Abstract
Context: Fumonisin B1 (FB1) is a mycotoxin produced by Fusarium verticillioides (Sacc.) Nirenberg (Nectriaceae) mold that contaminates maize and other agricultural products. Although the effects of FB1 on sphingolipid metabolism are clear, little is known about early molecular changes associated with FB1 carcinogenicity.
Objective: Alteration on DNA methylation, as an early event in non-genotoxic carcinogenesis, may play an important role in the mechanism of FB1 toxiciy.
Materials and methods: Dose-related effects of FB1 (1–50 µM for 24 h) on global DNA methylation by using high-performance liquid chromatography with UV-diode array detection (HPLC-UV/DAD) and CpG promoter methylation by methylation-specific PCR (MSP) were performed in rat liver (Clone 9) and rat kidney (NRK-52E) epithelial cells.
Results: Cell viability reduction is 39% and 34% by the XTT test and LDH release in the growth medium is 32% and 26% at 200 µM of FB1 treatment in Clone 9 and NRK-52E cells, respectively. No significant dose-related effects of FB1 on global DNA methylation which ranged from 4 to 5% were observed in both cells compared with controls. Promoter regions of c-myc gene were methylated (>33%) at 10 and 50 µM of FB1 treatment in Clone 9 cells while it was unmethylated in NRK-52E cells. Promoter regions of p15 gene were unmethylated while VHL gene were found to be methylated (>33%) at 10, 25, and 50 µM and 10 and 50 µM of FB1 treatment in Clone 9 and NRK-52E cells, respectively.
Discussion and conclusion: Alteration in DNA methylation might play an important role in the toxicity of FB1 in risk assessment process.
Introduction
Fumonisins are mycotoxins produced by the Fusarium verticillioides (Sacc.) Nirenberg (Nectriaceae) and Fusarium proliferatum (Matsush.) Nirenberg (Nectriaceae) which commonly contaminate maize and maize-based products worldwide. FB1 was considered as a natural cause of leukoencephalomalacia in horses (Kellerman et al., Citation1990; Marasas et al., Citation1988) and pulmonary edema in pigs (Harrison et al., Citation1990). Studies in laboratory animals showed a relationship between FB1 intake and hepatotoxicity, nephrotoxicity, and carcinogenicity (Gelderblom et al., Citation1991; Marasas et al., Citation2004; National Toxicology Program (NTP), Citation2001; Rilery et al., Citation1994; Voss et al., Citation1995). Also, consumption of corn-derived food products contaminated with fumonisins has been correlated with increased risk of human esophageal cancer in epidemiological studies in South Africa and China (Sydenham et al., Citation1990). The International Agency for Research on Cancer (IARC) classified FB1 as a possible carcinogen to humans (group 2B) (IARC, Citation2002). In 2002, the Joint FAO/WHO Expert Committee on Food Additives allocated a group provisional maximum tolerable daily intake of 2 µg/kg body weight to FB1, FB2, and FB3, alone or in combination [World Health Organization (WHO), Citation2002].
Due to its structural similarities with sphingoid bases, FB1 exerts its toxic effects by disruption of sphingolipid biosythesis and accumulation of sphinganine which play a major role in the modulation of apoptotic and cell proliferative pathways related to cancer development (Gelderblom & Marasas, Citation2012; Müller et al., Citation2012; Riley et al., Citation2001; Stockmann-Juvala & Savolainen, Citation2008). FB1 also disrupts the oxidative status of cells that results lipid peroxidation in liver cells (Abel & Gelderblom, Citation1998). FB1 lacks DNA interactive reactivity in different short-term genotoxicity assays utilizing bacteria (Aranda et al., Citation2000; Gelderblom & Snyman, Citation1991; Knasmueller et al., Citation1997) and in vivo and in vitro DNA repair assays in rat liver and primary hepatocytes (Domijan et al., Citation2006, Citation2007, Citation2008; Gelderblom et al., Citation1992; Norred et al., Citation1992).
Non-genotoxic (epigenetic) mechanisms including promoter DNA methylation, histone modifications, and microRNAs play an important role in the modulation of functional pathways that are key to neoplastic development and the expression profile of many tumor suppressor genes in tumor cells. DNA methylation, a well-known primary epigenetic regulator of gene expression, is an important event involved in chemical carcinogenesis (LeBaron et al., Citation2010; Moggs et al., Citation2004). FB1 can be considered as an epigenetic carcinogen in risk assessment process (Gelderblom et al., Citation2008; Müller et al., Citation2012; Stockmann-Juvala & Savolainen, Citation2008). Several studies showed that folate deficiency by FB1 (Abdel Nour et al., Citation2007; Gelineau-van Waes et al., Citation2005; Stevens & Tang, Citation1997) can cause disruption of DNA methylation and FB1-induced carcinogenesis therefore should be investigated as a possible epigenetic mode of action that should impact on the current risk assessment process for fumonisins (Gelderblom & Marasas, Citation2012). More recently, Chuturgoon et al. (Citation2014) showed that FB1 significantly decreased the methyltransferase activities and significantly up-regulated the demethylases, also increased DNA hypomethylation in human hepatocellular carcinoma (HepG2) cells. Therefore, to investigate the effects of FB1 on DNA methylation changes, we performed the experiments on well-characterized cell lines (Clone 9 and NRK-52E cells) treated with FB1 for 24 h. For that, analysis of global DNA methylation was performed by using HPLC/UV-DAD and alteration on gene-specific methylation was performed on the most tumor-related genes (p15, p16, VHL, e-cadherin, and c-myc) which are related to chemical carcinogenesis by MSP.
Material and methods
Chemicals
FB1 (99% purity) was obtained from Sigma-Aldrich (St Louis, MO). A stock solution of FB1 was prepared dissolving it in phosphate buffer solution (PBS, Ca2+, Mg2+-free, Wisent Bioproducts, Saint-Jean-Baptiste, QC, Canada) and kept at −20 °C. Ultra pure grade deoxyribonucleosides were 2′-deoxycytidine (dC, Sigma, St Louis, MO), 5-methyl-2′-deoxycytidine (5-mdC, MP Biomedicals, Athens, OH), 2′-deoxythymidine (2-dT, Amresco, Solon, OH), 2′-deoxyguanosine monohydrate (2-dG, Amresco, Solon, OH), and 2′-deoxyadenosine monohydrate (2-dA, Amresco, Solon, OH). Cell culture media and all other supplements were purchased from Wisent Bioproducts (Saint-Jean-Baptiste, QC, Canada) and sterile plastic materials were purchased from Greiner (Frickenhausen, Germany).
Cell culture and FB1 treatment
The rat liver epithelial cell line (Clone 9) and rat kidney proximal tubular epithelial cell line (NRK-52E) were obtained from the American Type Culture Collection (ATCC). Cells were maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with high glucose (4.5 g/L), 10% fetal bovine serum, and penicillin-streptomycin (100 U–100 µg/mL) at 37 °C in a humidified atmosphere with 5% CO2. Subculturing was performed when the cells reached 70–80% confluence (every 2 d) using trypsinization. For cytotoxiciy assays, cells (1 × 104 in 200 µL medium) were seeded in 96-well plates, then exposed to the FB1 in concentrations of 1, 5, 10, 50, 100, and 200 µM, and phosphate buffer saline (PBS, 1%) as a control for 24 h. For the DNA methylation analysis, cells (1.5 × 106 in 10 mL medium) were seeded in 25 cm2 tissue culture flasks, then exposed to the FB1 in concentrations of 1, 5, 10, 25, and 50 µM and PBS (1%) as a control for 24 h. For all concentrations, it was tested in triplicates and each test was repeated twice. The concentrations of FB1 used in these experiments are similar to the concentrations used in previous studies of toxicity mechanisms of FB1 (Domijan & Abramov, Citation2011; Galvano et al., Citation2002; Mobio et al., Citation2003; Stockmann-Juvala et al., Citation2004; Yu et al., Citation2004).
Cytotoxicity
After 24 h of incubation with FB1, cytotoxicity tests were performed using the Cytotox-LDH-XTT 2 Parameter Cytotoxicity Kit (Xenometrix AG, Allschwil, Switzerland), which measures membrane integrity (lactate dehydrogenase activity, LDH test) and mitochondrial activity (2,3-bis-[2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxyanilide salt, XTT test) in Clone 9 and NRK-52E cells. Briefly, after 24 h of exposure to FB1, a 96-well plate was removed from the incubator and 20 µL of cultured medium was transferred to a new 96-well plate (the original plate was replaced to the incubator for the XTT test). LDH II (NADH) and LDH III (pyruvate) solutions were mixed and 240 µL of this mixture was added to each well. Immediately, the optical density of the plate was read kinetically at 340 nm for 1 h at 37 °C for LDH assay. The 96-well plate was removed from the incubator for the XTT test. The cells were washed with PBS and 200 µL/well of fresh culture medium was added. XTT I (2,3-bis[2-methoxy-4-nitro-5-sulfopheny]-2H-tetrazolium-5-carboxyanilide salt) and XTT II (buffer) solutions were mixed at 1:100 ratio. Then, 50 µL of this mixture was added to all wells. The plate was incubated at 37 °C, 5% CO2 for 3 h. After 3 h, the content of the well was mixed by pipetting up and down. Then, the optical density of the plate was read at 480 nm with a reference wave length at 680 nm. The % inhibition of cell viability for XTT test and % LDH release of each concentration of FB1 were calculated in both cells.
Genomic DNA isolation
Genomic DNA was isolated from Clone 9 and NRK-52E cells using the High Pure PCR Template Preparation Kit (Roche Diagnostics, Mannheim, Germany) according to the instructions provided by the manufacturer. DNA concentration (OD260) and quality (OD260/280) were checked by spectrophotometric measurement.
Global DNA methylation analysis
DNA hydrolysis was performed as previously described by Chen et al. (Citation2010) with some modifications. First, RNA contamination of isolated DNA was removed by treating with 100 µg/mL RNase A and 2000 U/mL RNase T in a volume of 100 µL at 37 °C for 2 h. Then, DNA was purified by chloroform/isoamyl alcohol extraction followed by ethanol precipitation. For DNA hydrolysis, 5 µg genomic DNA was first denaturated by heating at 100 °C for 3 min and chilled on ice. After treating with 5 U nuclease P1 (Sigma-Aldrich, St. Louis, MO) for 2 h at 37 °C, 1 U alkaline phosphatase (Calbiochem, Darmstadt, Germany) was added to the sample and incubated for 1 h at 37 °C. The hydrolyzed DNA was transferred onto a Centrifugal Filter Unit (Amicon Ultra-0.5, Millipore, Darmstadt, Germany) and centrifuged for 20 min at 4 °C and 14 000 rpm. The hydrolyzed DNA samples were diluted with ddH2O to yield 100 µL and now contained deoxyribonucleosides at approximately 5 µg/100 µL. The samples were either analyzed immediately on HPLC or stored at −20 °C until analysis.
For analysis of deoxyribonucleosides, 50 µL of hydrolyzed DNA sample was injected into the HPLC system. HPLC-UV/DAD analysis was performed using a Shimadzu (Kyoto, Japan) HPLC system (LC-20 A) equipped with a gradient pump (LC-20AD), an automatic sampler (SIL-20AHT), and a photodiode array UV–VIS detector (SPD-M20A). Separation was carried out on a Snergy, 4 µm, C18 (Polar RC 80 A, 250 mm × 4.6 mm, Phenomenex, Torrance, CA) column by gradient elution with 50 mM potassium dihydrogen phosphate, pH 4.1 (solvent A) and methanol (solvent B) using the following conditions: 97.5% A and 2.5% B (starting conditions) for 15 min, followed by an increase to 10% B in 5 min, and continued for 10 min at a flow rate of 1 mL/min. The column temperature was kept at 35 °C. Analytes were detected in the 278 nm which was close to the λmax of deoxyribonucleosides and the retention times of deoxyribonucleosides were about 4.9 min for dC, 6.8 min for 5-mdC, 10.7 min for dG, 11.9 min for dT, and 22.6 min for dA using the same chromatographic conditions. Since guanosine base pairs with cytidine, the amount of 2-dG in a sample represents the total amount of methylated and unmethylated 2-dC. Global DNA methylation was expressed as the percentage of methylated deoxycytidine in total deoxycytidine. Quantitation of 2-dG and 5-mdC was performed using external standards. For the calibration curves, standard solutions of 5-mdC in the concentration range of 0.02, 0.04, 0.1, 0.2, and 0.4 µM and 0.5, 1, 2.5, 5, and 10 µM for dC, dG, dT, and dA were prepared in water.
Methylation-specific polymerase chain reaction
Methylation-specific polymerase (MSP) analyses of DNA extracted from control and FB1-treated Clone 9 and NRK-52E cells were carried out as described by Herman et al. (Citation1996). In MSP, genomic DNA is modified by treatment with sodium bisulfite, which converts all methylated cytosines to urasil, then to thymidine during the subsequent PCR step (Frommer et al., Citation1992). Bisulfite modification of DNA samples was carried out using Imprint DNA Modification Kit from Sigma (St. Louis, MO) according to the instructions of the manufacturer. Briefly, genomic DNA (1 µg/10 µL) was incubated with DNA modification solution at 99 °C for 6 min. After the first incubation step, immediately followed by second incubation at 65 °C for 90 min, and then proceeded to the post-modification DNA clean-up. After bisulfite modification, DNA was eluted in a total volume of 20 µL elution buffer and either was used immediately or stored at −80 °C.
Two sets of primers are used to amplify each region of interest. One pair recognizes a sequence in which CpG sites are unmethylated, and the other pair recognizes a sequence in which CpG sites are methylated. Primer sequences for p15, VHL, and c-myc genes were designed with primer design programs for bisulfite-modified DNA [Methyl Primer Express® Software v1.0. free software from Applied Biosystems, San Francisco, CA, and MethPrimer-Design Primers for Methylation PCRs (Li & Dahiya, 2002)]. Primer sequences for p16 and e-cadherin genes were obtained from previous publications (Niwa et al., Citation2005; Swafford et al., Citation1997, respectively). Primer sequences are listed in .
Table 1. Primer sets for MSP analysis.
Each MSP mixture contained 12.5 µL 2×Thermo-Start® PCR Master Mix (Thermo Scientific, Waltham, MA). About 2 µL of 10 pmol/µL of forward and reverse primers each, ∼50 ng/µL DNA template and 7.5 µL nuclease-free water, results in a total volume of 25 µL. MSP conditions were (i) enzyme activation at 95 °C for 15 min; (ii) 35 cycles of denaturation at 95 °C for 30 s annealing at the primer-specific annealing temperature for 30 s and extension at 72 °C for 30 s; (iii) a final extension at 72 °C for 7 min, and (iv) cooling down to 4 °C. Annealing temperatures were optimized for each primer pair and are listed in . The MSP products were analyzed by 8% acrylamide gel electrophoresis, stained with ethidium bromide, and visualized under UV light (QuantumST4-Vilber Lourmat, Torcy, France). To confirm the specificity, DNA was artificially methylated by SssI (CpG) methyltransferase in the presence of s-adenosyl methionine (all from New England Biolabs GmbH, Frankfurt am Main, Germany) according to the instructions of the manufacturer and used as a positive control for the methylated primer sequence. Negative control reactions were performed by using both sets of modified primers with untreated DNA confirm that unmodified DNA is not amplified in the event of incomplete bisulfite reactions.
Statistical analysis
Global methylation levels and cytotoxicity results were compared using ANOVA followed by Dunnett's multiple comparison test; p values <0.05 and <0.001 were considered statistically significant.
Results
Cytotoxicity
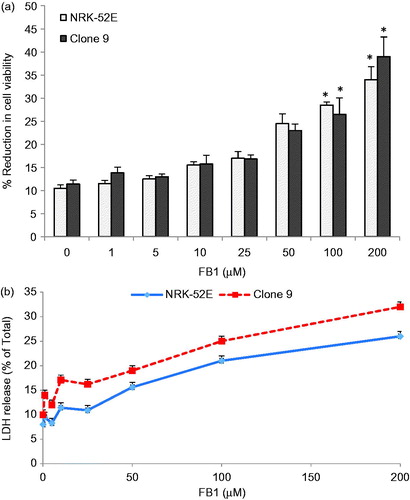
Cell viability was determined by XTT assay in the FB1 (1–200 µM)-treated Clone 9 and NRK-52E cells. It showed that 100 and 200 µM FB1 reduced the XTT activity significantly in Clone 9 and NRK-52E cells (p < 0.001) (). In 200 µM FB1 treatment, the cell viability reduction is 39% and 34% in Clone 9 and NRK-52E, respectively, by the XTT test. In addition, we also observed LDH release in the growth medium above the control cultures at the 100 µM (25% and 21%, p < 0.001) and 200 µM (32% and 26%, p < 0.001) concentration levels of FB1 in Clone 9 and NRK-52E cells, respectively (). Based on these data, we selected 0–50 µM as the FB1 concentration in our DNA methylation study for which there was no obvious decrease on cell viability. This is consistent with the previous studies on the toxicity mechanisms of FB1 (Domijan et al., Citation2011; Galvano et al., Citation2002; Mobio et al., Citation2003; Stockmann-Juvala et al., Citation2004; Yu et al., Citation2004).
Global DNA methylation
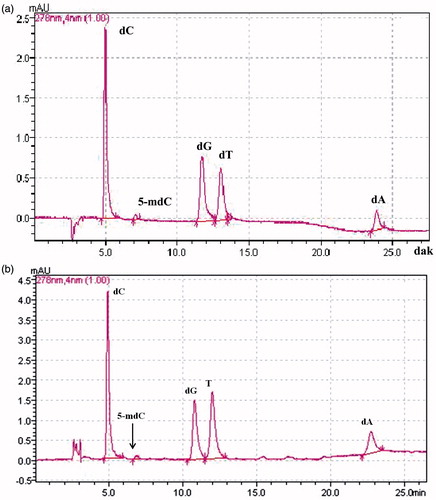
To establish the global levels of cytosine methylation in the FB1-treated Clone 9 and NRK-52E cells, reverse-phase HPLC/UV-DAD was performed. shows the separation of all deoxyribonucleosides in a standard mixture. As it can be seen in , lack of RNA contamination was successful in hydrolyzed DNA of NRK-52E cells. The identity of the peaks was confirmed by co-elution with the corresponding authentic 2′-deoxynucleoside standards. Linear calibration curves were obtained in the concentration range of 0.02–0.4 µM for 5-mdC and 0.5–10 µM for dC, dG, dT, and dA with a correlation coefficient higher than 0.9990. The detection limit of 5 mdC was 0.005 µM (S/N = 3).
Figure 2. A representative HPLC chromatogram of all five deoxyribonucleosides in standard mixture (a) and in hydrolyzed DNA of NRK-52E cells (b). All deoxyribonucleosides were well separated based on established retention times with standard chemicals. dC, 2′-deoxycytidine; 5-mdC, 5-methyl-2′-deoxycytidine;2-dT, 2′-deoxythymidine; 2-dG, 2′-deoxyguanosine monohydrate; 2-dA, 2′-deoxyadenosine monohydrate.
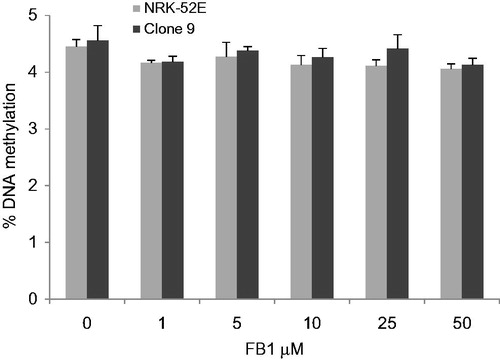
shows the relationship between DNA methylation levels and FB1 treatment in NRK-52E and Clone 9 cells. Global DNA methylation in control samples ranged from 4 to 5%. No significant dose-related effects on global DNA methylation were observed in both cells compared with controls by using HPLC with UV-DAD.
Figure 3. Effects of FB1 (1, 5, 10, 25, and 50 µM) on global DNA methylation in NRK-52E and Clone 9 cells after 24 h incubation. Data are presented as mean ± standard deviation (n = 6). Genomic DNA was extracted and hydrolyzed to deoxyribonucleosides. Global methylation status was quantified by HPLC-UV/DAD.
Promoter DNA methylation
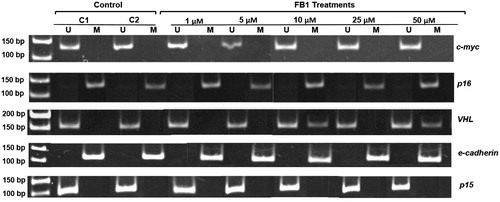
Using MSP, a total of five genes (e-cadherin, c-myc, VHL, p16, and p15) were analyzed for the possible effects of FB1 on their CpG promoter methylation. Representative profiles of MSP for the investigated genes using both methylated (M)- and unmethylated (U)-specific primers in Clone 9 cells which treated with FB1 at the concentrations of 1, 5, 10, 25, and 50 µM are shown in . In Clone 9 cells, the promoter regions of e-cadherin gene were methylated in both control and treated cells. CpG promoter methylation of c-myc and VHL genes was observed in Clone 9 cells by FB1 at concentrations of 10 and 50 µM and 10, 25, and 50 µM, respectively. However, p16 and p15 genes were unmethylated in their CpG promoter regions in response to treatment with FB1 in Clone 9 cells. The methylation status of the investigated genes in NRK-52E cells is shown in . In NRK-52E cells, the promoter regions of e-cadherin and p16 genes were methylated in both control and treated cells, whereas p15 and c-myc genes were unmethylated in their CpG promoter regions by FB1. In NRK-52E cells, only promoter regions of VHL gene were found to be methylated in response to treatment with FB1 at the concentrations of 10 and 50 µM.
Figure 4. Effect of FB1 on methylation status of c-myc, p16, VHL, e-cadherin, and p15 genes in Clone 9 cells. A representative sample of Clone 9 cells treated with FB1 (1, 5, 10, 25, and 50 µM) for 24 h is shown. Methylation was determined by bisulfite modification of the genomic DNA and MSP using primers for the unmethylated (U) or methylated (M) promoter sequence. C1 and C2 = PBS (1%) as a control instead of FB1 treatment.
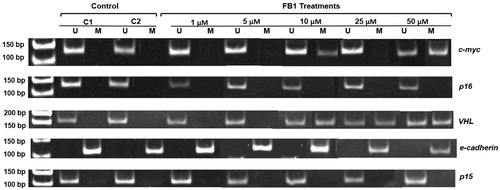
Figure 5. Effect of FB1 on methylation status of c-myc, p16, VHL, e-cadherin, and p15 genes in NRK-52E cells. A representative sample of NRK-52E cells treated with FB1 (1, 5, 10, 25, and 50 µM) for 24 h is shown. Methylation was determined by bisulfite modification of the genomic DNA and MSP using primers for the unmethylated (U) or methylated (M) promoter sequence. C1 and C2 = PBS (1%) as a control instead of FB1 treatment.
Discussion
Epigenetic alterations may be early indicators in chemical carcinogenesis and may be used as biomarkers in the assessment of the carcinogenic potential of environmental chemical agents (Koturbash et al., Citation2011; Rasoulpour et al., Citation2011). DNA methylation, as an epigenetic mechanism, is critical for the regulation of both gene expression patterns and chromatin structure, and alterations in DNA methylation are important factors in chemical carcinogenesis (Fragou et al., Citation2011; Bombail et al., Citation2004; Pereira et al., Citation2004; Pogribny et al., Citation2008). FB1 is generally regarded as a non-genotoxic carcinogen, because it lacks activity in mutagenecity (Gelderblom & Snyman, Citation1991) and genotoxicity assays (Norred et al., Citation1992) and appears not to bind directly to DNA. Epigenetic alterations may play an important role in the mechanism of FB1 carcinogenicity (Gelderblom & Marasas, Citation2012). In terms of DNA methylation, there is no clear evidence to suggest that FB1 could alter either global DNA methylation levels or promoter methylation status of CpG islands of specific genes. Recently, Chuturgoon et al. (Citation2014) showed that FB1 significantly decreased the methyltransferase activities and significantly up-regulated the demethylases, besides FB1 increased DNA hypomethylation in HepG2 cells at the 200 µM concentration after 24 h treatment. In the present study, we investigated the alteration on global and gene-specific DNA methylation in liver and kidney cell cultures after low-dose FB1 exposure. FB1 reduced the cell viability at the high concentrations (100 and 200 µM) in Clone 9 and NRK-52E cells by the XTT test. In 200 µM FB1 treatment, the cell viability reduction is 39% and 34% in Clone 9 and NRK-52E, respectively, by the XTT test. In accordance, we also observed LDH release in the growth medium above the control cultures at the 100 µM (25% and 21%) and 200 µM (32% and 26%) concentration levels of FB1 in Clone 9 and NRK-52E cells, respectively. This is totally in agreement with the data previously published by Kouadio et al. (Citation2005). Abel and Gelderblom (Citation1998) also found that at the 250 µM FB1 concentration LDH release was %30–35 in primary rat hepatocytes.
In the present study, global DNA methylation in control samples ranged from 4 to 5%. This is consistent with the previous study in which approximately 2–5% of cytosine residues in the mammalian genome were shown to be methylated (Bombail et al., Citation2004). We did not reveal any modulation of global DNA methylation by FB1 in both Clone 9 and NRK-52 E cells at the concentration of 1–50 µM. However, Kouadio et al. (Citation2007) showed that in Caco-2 cells, FB1 increased the level of 5-mdC from 5% to 9%, 9.5%, and 8% at concentrations of 5, 10, 20, and 40 µM, respectively. Moreover, FB1 induced significant hypermethylation at the concentrations of 9 and 18 µM in C6 glioma cells (Mobio et al., Citation2000). Since we did not observe any alterations on the global DNA methylation, we aimed to investigate the effects of FB1 on the CpG island methylation in promotor regions of key tumor-suppressor genes (e-cadherin, VHL, p16, and p15) and oncogene (c-myc) by MSP following the bisulfite DNA modification. Promoter hypermethylation-mediated gene silencing has been reported for several genes which play an important role in the regulation of DNA repair, cell cycle, apoptosis, cell–cell adhesion, metastasis, and various signalling pathways in cancers (Baylin et al., Citation1998; Jones & Baylin, Citation2002). Selected genes in the present study (p16, p15, VHL, and e-cadherin) are known to be widely hypermethylated in various human cancers (Esteller, Citation2002). Moreover, it has been shown that non-genotoxic carcinogens influence DNA methylation of these genes involved in cell cycle and apoptosis (Baccarell & Bollati, Citation2009; Watson & Goodman, Citation2002). Kato et al. (Citation2006) suggest that aberrant DNA methylation of e-cadherin and p16 genes may play important roles in the development of lung adenocarcinomas induced by N-nitrosobis(2-hydroxypropyl)amine in rats. Du et al. (Citation2009) showed that diethylinitrosamine induced DNA hypermethylation of p16 gene and hypomethylation of c-myc gene and this correlated with the alteration on gene expression.
E-cadherin gene, a cell adhesion molecule, was found to be methylated in both Clone 9 and NRK-52E cells for both control and treated groups, showing aberrant methylation of e-cadherin gene could not be detected by FB1 for both cells. p16 tumor suppression gene is an inhibitor of cyclin D-dependent protein kinases which inhibits cell-cycle progression. The present study showed that p16 gene was found to be methylated in only NRK-52E cells for both control and treated groups. However, p16 gene was unmethylated in its CpG promoter regions in response to treatment with FB1 in Clone 9 cells. Contrary to our results, Asada et al. (Citation2006) showed that p16 gene was partly methylated in Clone 9 and tumor cell line MH1C1. It can be explained that hypermethylation of the p16 gene in the normal kidney cells was achieved during in vitro growth as indicated by Asada et al. (Citation2006) for normal liver cells. c-myc Proto-oncogene encodes for a transcription factor that is involved to regulate cell proliferation, differentiation, and apoptosis. In our study, CpG promoter methylation of c-myc gene was observed in Clone 9 cells at concentrations of 10 and 50 µM, while it was unmethylated in NRK-52E cells in response to FB1. Hypermethylation of c-myc gene may be related to downregulation of this gene by FB1. It may be indicative of potential suppression of c-myc functions by FB1 in Clone 9 cells. Methylation of the CpG island of the VHL tumor suppressor gene is associated with transcriptional inactivation in a subset of clear cell renal carcinomas (Herman et al., Citation1994; McRonald et al., Citation2009). CpG promoter methylation of VHL gene was observed in Clone 9 and NRK-52 E cells by FB1 at concentrations of 10, 25, and 50 µM and 10 and 50 µM, respectively. CpG promoter DNA methylation was not detected in p15 gene, cyclin-dependent kinase inhibitor gene, in controls and FB1-treated Clone 9 and NRK-52E cells.
This is the first study that investigates the gene-specific DNA methylation in the toxicity mechanisms of FB1 for the risk assessment process. In a recent study on the epigenetic mechanisms in FB1 toxicity, FB1 can alter histone modifications which lead to heterochromation disorganization at low doses (Pellanda et al., Citation2012). In this work, it was shown that H4K20me3 significantly decreased, while H3K9me3 significantly increased in the fetuses when pregnant dams were exposed to methyl-deficient diet and FB1 (Pellenda et al., Citation2012).
In conclusion, results from this study suggest that CpG promoter DNA methylation as an epigenetic mechanism might play an important role in the FB1 toxicity. This work will not only contribute to a deeper understanding of the early molecular events involved in the mechanism of toxicity of non-genotoxic carcinogens but may also help in the design of methods for early detection of non-genotoxic carcinogenesis for risk assessment process. It should be also noted that this work would be expanded in the future to investigate the effects of FB1 on genome-wide DNA methylation, gene regulation, histone modifications, and down-regulation of microRNAs in vitro and in vivo, which are also critical events for epigenetic control of gene expression.
Declaration of interest
The authors report that there are no declarations of interest. The authors alone are responsible for the content and writing of the paper. This work was supported partly by Scientific Research Projects Coordination Unit of Istanbul University (Project nos. TP-11059, UDP-21364, BYP-9845, and YADOP-13242) and TUBITAK (Project no. SBAG-109S187, Grant for Göksun Demirel).
References
- Abdel Nour AM, Ringot D, Gue'ant JL, Chango A. (2007). Folate receptor and human reduced folate carrier expression in HepG2 cell line exposed to fumonisin B1 and folate deficiency. Carcinogenesis 28:2291–7
- Abel S, Gelderblom WC. (1998). Oxidative damage and fumonisin B1-induced toxicity in primary rat hepatocytes and rat liver in vivo. Toxicology 131:121–31
- Aranda M, Perez-Alzola LP, Ellahuene MF, Sepulveda C. (2000). Assessment of in vitro mutagenicity in Salmonella and in vivo genotoxicity in mice of the mycotoxin fumonisin B1. Mutagenesis 15:469–71
- Asada K, Asada R, Yoshiji H, et al. (2006). DNA cytosine methylation profile in various cancer-related genes is altered in cultured rat hepatocyte cell lines as compared with primary hepatocytes. Oncol Rep 15:1241–8
- Baccarelli A, Bollati V. (2009). Epigenetics and environmental chemicals. Curr Opin Pediatr 21:243–51
- Baylin SB, Herman JG, Graff JR, et al. (1998). Alterations in DNA methylation: A fundamental aspect of neoplasia. Adv Cancer Res 72:141–96
- Bombail V, Moggs JG, Orphanides G. (2004). Perturbation of epigenetic status by toxicants. Toxicol Lett 149:51–8
- Chen T, Mally A, Ozden S, Chipman JK. (2010). Low doses of the carcinogen furan alter cell cycle and apoptosis gene expression in rat liver independent of DNA methylation. Environ Health Perspect 118:1597–602
- Chuturgoon AA, Phulukdaree A, Moodley D. (2014). Fumonisin B1 induces global DNA hypomethylation in HepG2 cells – An alternative mechanism of action. Toxicology 315:65–9
- Domijan AM, Zeljezic D, Kopjar N, Peraica M. (2006). Standard and Fpg-modified comet assay in kidney cells of ochratoxin A- and fumonisin B1-treated rats. Toxicology 222:53–9
- Domijan AM, Zeljezic D, Milic M, Peraica M. (2007). Fumonisin B(1): Oxidative status and DNA damage in rats. Toxicology 232:163–9
- Domijan A, Zeljezic D, Peraica M, et al. (2008). Early toxic effects of fumonisin B1 in rat liver. Hum Exp Toxicol 27:895–900
- Domijan AM, Abramov AY. (2011). Fumonisin B1 inhibits mitochondrial respiration and deregulates calcium homeostasis-implication to mechanism of cell toxicity. Int J Biochem Cell Biol 43:897–904
- Du YP, Peng JS, Sun A, et al. (2009). Assessment of the effect of betaine on p16 and c-myc DNA methylation and mRNA expression in a chemical induced rat liver cancer model. BMC Cancer 9:1–9
- Esteller M. (2002). CpG island hypermethylation and tumor suppressor genes: A booming present, a brighter future. Oncogene 21:5427–40
- Fragou D, Fragou A, Kouidou S, et al. (2011). Epigenetic mechanisms in metal toxicity. Toxicol Mech Methods 21:343–52
- Frommer M, McDonald LE, Millar DS, et al. (1992). A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. Proc Natl Acad Sci USA 89:1827–31
- Galvano F, Campisi A, Russo A, et al. (2002). DNA damage in astrocytes exposed to fumonisin B1. Neurochem Res 27:345–51
- Gelderblom WCA, Kriek NPJ, Marasas WFO, Thiel PG. (1991). Toxicity and carcinogenicity of the Fusarium moniliforme metabolite, fumonisin B1, in rats. Carcinogenesis 12:1247–51
- Gelderblom WC, Snyman SD. (1991). Mutagenicity of potentially carcinogenic mycotoxins produced by Fusarium moniliforme. Mycotoxin Res 7:46–52
- Gelderblom WC, Semple E, Marasas WF, Farber E. (1992). The cancer-initiating potential of the fumonisin B mycotoxins. Carcinogenesis 13:433–7
- Gelderblom WC, Marasas WF, Lebepe-Mazur S, et al. (2008). Cancer initiating properties of fumonisin B1 in a short-term rat liver carcinogenesis assay. Toxicology 250:89–95
- Gelderblom WC, Marasas WF. (2012). Controversies in fumonisin mycotoxicology and risk assessment. Hum Exp Toxicol 31:215–35
- Gelineau-van Waes J, Starr L, Maddox J, et al. (2005). Maternal fumonisin exposure and risk for neural tube defects: Mechanisms in an in vivo mouse model. Birth Defects Res 73:487–97
- Harrison LR, Colvin BM, Greene JT, et al. (1990). Pulmonary edema and hydrothorax in swine produced by fumonisin B1, a toxic metabolite of Fusarium moniliforme. J Vet Diagn Invest 2:217–21
- Herman JG, Graff JR, Myöhänen S, et al. (1996). Methylation-specific PCR: A novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA 93:9821–6
- Herman JG, Latif F, Weng Y, et al. (1994). Silencing of the VHL tumor-suppressor gene by DNA methylation in renal carcinoma. Proc Natl Acad Sci USA 91:9700–4
- International Agency for Research on Cancer. (2002). Fumonisin B1, IARC monographs on the evaluation of carcinogenic risks to humans: Some traditional medicines, some mycotoxins, naphthalene and styrene. IARC 82:301–66
- Jones PA, Baylin SB. (2002). The fundamental role of epigenetic events in cancer. Nat Rev Genet 3:415–28
- Kato A, Shimizu Y, Fujii H, et al. (2006). Aberrant DNA methylation of E-cadherin and p16 genes in rat lung adenocarcinomas induced by N-nitrosobis(2-hydroxypropyl)amine. Mol Carcinog 45:106–11
- Kellerman TS, Marasas WF, Thiel PG, et al. (1990). Leukoencephalomalacia in two horses induced by oral dosing of fumonisin B1. Onderstepoort J Vet Res 57:269–75
- Knasmueller S, Bresgen N, Kassie F, et al. (1997). Genotoxic effects of three Fusarium mycotoxins, fumonisin B1, moniliformin and vomitoxin in bacteria and in primary cultures of rat hepatocytes. Mutat Res-Genet Toxicol Environ Mutagen 391:39–48
- Koturbash I, Beland FA, Pogribny IP. (2011). Role of epigenetic events in chemical carcinogenesis – A justification for incorporating epigenetic evaluations in cancer risk assessment. Toxicol Mech Methods 21:289–97
- Kouadio JH, Dano SD, Moukha S, et al. (2007). Effects of combinations of Fusarium mycotoxins on the inhibition of macromolecular synthesis, malondialdehyde levels, DNA methylation and fragmentation, and viability in Caco-2 cells. Toxicon 49:306–17
- Kouadio JH, Mobio TA, Baudrimont I, et al. (2005). Comparative study of cytotoxicity and oxidative stress induced by deoxynivalenol, zearalenone or fumonisin B1 in human intestinal cell line Caco-2. Toxicology 213:56–65
- LeBaron MJ, Rasoulpour RJ, Klapacz J, et al. (2010). Epigenetics and chemical safety assessment. Mutat Res 705:83–95
- Li LC, Dahiya R. (2002). MethPrimer: Designing primers for methylation PCRs. Bioinformatics 18:1427–31
- Marasas WF, Riley RT, Hendricks KA, et al. (2004). Fumonisins disrupt sphingolipid metabolism, folate transport, and neural tube development in embryo culture and in vivo: A potential risk factor for human neural tube defects among populations consuming fumonisin-contaminated maize. J Nutr 134:711–16
- Marasas WFO, Kellerman TS, Gelderblom WCA, et al. (1988). Leukoencephalomalacia in a horse induced by fumonisin B1 isolated from Fusarium moniliforme. Onderstepoort J Vet Res 55:197–203
- McRonald FE, Morris MR, Gentle D, et al. (2009). CpG methylation profiling in vhl related and VHL unrelated renal cell carcinoma. Mol Cancer 8:1–11
- Methyl Primer Express Software 1. Applied Biosystems (Designed and created by M.F. Fraga, C. Ferrero, M. Esteller)
- Mobio TA, Anane R, Baudrimont I, et al. (2000). Epigenetic properties of fumonisin B(1): Cell cycle arrest and DNA base modification in C6 glioma cells. Toxicol Appl Pharmacol 164:91–6
- Mobio TA, Tavan E, Baudrimont I, et al. (2003). Comparative study of the toxic effects of fumonisin B1 in rat C6 glioma cells and p53-null mouse embryo fibroblasts. Toxicology 183:65–75
- Moggs JG, Goodman JI, Trosko JE, Roberts RA. (2004). Epigenetics and cancer: Implications for drug discovery and safety assessment. Toxicol Appl Pharmacol 196:422–30
- Müller S, Dekant W, Mally A. (2012). Fumonisin B1 and the kidney: Modes of action for renal tumor formation by fumonisin B1 in rodents. Food Chem Toxicol 50:3833–46
- Niwa T, Yamashita S, Tsukamoto T, et al. (2005). Whole-genome analyses of loss of heterozygosity and methylation analysis of four tumor-suppressor genes in N-methyl-N′-nitro-N-nitrosoguanidine-induced rat stomach carcinomas. Cancer Sci 96:409–13
- Norred WP, Plattner RD, Vesonder RF, et al. (1992). Effects of selected secondary metabolites of Fusarium moniliforme on unscheduled synthesis of DNA by rat primary hepatocytes. Food Chem Toxicol 30:233–7
- National Toxicology Program. (2001). Toxicology and carcinogenesis studies of fumonisin B1 (CAS no. 116355-83-0) in F344/N rats and B6C3F1 mice (feed studies). Natl Toxicol Program Tech Rep Ser 496:1–352
- Pellanda H, Forges T, Bressenot A, et al. (2012). Fumonisin FB1 treatment acts synergistically with methyl donor deficiency during rat pregnancy to produce alterations of H3- and H4-histone methylation patterns in fetuses. Mol Nutr Food Res 56:976–85
- Pereira MA, Wang W, Kramer PM, Tao L. (2004). DNA hypomethylation induced by non-genotoxic carcinogens in mouse and rat colon. Cancer Lett 212:145–51
- Pogribny IP, Tryndyak VP, Boureiko A, et al. (2008). Mechanisms of peroxisome proliferator-induced DNA hypomethylation in rat liver. Mutat Res 644:17–23
- Rasoulpour RJ, LeBaron MJ, Ellis-Hutchings RG, et al. (2011). Epigenetic screening in product safety assessment: Are we there yet? Toxicol Mech Methods 21:298–311
- Riley RT, Enongene E, Voss KA, et al. (2001). Sphingolipid perturbations as mechanisms for fumonisin carcinogenesis. Environ Health Perspect 109:301–8
- Riley RT, Hinton DM, Chamberlain WJ, et al. (1994). Dietary fumonisin B1 induces disruption of sphingolipid metabolism in Sprague–Dawley rats: A new mechanism of nephrotoxicity. J Nutr 124:594–603
- Stevens VL, Tang J. (1997). Fumonisin B1-induced sphingolipid depletion inhibits vitamin uptake via the glycosylphosphatidylinosotol-anchored folate uptake. J Biol Chem 272:18020–5
- Stockmann-Juvala H, Mikkola J, Naarala J, et al. (2004). Oxidative stress induced by fumonisin B1 in continuous human and rodent neural cell cultures. Free Radic Res 38:933–42
- Stockmann-Juvala H, Savolainen K. (2008). A review of the toxic effects and mechanisms of action of fumonisin B1. Hum Exp Toxicol 27:799–809
- Swafford DS, Middleton SK, Palmisano WA, et al. (1997). Frequent aberrant methylation of p16INK4a in primary rat lung tumors. Mol Cell Biol 17:1366–74
- Sydenham EW, Thiel PG, Marasas WFO, et al. (1990). Natural occurrence of some Fusarium mycotoxins in corn from low and high esophageal cancer prevalence areas of the Transkei, southern Africa. J Agric Food Chem 38:1900–3
- Voss KA, Chamberlain WJ, Bacon CW, et al. (1995). Subchronic feeding study of the mycotoxin fumonisin B1 in B6C3F1 mice and Fischer 344 rats. Fundam Appl Toxicol 24:102–10
- Watson RE, Goodman JI. (2002). Epigenetics and DNA methylation come of age in toxicology. Toxicol Sci 67:11–16
- World Health Organization. (2002). Evaluation of Certain Mycotoxins in Food. Fifty-sixth Report of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). WHO Technical Report 906:16–27
- Yu MU, Yoo JM, Lee YS, et al. (2004). Altered de novo sphingolipid biosynthesis is involved in the serum deprivation-induced cell death in LLC-PK1 cells. J Toxicol Environ Health A 67:2085–94