfusing the heart during 30 min
– (Els + S): Electrolysis of tyrode solution in the presence of saffron (10 µg/ml) during 30 min
– (Els + Cat): Electrolysis of tyrode solution in the presence of catalase (150 IU) during 30 min
– (Doxo): Doxo (30 μM) was added to the tyrode solution during 30 min
– (Doxo + S): Doxo (30 μM) and saffron (10 μg/ml) were added to the tyrode solution during 30 min
– (Doxo + Cat): Doxo (30 μM) and catalase (150 IU) were added to the tyrode solution during 30 min
Figure 1. Experimental protocol. Control hearts perfused with standard Tyrode solution (1); hearts perfused with Tyrode plus saffron extracts at different concentrations (2); hearts subjected to electrolysis; (3) hearts subjected to electrolysis in the presence of saffron (4); or catalase (5); hearts perfused with doxorubicin added to the tyrode solution (6); hearts perfused with doxorubicin added to the tyrode solution in the presence of saffron (7); or catalase (8). C, control; Cat, catalase; Doxo, doxorubicin; Els, electrolysis; Stab, period of stabilization; and S, saffron.
Biochemical study
At the end of each protocol, hearts used for mechanical studies were immersed in liquid nitrogen and conserved at −80 °C. Lipid peroxidation and superoxide dismutase activities (SOD) were determined by spectrophotometry.
The malondialdehyde (MDA) levels, used as a marker of lipid peroxidation, were measured in heart tissues. MDA reacts with thiobarbituric acid (TBA) as a TBA reactive substance to produce a red-colored complex with a peak absorbance at 532 nm. A total of 3 ml phosphoric acid (1%) and 1 ml TBA (0.6%) were added to 0.5 ml of 10% heart homogenate in a centrifuge tube, and the mixture was heated for 45 min in a boiling water bath. After cooling, 4 ml of n-butanol was added to the mixture and vortex-mixed for 1 min followed by centrifugation at 70 000 rpm for 20 min. The organic layer was transferred to a fresh tube and its absorbance was measured at 532 nm, and the MDA levels were expressed as nmol/g tissue (Draper & Hadley, Citation1990).
SOD activity was assessed by measuring the dismutation of superoxide radicals generated by xanthine oxidase and hypoxanthine. Using this enzyme, the standard curve generated serves to accurately quantify the activity of all three types of SOD (Cu/Zn-, Mn- and Fe-SOD). We used the SOD assay kit (catalog No. 706002) from Cayman Chemical Company, Ann Arbor, MI.
Pathological study
Hearts intended for anatomopathology were introduced in 10% formalin at the end of the experiment. These hearts were fixed with paraffin, stained with hematoxylin–eosin and thinly sliced with a microtome for visualization by light microscopy.
Chemicals
All chemicals in this study were of analytical grade and were purchased from Sigma (Gillingham, UK), with the exception of the SOD kit. All solutions were prepared in deionized water.
Statistics
The results are expressed as mean ± standard deviation. Statistical studies were performed by variance analysis followed by Duncan’s test. These results are considered statistically significant if the p value is less than 0.05.
Results
Determination of the non-toxic dose of saffron
Three solutions of different concentrations of saffron (0.01, 0.1 and 1 mg/ml) were tested for their direct influence on the activity of the isolated heart. Saffron itself is toxic to the heart at concentrations greater than 1 mg/ml. It depresses the LVP, HR and CF as it slightly increases LVEDP. At a concentration of 0.1 mg/ml, saffron has no significant effects on these parameters up to 10 min of perfusion. It can also be infused at a concentration of 10 μg/ml for 60 min without any significant effect on these parameters (data not shown). Therefore, 10 μg/ml was chosen as the maximal non-toxic dose of saffron.
Cardiodynamic parameters
Regarding the control hearts, LVP was stable at around 120 mm Hg, LVEDP was stable near 10 mmHg, HR also remains stable at around 160 beats/min and CF was stable around 24 ml/min.
Electrolysis of the tyrode solution, perfusing the isolated heart for 30 min, generates free radicals in large quantities and produces alterations in heart manifested as a fall in LVP, HR and CF with an increase in LVEDP. Saffron (10 μg/ml) infused 1 min before and during electrolysis protects the myocardium against the deleterious effects of free radicals. The hemodynamic parameters were significantly improved by saffron and catalase; however, a total recovery of the heart was not observed (; ).
Figure 2. Percent changes of the cardiodynamic parameters in response to different treatments. Data represent the percentages of the baseline values. LVP, left ventricular pressure; LVEDP, end diastolic pressure; CF, coronary flow; HR, heart rate; C, control; Cat, catalase; Doxo, doxorubicin; Els, electrolysis; Stab, period of stabilization; S, saffron.
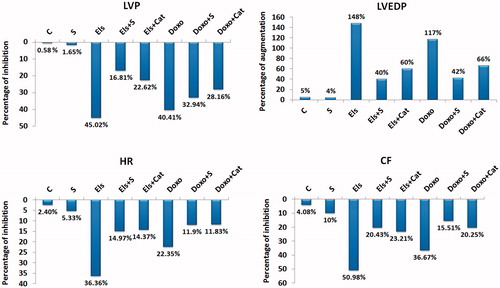
Table 1. The effect of different treatments on the cardiodynamics of the isolated rabbit heart.
Table 2. Arrhythmias quantification.
Doxo perfused at 30 μM for 30 min induces, as electrolysis does, a significant decrease in LVP, HR and CF with an increase in LVEDP. Saffron administered 1 min before and during Doxo perfusion provides partial protection on hemodynamic parameters. In addition, catalase, a known free radical scavenger, provides approximately 50% protection at the optimal dose of 150 IU (; ).
Arrhythmias quantification
In the control heart, none or very few rare premature ventricular contractions (PVCs) were observed; in hearts perfused with saffron (10 μg/ml), few PVCs were seen, with no significant difference versus the control. Electrolyzed heart manifested mostly ventricular fibrillation (VF) and/or tachycardia (VT), whereas hearts perfused with Doxo exhibited mostly PVCs and rarely VT/VF. Saffron, as catalase, was more effective on electrolysis than against Doxo in reducing the incidence of arrhythmias (Table 2).
Oxidative enzymes markers
In isolated rabbit heart, electrolysis (3 mV for 30 min) significantly increased lipid peroxidation of the myocardium to 70.58 nmol/g of tissue in comparison to 32.58 nmol/g of tissue for the control group. Similarly, perfusion of Doxo at 30 μM for 30 min significantly increased lipid peroxidation of the myocardium to 50.16 nmol/g of tissue, which is less than that observed for electrolysis (). In addition, we observed a reduction in SOD activity to 7.3 U/mg of tissue for the electrolyzed group and to 7.66 U/mg of tissue for the Doxo-treated group in comparison to 12.44 U/mg of tissue for the control group ().
Figure 3. Changes in the levels of lipid peroxidation (LP) and the activity of superoxide dismutase (SOD) in the tissue of the control hearts and hearts subjected to electrolysis or to doxorubicin alone or in presence of saffron extracts or catalase. #p < 0.05 versus control and *p < 0.05 versus Els or Doxo, n = 5. C, control heart; S, saffron; Els, electrolysis; Doxo, doxorubicin; and Cat, catalase.
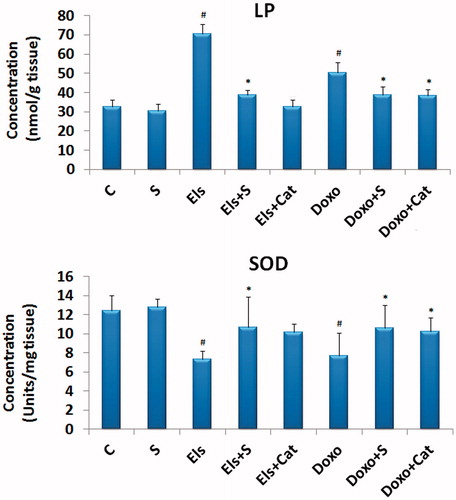
The administration of saffron along with electrolysis or Doxo treatments causes a significant decrease in lipid peroxidation, to respectively, 38.58 and 38.7 nmol/g of tissue, comparable to that of the control group and the Doxo + Cat-treated group. We also observed an increased level of antioxidant enzyme SOD to 10.58 and 10.64 U/mg of tissue, respectively, for the Doxo + S- and Els + S-treated groups, comparable to that of the control group ().
Anatomopathological studies
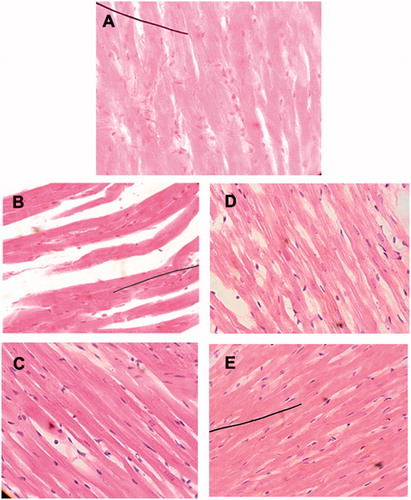
Histopathological sections showed, on the one hand, a deformed appearance of cardiomyocytes with wider intercellular spaces for the electrolyzed heart in comparison to the control group. On the other hand, myocardial architecture is strongly affected by increased intercellular spaces, less adhesion between cardiomyocytes and the formation of edema (indicated by arrows) for hearts perfused with Doxo (). The general appearance of cardiac tissue appeared closer to normal, and the spaces between cardiomyocytes seem significantly reduced in saffron-treated groups along with Doxo or electrolysis.
Figure 4. Histological examination via light microscopy (×400). (A) control heart; (B) electrolyzed heart (note the distorted appearance of the cardiomyocytes with wider intercellular spaces); (C) heart electrolyzed plus saffron (the cardiomyocytes have an appearance closer to normal and the spaces between the cells are significantly reduced); (D) heart perfused with doxorubicin (formation of edema and less adhesion between the cardiomyocytes); and (E) heart perfused with doxorubicin plus saffron (the general appearance of the cardiac tissue is similar to the control heart).
Discussion
The widely accepted hypothesis that anthracycline-induced cardiotoxicity is associated with the formation of free radicals has led to research on the use of antioxidants to limit the production of these radicals. Several compounds with antioxidant properties have proven to be cardioprotective in in vitro and in vivo trials (Mokni et al., Citation2012; Yalçin et al., Citation2010); however, clinical approaches have generated quite disappointing results.
The role of ROS in the cardiac toxicity of anthracyclines was confirmed by the use of transgenic mice overexpressing enzymes or proteins with antioxidant activity. It has been shown in animals overexpressing catalase (Kang et al., Citation1996) or manganese superoxide dismutase (Yen et al., Citation1996), that the cardiotoxic effects of Doxo were attenuated; catalase also limits the degree of cardiac damage after incubation of cells in the presence of Doxo (Lee et al., Citation1991). Furthermore, N-acetylcysteine, coenzyme Q, vitamin C (ascorbic acid) and vitamin E (α-tocopherol) have beneficial effects in a model of anthracycline-induced cardiotoxicity in animals. However, they do not prevent myocardial damage in humans (Dorr, Citation1991). Currently, the antioxidant, dexrazoxane, is the only drug approved by the US Food and Drug Administration for clinical use in conjunction with Doxo to alleviate cardiotoxicity. Despite the utility of dexrazoxane, its administration is often limited due to the controversial results associating its use with the risk of secondary malignancies (Tebbi et al., Citation2007).
Since this line of research is not fully understood, we therefore undertook our research on extracts from Lebanese saffron, which is a natural product that showed promising results from our laboratory (Makhlouf et al., Citation2011).
In a first step, we prepared extracts from saffron, which is newly cultivated in Lebanon, in such a way to ensure that our product contains the active metabolites in standard concentrations; we then perfused it in isolated rabbit heart to find the therapeutic nontoxic dose. It should be noted that saffron, at a dose greater than 100 μg/ml, is toxic to the heart. Saffron is therefore a biphasic, dose-dependent substance; at a certain dose, it has anticancer properties and at other doses, it acts as a scavenger against ROS, which makes it a very interesting substance to pursue as a therapeutic, anticancer agent (Abdullaev, Citation2002; Molnar et al., Citation2000).
In a second step, we used the electrolysis system for ROS generation in the isolated heart to ensure that saffron is able to trap radicals formed. In fact, electrolysis produces a cascade of radicals as confirmed by the electron paramagnetic resonance technique (Lecour et al., Citation1998).
In the final step, we perfused Doxo alone to measure the magnitude of cardiac damage it causes and the protection that saffron is able to provide. It should be noted that the dose of Doxo administered to the rabbit heart was chosen at a concentration that can reach the heart via the circulation following a dose used in chemotherapy.
It is obvious that the generated free radicals, via electrolysis or Doxo, reacts with lipids, proteins and other cellular constituents causing DNA breaks, lipid peroxidation and injury to mitochondria and cell membranes of the heart muscle cells (Dziegiel et al., Citation2002). Cardiac tissue is sensitive to oxidative damage because of its highly oxidative metabolism and lower antioxidant defenses in this organ compared with others. The high level of oxidative stress during chemotherapy may overcome the antioxidant defenses in cardiac tissue leading thereby to toxicity (Dalloz et al., Citation1999).
As we expected, saffron administered during electrolysis, where the generation of free radicals is abundant, protects the heart against the deleterious effects of these free radicals. This protection is highly significant as confirmed by cardiodynamic, biochemical, and pathological parameters. Saffron was administered in conjunction with Doxo to test its ROS scavenger effect. Under our experimental conditions, saffron offers significant protection against the cardiotoxicity of Doxo in reference to the cardiodynamic parameters and biochemical results. However, this protection was not as important as in electrolysis. It is noteworthy that catalase, a reference product used in the optimal dose, did not give full protection in our system, thus suggesting that another mechanism beside the generation of free radicals might be implicated in Doxo cardiotoxicity. Saffron, like catalase, is not able to fully oppose the toxicity of Doxo as used in this model. To place this into the proper perspective, and as a logical extension of this study, saffron will be tested in a chronic model of Doxo cardiotoxicity.
By way of clinical extrapolation, these results confirm the unexpected complexity of the subject and any “blind” supplements of antioxidants in the context of the chemotherapy must follow a strict test for biological markers of oxidative stress. Antioxidants are undoubtedly beneficial, but care should be exercised in their use based on the current state of knowledge about these substances. Such strategies can be employed in a medical environment, and directed seriously by the relevant biological test. Therefore, it would be ideal to supplement a treatment regimen with antioxidants for patients treated with radio and/or chemotherapy, based on a preliminary laboratory diagnosis of oxidative stress.
Conclusions
The Lebanese saffron is beneficial in improving Doxo-induced cardiac dysfunction and damage in the rabbit heart when used within the therapeutic dose range. The mechanism of this cardioprotective effect may involve prevention of lipid peroxidation and the preservation of antioxidant enzymes (SOD) as well as scavenging of free radicals.
Declaration of interest
The authors have no conflicts of interest. This work was supported by grants from the Lebanese University – Research Group 007.
References
- Abd-Allah A, Al-Majed A, Mostafa A, et al. (2002). Protective effect of Arabic gum against cardiotoxicity induced by doxorubicin in mice: A possible mechanism of protection. J Biochem Mol Toxicol 16:254–9
- Abdullaev FI. (2002). Cancer chemopreventive and tumoricidal properties of saffron (Crocus sativus). Exp Biol Med 227:20–5
- Bagchi D, Sen CK, Ray SD, et al. (2003). Molecular mechanisms of cardioprotection by a novel grape seed proanthocyanidin extract. Mutat Res 523–524:87–97
- Breitbart E, Lomnitski L, Nyska A, et al. (2001). Effects of water-soluble antioxidant from spinach, NAO, on doxorubicin-induced heart injury. Hum Exp Toxicol 20:337–45
- Chopra S, Pillai KK, Husain SZ, Giri DK. (1995). Propolis protects against doxorubicin-induced myocardiopathy in rats. Exp Mol Pathol 62:190–8
- Dalloz F, Maingon P, Cottin Y, et al. (1999). Effects of combined irradiation and doxorubicin treatment on cardiac function and antioxidant defenses in the rat. Free Radic Biol Med 26:785–800
- DeAtley SM, Aksenov MY, Aksenova MV, et al. (1999). Antioxidants protect against reactive oxygen species associated with adriamycin-treated cardiomyocytes. Cancer Lett 136:41–6
- Dorr RT. (1991). Chemoprotectants for cancer chemotherapy. Semin Oncol 18:48–58
- Draper HH, Hadley M. (1990). Malondialdehyde determination as an index of lipid peroxidation. Meth Enzymol 186:421–31
- Dziegiel P, Surowiak P, Zabel M. (2002). Correlation of histopathological and biochemical appraisal of anthracyclin-induced myocardium damage. Folia Histochem Cytobiol 40:127–8
- Fatehi M, Rashidabady T, Fatehi-Hassanabad Z. (2003). Effects of Crocus sativus petal’s extract on rat blood pressure and on responses induced by electrical field stimulation in the rat isolated vas deferens and guinea-pig-ileum. J Ethnopharmacol 84:199–203
- Hu ML. (2011). Dietary polyphenols as antioxidants and anticancer agents: More questions than answers. Chang Gung Med J 34:449–60
- Jensen BV, Skovsgaard T, Nielsen SL. (2002). Functional monitoring of anthracycline cardiotoxicity: A prospective, blinded, long-term observational study of outcome in 120 patients. Ann Oncol 13:699–709
- Kang YJ, Chen Y, Epstein PN. (1996). Surexpression of doxorubicin cardiotoxicity by over-expression of catalase in the heart of transgenic mice. J Biol Chem 271:12610–16
- Lecour S, Baouali AB, Maupoil V, et al. (1998). Demonstration of the production of oxygen-centered free radicals during electrolysis using E.S.R. spin-trapping techniques: Effects on cardiac function in the isolated rat heart. Free Radic Biol Med 24:573–9
- Lee V, Randhawa AK, Singal PK. (1991). Adriamycin-induced myocardial dysfunction in vitro is mediated by free radicals. Am J Physiol 261:H989–95
- Makhlouf H, Saksouk M, Habib J, Chahine R. (2011). Determination of antioxidant activity of saffron taken from the flower of Crocus sativus grown in Lebanon. Afr J Biotechnol 10:8093–100
- Mokni M, Hamlaoui-Guesmi S, Amri M, et al. (2012). Extract protects against acute chemotherapy toxicity induced by doxorubicin in rat heart. Cardiovasc Toxicol 24:84–99
- Molnar J, Szabo D, Pusztai R, et al. (2000). Membrane associated antitumor effects of crocin-, ginsenoide- and cannabinoid-derivatives. Anticancer Res 20:861–7
- Nemr R, Lasserre B, Chahine R. (2003). Effects of nicotine on thromboxane/prostacyclin balance in myocardial ischemia. Prostaglandins Leukot Essent Fatty Acids 68:191–5
- Quiles JL, Huertas JR, Battino M, et al. (2002). Antioxidant nutrients and adriamycin toxicity. Toxicology 180:79–95
- Sarvazyan N. (1996). Visualization of doxorubicin-induced oxidative stress in isolated cardiac myocytes. Am J Physiol 271:H2079–85
- Shimpo K, Nagatsu T, Yamada K, et al. (1991). Ascorbic acid and adriamycin toxicity. Amer J Clin Nutr 54:1298S–301S
- Simůnek T, Stérba M, Popelová O, et al. (2009). Anthracycline-induced cardiotoxicity: Overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacol Rep 61:154–71
- Swain M, Whaley F, Ewer MS. (2003). Congestive heart failure in patients treated with doxorubicin. Cancer 97:2869–79
- Tebbi CK, London WB, Friedman D, et al. (2007). Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin’s disease. J Clin Oncol 25:493–500
- Visioli F, De La Lastra CA, Andres-Lacueva C, et al. (2011). Polyphenols and human health: A prospectus. Crit Rev Food Sci Nutr 51:524–46
- Volkova M, Russell R 3rd. (2011). Anthracycline cardiotoxicity: Prevalence, pathogenesis and treatment. Curr Cardiol Rev 7:214–20
- Wang S, Konorev EA, Kotamraju S, et al. (2004). Doxorubicin induces apoptosis in normal and tumor cells via distinctly different mechanisms. Intermediacy of H(2)O(2)- and p53-dependent pathways. J Biol Chem 279:25535–43
- Wattanapitayakul SK, Chularojmontri L, Herunsalee A, et al. (2005). Screening of antioxidants from medicinal plants for cardioprotective effect against doxorubicin toxicity. Basic Clin Pharmacol Toxicol 96:80–7
- Yalçin E, Oruç E, Cavuşoğlu K, Yapar K. (2010). Protective role of grape seed extract against doxorubicin-induced cardiotoxicity and genotoxicity in albino mice. J Med Food 13:917–25
- Yen HC, Oberley TD, Vichitbandhas S, et al. (1996). The protective role of manganese superoxide dismutase against adriamycin-induced acute cardiac toxicity in transgenic mice. J Clin Invest 98:1253–60
- Zhou S, Palmeira CM, Wallace KB. (2001). Doxorubicin-induced persistent oxidative stress to cardiac myocytes. Toxicol Lett 121:151–7