Abstract
Context: Ferulic acid (FA) is a potent ubiquitous plant antioxidant found in cereals such as brown rice, whole wheat, and oats. Phytochemical-based antioxidants are shown to be effective in neurodegenerative diseases. This study hypothesizes that supplementation of FA might combat oxidative stress-induced Parkinson’s disease (PD).
Objective: To explore the effect of FA on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine hydrochloride (MPTP)-induced neurotoxicity.
Materials and methods: Mice were randomized into five groups: Group I mice served as control. Group II mice received 5 × MPTP [25 mg/kg body weight (i.p.)] in saline 24 h apart starting from the 3rd day and continued till the last day of the experimental period of 7 d. In addition to MPTP injections, mice in Groups III, IV, and V were given FA at a dose of 20, 40, and 80 mg, respectively, for 7 d. Mice were subjected to a battery of behavioral tests along with histological investigations.
Results: Our histological findings revealed that MPTP administration enhanced Bax/Bcl2 ratio and microglial cells activation reflecting induction of apoptosis and inflammation, respectively. This dopaminergic neuronal loss caused impairment in motor balance and coordination in MPTP mice as assessed by various behavioral tests. FA at a dose of 40 mg/kg/d body weight effectively attenuated MPTP-induced neurotoxicity.
Discussion: Antioxidant, free-radical quenching, and anti-inflammatory activities of FA could contribute to its neuroprotective effect.
Conclusion: This study provides elementary evidence for the neuroprotective action of FA against MPTP-induced PD in mice and warrants further studies.
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder affecting approximately 30 million people worldwide (Benetti et al., Citation2012) and its prevalence is expected to double within the next two decades in parallel with the aging population. PD is characterized by the degeneration of the neuromelanin containing neurons in the brainstem (Fahn, Citation2003), particularly those in the pars compacta of the substantia nigra (SNpc). This specific neurodegeneration leads to clinical signs including tremor at rest, rigidity on passive movement, bradykinesia, and hypokinesia reflecting a threshold reduction of ∼80% dopamine (DA) in the striatum (Dauer & Przedborski, Citation2003). Neuropathological hallmarks of PD are loss of dopaminergic neurons in the substantia nigra and the formation of intraneuronal protein inclusions termed Lewy bodies, composed primarily of α-synuclein (Stefanis, Citation2012; Yasuda et al., Citation2013). Postural instability, orthostatic hypotension, and dementia are invariably developed with the progression of the disease (Martínez-Banaclocha, Citation2012). At present, there is no effective strategy for reducing the incidence or progression of PD in high-risk populations. Although levodopa therapy is considered as the gold standard for the treatment of PD, its toxicity is a limiting factor.
The 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine hydrochloride (MPTP) model of PD has been extensively used to elucidate the basal ganglia response to nigrostriatal deficits as well as to examine the validity of novel drug treatments for PD. Mice systemically administered with MPTP are shown to display signs of akinesia and catalepsy (CAT) due to bilateral degeneration of the nigrostriatal tract (Sedelis et al., Citation2001). Further, sub-acute dosing with MPTP (i.e., 25–40 mg/kg/d) for 5 d is reported to cause 76% loss in striatal DA and 60% SNpc cell loss and it is also claimed that more persistent hypoactivity is evident within 3 h post-treatment that lasts for at least 10 d (Pothakos et al., Citation2009). Astrocytes play a critical role in MPTP-induced neuronal injury through their ability to convert the innocuous MPTP into the toxic pyridinium metabolite MPP+ by a two-step reaction that requires the enzyme monoamine oxidase B (MAO B). The toxic product is actively sequestrated within dopaminergic neurons by monoaminergic transporters (Rappold & Tieu, Citation2010). Inside mitochondria MPP+ can inhibit mitochondrial complex I activity, and its toxicity has been attributed to the subsequent mitochondrial depolarization and generation of reactive oxygen species (Nakamura et al., Citation2000).
Since increased oxidative stress, chronic inflammation, and mitochondrial dysfunction play an important role in the degeneration of DA neurons, dietary antioxidants are gaining much attention in the prevention of PD. Ferulic acid (FA) is a potent ubiquitous plant antioxidant found in wheat, oats, rice, peanuts, artichokes, and coffee. Fruits such as apples, pineapples, and oranges are also great sources of FA. FA has been shown to be beneficial in the prevention and/or treatment of disorders linked to oxidative stress and inflammation (Kim et al., Citation2004; Sultana, Citation2012). FA was proposed as a novel antioxidant compound endowed with a strong cytoprotective activity due to both the ability to scavenge free radicals and the ability to activate cell stress response (Mancuso & Santangelo et al., Citation2014). FA has also been shown to have a variety of protective effects in several disease models, including reduced blood pressure (Ardiansyah et al., Citation2008), blood glucose (Prabhakar et al., Citation2013), oxidative stress (Trombino et al., Citation2013), amyloid load, and improved cognitive behavior in Alzheimer disease in mice (Yan et al., Citation2013). Nevertheless, the effect of FA against the MPTP toxicity in C57BL/6 mice has not been previously investigated. Thus, a preliminary study is done to explore the impact of FA on MPTP-induced motor impairment and pathological alterations in C57BL/6 mice.
Materials and methods
Ethics statement
Experimental procedures involving the use of animals or animal tissue were performed in accordance with the Indian National Law on animal care and use and approved by the Institutional Animal Ethics Committee at the SASTRA University (CPCSEA approval no: 276/SASTRA/IAEC/RPP).
Animals and diet
Male C57BL/6 mice (25–30 g) were purchased from the Biogen Laboratory animal facility, Bangalore, Karnataka, India, and were maintained in an air conditioned room (22 ± 1 °C) with 12 h light/dark and 60% humidity at Central Animal Facility, SASTRA University, Tamil Nadu, India. Standardized pellet diet was purchased from M/S Provimi Nutrition India PVT Ltd, Bangalore, Karnataka, India. Feed and water were provided ad libitum to all the animals. Animals were acclimatized for a period of 1 week before initiating the experimental protocol.
Chemicals
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine hydrochloride (MPTP), FA, and Luxol fast blue were purchased from Sigma Chemical Co., St. Louis, MO. Bax and Bcl2 antibodies were purchased from Santa Cruz Biotechnology, Inc. Dallas, TX. Secondary detection kit was obtained from Bio Genex Laboratories, Inc., San Ramon, CA. All other chemicals were of analytical grade.
MPTP injection
MPTP was purchased as MPTP.HCl, where HCl (MW: 35.4) represents 17% of the MPTP. Hence, 23.4 mg/kg (20 mg/kg × 1.17) of MPTP.HCl was used to make 20 mg/kg dose of free base according to the protocol reported by Jackson-Lewis and Przedborski (Citation2007). The total volume of MPTP needed for the experiment was estimated by adding the weight of all mice to be injected with MPTP dissolved in sterile saline.
Experimental design
For this preliminary study, adult male C57BL/6 mice were randomized and divided into five groups of six animals each. Group I mice received carboxymethyl cellulose (CMC) p.o. and saline s.c. and served as control. Group II mice received 5 × MPTP (25 mg/kg body weight. i.p.) in saline 24 h apart starting from the 3rd day continued to the 7th day of the experimental period. In addition to MPTP, mice in Groups III, IV, and V received FA via intragastric intubation at a dose of 20, 40, and 80 mg/kg body weight, respectively, for 7 d. Mice were trained on all behavioral apparatus before the start of the experiment in order to reach the ceiling performance. Behavioral tests namely open field test (OFT), rotarod test (RRT), and CAT were done on the 3rd, 5th, and 7th day whereas the forced swim test (FST), pole test, and grip test were done on the 2nd, 6th, and 8th day of the experimental period in a blind manner. Behavioral assessments were done by taking the average score of all three performances of each test.
Tissue processing
At the end of the experiment (8th day) after performing behavioral parameters, mice were sacrificed by CO2 asphyxiation and intracardial perfusion with PBS (pH 7.4). Brain was collected and post-fixed in 4% PFA for 24 h at 4 °C, embedded in paraffin (Leica EG1150H, Leica Microsystems, Heerbrugg, Switzerland), and cut into 5 µm sections (Leica RM2125 RTS, Leica Microsystems, Heerbrugg, Switzerland) encompassing the entire ST and SN regions with the aid of the mouse brain atlas for histological investigations.
Behavioral assessments
Open field test
The OFT apparatus was constructed of white plywood (72 × 72 cm2) with 36 cm wall and one of the walls was clear Plexiglas. Blue lines were drawn on the floor with a marker and were visible through the clear Plexiglas floor. The lines divided the floor into sixteen 18 × 18 cm2. A central square (18 cm × 18 cm) was drawn in the middle of the open field (Brown et al., Citation1999). Mice were transported to the testing place and immediately placed into the open field chamber handled by the base of their tails and its behaviors were observed for 5 min as follows: (a) line crossing: frequency with which the mice crossed one of the grid lines with all four paws. (b) Center square entries: frequency with which the mice crossed one of the red lines with all four paws into the central square. (c) Rearing: frequency with which the mice stood on their hind legs in the maze. (d) Grooming: The number of times of licking the fur, washing face, or scratching behavior. After 5 min, mice were returned back to home cages and the OFT apparatus was cleaned with 70% ethanol and dried between tests.
Rotorod test
The RRT is used as an index of motor coordination. The length of time that a given animal stays on the rotating rod is a measure of their balance, coordination, physical condition, and motor planning. Digital rotarod apparatus with four compartments was used in the study to test four mice at a time. Mice were placed at the center of the rod in each lane handled by tail at 45 °C and were allowed to adjust their posture in order to maintain their balance on a rotating rod at speeds of 5, 10, and 20 rpm. The latency for each mouse to fall off the rod was recorded as described previously (Rozas et al., Citation1998).
Forced swim test
The FST was carried out using a procedure modified from the original report by Porsolt et al. (Citation1977). Briefly mice were individually placed into a glass cylinder (height: 25 cm, diameter: 10 cm) filled with water (depth: 8 cm, temperature: 22–24 °C). The mice were forced to swim for 6 min and the total duration of immobility during the last 4 min of a single 6-min test session was recorded. Immobility was defined as no body or limb movement other than a minimal forelimb movement required for keeping the head above water. The mouse was then removed from water and allowed to dry with a towel before returning to the home cage. The water was changed between each subject.
CAT
CAT or freezing reaction is a state of postural immobility (akinesia) with muscular rigidity and was evaluated using a bar test (Ferré et al., Citation1990). Briefly, CAT was measured as the time the animal maintained an imposed position with both front limbs extended and resting on a 4 cm high bar. The endpoint of CAT was considered to occur when both front paws were removed from the bar or if the animal moved its head in an exploratory manner. The animal is lifted by its tail and is allowed to place its forepaws on a horizontal wooden bar. The duration taken for the first movement of paws was measured as cataleptic time.
Pole test
The Pole test method was adapted from the protocol originally described by Ogawa et al. (Citation1985). Briefly, animals were placed head-up on top of a vertical wooden pole 50 cm long (1 cm diameter). The base of the pole was placed in the home cage. When placed on the pole, animals orient themselves downward and descend the length of the pole back into their home cage. The time to orient downward (t-turn) and the total time to descend (t-total) were measured.
Grip test
Grip test was performed as described previously (Khuwaja et al., Citation2011). Briefly, the apparatus consists of a string of 50 cm length, pulled tight between two vertical supports and elevated 40 cm from a flat surface was used. Mouse was placed on the string at a point midway between supports and evaluated according to the following scale; 0 = fall off, 1 = hangs onto string by two forepaws, 2 = as for 1 but attempts to climb on string, 3 = hangs onto string by two forepaws plus one or both hind paws, 4 = hangs onto string by all forepaws plus tail wrapped around string and 5 = escape.
Histological investigations
Luxol fast blue and cresyl violet staining
Paraffin-embedded tissue sections were incubated in an oven at 60 °C for 60 min, then immersed in xylene for 25 min, and hydrated in 95% ethanol. Then the tissue sections were incubated in Luxol fast blue solution over 1.5 h, washed in 95% alcohol, rinsed in deionized water, and differentiated in 0.05% lithium carbonate for 6 s followed by 70% alcohol for 2 min. Sections were then incubated in cresyl violet for 10 min. Finally, the slides were dehydrated with 95% and 100% ethanol for 30 s followed by one change in xylene and mounted in a neutral deparaffinized xylene (DPX). Images were captured using a bright field microscope (Nikon, Cis, Meillville, NY) at 40 × magnification.
Immunohistochemistry
Briefly 4 μm thick paraffin-embedded sections were heated for 60 min at 60 °C, deparaffinized in xylene, and rehydrated through graded alcohols at room temperature. Tris–HCl buffer (0.05 M; pH 7.6) was used to prepare solutions and for washes between various steps. Sections were treated for 40 min at room temperature with 2% bovine serum albumin and incubated with primary antibodies against Bax and Bcl2 for 1 h. Horseradish peroxidase activity was visualized after treatment with H2O2 and diaminobenzidine hydrochloride for 5 min using the labeled streptavidin–biotin method (BioGenex Kit, San Raman, CA). Finally, sections were incubated with biotinylated secondary antibody and streptavidin peroxidase. The color was developed with 3,3-diaminobenzidine and the sections were mounted with DPX after dehydrating them in graded ethanol and mounted (Singh et al., Citation2009). Images were captured using a bright field microscope (Nikon, Cis) at 20 × magnification. The percentage area of immunopositivity was analyzed and quantified by using Image J software (National Institute of Mental Health, Bethesda, MD).
Statistical analysis
All the experimental data were analyzed using one-way analysis of variance (ANOVA) using SPSS version 10.0 software (SPSS Inc., Chicago, IL) and individual comparisons were obtained using DUNCAN’S Multiple Range Test (DMRT). Values were considered statistically significant if p < 0.05. Analyzed data are expressed as mean ± SD of the number of experiments (n = 6). Bar graphs for the analyzed experimental data were prepared using Sigma plot (SPW 10) software (Systat Software, Inc., San Jose, CA).
Results
FA improves behavioral deficits in MPTP-administered mice
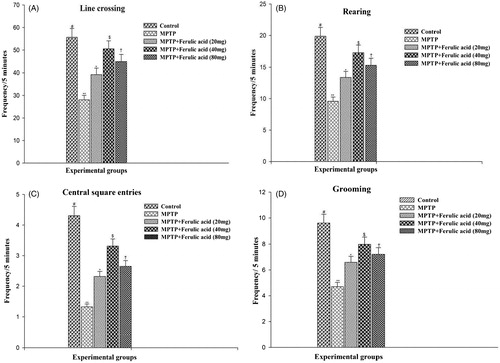
OFT is used to assess locomotor, exploratory, and anxiety like behaviors. A high frequency of these behaviors indicates increased locomotion and exploration and/or a lower level of anxiety. shows the degree of OFT performance of experimental mice. MPTP-treated mice showed significant reduction in OFT parameters as compared with control mice. FA at a dose of 40 mg/kg body weight exerts more pronounced effect than 20 and 80 mg, respectively.
Figure 1. Open field test performance of experimental mice: (A) Line crossing, (B) rearing, (C) central square entries, and (D) grooming. Values are given as mean ± SD for six mice in each group. Error bars sharing common symbol do not differ significantly at p < 0.05. #Significantly p < 0.05 differ from MPTP and MPTP + ferulic acid-treated groups. **Significantly p < 0.05 differ from control and MPTP + ferulic acid groups. *Significantly p < 0.05 differ from control, MPTP, and MPTP+ferulic acid (40 mg/kg and 80 mg/kg body weight). $Significantly p < 0.05 differ from control, MPTP, and MPTP+ferulic acid (20 mg/kg and 80 mg/kg body weight) groups. †Significantly p < 0.05 differ from control, MPTP, and MPTP+ferulic acid (20 mg/kg and 40 mg/kg body weight) groups.
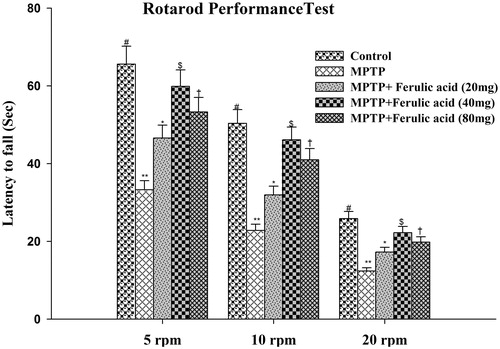
To evaluate MPTP-induced impairment of motor coordination, mice were subjected to RRT performance (the time spent on the rotating rod) at 5, 10, and 20 rpm. The average time taken by the mice to fall in each experimental group is plotted against different rpm as shown in . MPTP alone-treated mice showed decreased retention time as compared with control mice. FA-supplemented MPTP-treated mice showed significantly increased retention time which is more pronounced at 40 mg/kg body weight.
Figure 2. Rotarod performance of experimental mice: values are given as mean ± SD for six mice in each group. Error bars sharing common symbol do not differ significantly at p < 0.05. #Significantly p < 0.05 differ from MPTP and MPTP + ferulic acid-treated groups. **Significantly p < 0.05 differ from control and MPTP + ferulic acid groups. *Significantly p < 0.05 differ from control, MPTP, and MPTP+ferulic acid (40 mg/kg and 80 mg/kg body weight). $Significantly p < 0.05 differ from control, MPTP, and MPTP+ferulic acid (20 mg/kg and 80 mg/kg body weight) groups. †Significantly p < 0.05 differ from control, MPTP, and MPTP+ferulic acid (20 mg/kg and 40 mg/kg body weight) groups.
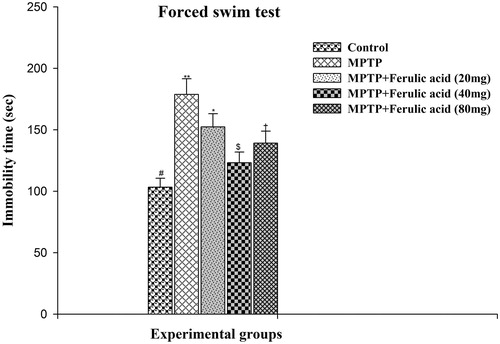
The effect of FA and/or MPTP on FST performance of experimental mice is shown in . As compared with MPTP alone-administered mice, the mean immobility time spent by MPTP-treated mice supplemented with FA was significantly reduced at different doses namely 20, 40, and 80 mg. The reduction in immobility period of mice treated with FA at a dose of 40 mg/kg body weight differs more significantly from the other two doses of FA.
Figure 3. Forced swim test performance of experimental mice: values are given as mean ± SD for six mice in each group. Error bars sharing common symbol do not differ significantly at p < 0.05. #Significantly p < 0.05 differ from MPTP and MPTP + ferulic acid-treated groups. **Significantly p < 0.05 differ from control and MPTP + ferulic acid groups. *Significantly p < 0.05 differ from control, MPTP, and MPTP+ferulic acid (40 mg/kg and 80 mg/kg body weight). $Significantly p < 0.05 differ from control, MPTP, and MPTP+ferulic acid (20 mg/kg and 80 mg/kg body weight) groups. †Significantly p < 0.05 differ from control, MPTP, and MPTP+ferulic acid (20 mg/kg and 40 mg/mg/kg body weight) groups.
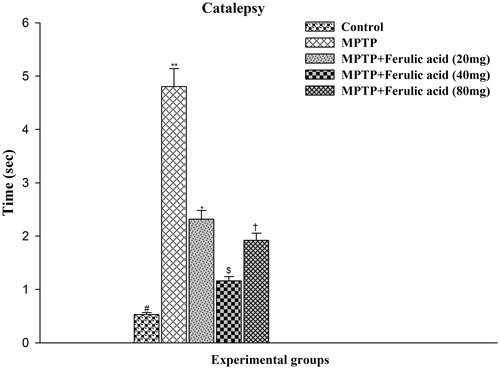
The time taken to correct the unusual posture imposed on animal is regarded as an index of the intensity of CAT. As shown in , control rats did not induce notable signs of CAT whereas MPTP-administered mice produced significant cataleptic response. The duration of CAT was significantly (p < 0.05) reduced by the prophylactic treatment of FA at different doses namely 20, 40, and 80 mg. Findings of this study showed that the duration of CAT was shorter at a dose of 40 mg/kg which is significantly different when compared with other two doses of FA (i.e., 20 mg/kg and 80 mg/kg body weight) but it do not differ significantly as compared with control mice reflecting its optimum protective effect.
Figure 4. Catalepsy test performance in experimental mice: Values are given as mean ± SD for six mice in each group. Error bars sharing common symbol do not differ significantly at p < 0.05. #Significantly p < 0.05 differ from MPTP and MPTP + ferulic acid-treated groups. **Significantly p < 0.05 differ from control and MPTP + ferulic acid groups. *Significantly p < 0.05 differ from control, MPTP, and MPTP+ferulic acid (40 mg/kg and 80 mg/kg body weight). $Significantly p < 0.05 differ from control, MPTP, and MPTP+ferulic acid (20 mg/kg and 80 mg/kg body weight) groups. †Significantly p < 0.05 differ from control, MPTP, and MPTP+ferulic acid (20 mg/kg and 40 mg/kg body weight) groups.
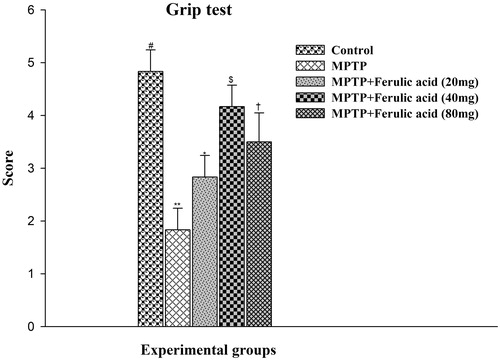
The grip test is used to measure the maximal muscle strength of forelimbs and combined forelimbs and hind limbs as a primary phenotype screen. As shown in , the mean score for grip test in MPTP alone-administered mice showed a significant decrease (p < 0.05) in motor strength as compared with control mice. FA supplementation significantly improves the motor strength as evidenced by the obtained mean score. FA supplementation at 40 mg/kg body weight significantly (p < 0.05) scored the optimum protective effect.
Figure 5. Grip test performance in experimental mice: Values are given as mean ± SD for six mice in each group. Error bars sharing common symbol do not differ significantly at p < 0.05. #Significantly p < 0.05 differ from MPTP and MPTP + ferulic acid-treated groups. **Significantly p < 0.05 differ from control and MPTP + ferulic acid groups. *Significantly p < 0.05 differ from control, MPTP, and MPTP + ferulic acid (40 mg/kg and 80 mg/kg body weight). $Significantly p < 0.05 differ from control, MPTP, and MPTP + ferulic acid (20 mg/kg and 80 mg/kg body weight) groups. †Significantly p < 0.05 differ from control, MPTP, and MPTP+ferulic acid (20 mg/kg and 40 mg/kg body weight) groups.
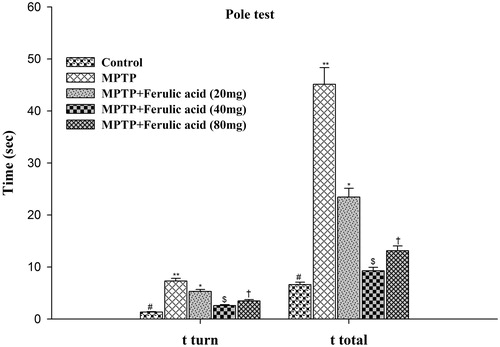
Pole test is a useful method for evaluating the mouse movement disorder caused by cortical damage. The time to orient down (t-turn) and the total time to descend the pole (t-total) were measured in experimental mice and the data are represented in . Statistical analysis of ANOVA and post hoc run by DMRT showed that the mean score taken by each experimental group for both parameters namely t-turn and t-total differ significantly at p < 0.05. FA supplementation to MPTP mice at a dose of 40 mg/kg body weight showed optimum improvement in pole test performance as compared with other two doses of FA supplementation.
Figure 6. Pole test performance in experimental mice: Values are given as mean ± SD for six mice in each group. Error bars sharing common symbol do not differ significantly at p < 0.05. #Significantly p < 0.05 differ from MPTP and MPTP + ferulic acid-treated groups. **Significantly p < 0.05 differ from control and MPTP + ferulic acid groups. *Significantly p < 0.05 differ from control, MPTP, and MPTP + ferulic acid (40 mg/kg and 80 mg/kg body weight). $Significantly p < 0.05 differ from control, MPTP, and MPTP+ferulic acid (20 mg/kg and 80 mg/kg body weight) groups. †Significantly p < 0.05 differ from control, MPTP, and MPTP+ferulic acid (20 mg/kg and 40 mg/kg body weight) groups.
FA prevents pathological alterations and neuronal cell death
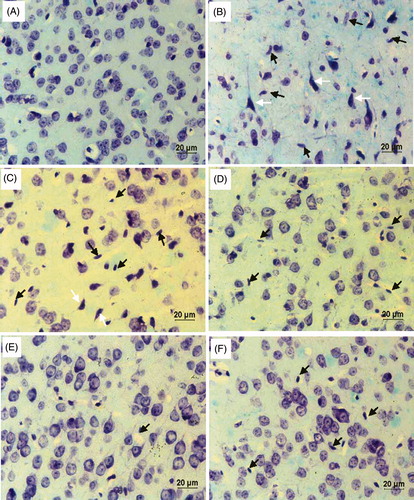
Luxol fast blue-cresyl violet (LFB-CV) staining is used to characterize the histology of SNpc and the representative photomicrographs are shown in . MPTP-injected mice showed a greater neuronal cell loss as compared with control mice. Further appearance of nuclear pyknosis and glial cell recruitment in the SNpc of MPTP administered mice reveals the onset of inflammation and apoptosis in dopaminergic neurons. Reduced number of glial cells and increased number of intact neuronal cells in FA-supplemented MPTP mice substantiate its neuroprotective effect.
Figure 7. Luxol fast blue-cresyl violet – coronal section; brain (40X): (A) substantia nigra (SN) of control mice showing normal histology. (B) and (C) SN of MPTP alone-administered mice showing loss of neurons along with nuclear pyknosis (white arrow) and increase in the number of spindle-shaped microglial cells (black arrow) indicating inflammatory response. (D–F) Coronol sections of ferulic acid (FA) supplemented MPTP-intoxicated mice at different doses 20 mg, 40 mg, and 80 mg/b.w., respectively, showing decreased microglial cells and increased intact neuronal cells.
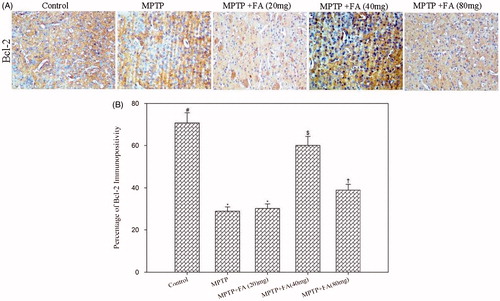
Immunostaining of Bax and Bcl2 in the neuronal cells is shown in and . Mice that received the MPTP injection showed an increased Bax/Bcl2 ratio as compared with control mice (2.38 versus 0.1) reflecting neuronal cell death and neurodegeneration. As compared with MPTP alone-administered mice, MPTP + FA (20, 40, and 80 mg/kg body weight) mice showed decreased Bax/Bcl2 ratio (1.26 versus 0.16 versus 0.92, respectively). The expression of antitapoptotic protein Bcl2 in the low dose FA (20 mg/kg body weight) supplemented group was not significantly different from the MPTP alone-administered group. However, FA supplementation at a dose of 40 and 80 mg/kg body weight remarkably increases the Bcl2 expression. The expression of proapoptotic protein Bax was significantly reduced by all the three tested doses but the more pronounced effect was observed at 40 mg/kg body weight. Thus, a differential dose–response effect of FA was observed with respect to apoptosis. Altogether, it is depicted that FA supplementation reduced the nigrostriatal dopaminergic neuron loss in MPTP-administered mice by alleviating MPP+-enhanced Bax/Bcl2 ratio.
Figure 8. (A) Immunohistochemistry of Bax (20×). (B) Quantification of Bax expression. Values are given as mean ± SD for six mice in each group. Error bars sharing common symbol do not differ significantly at p < 0.05. #Significantly p < 0.05 differ from MPTP and MPTP + ferulic acid-treated groups. *Significantly p < 0.05 differ from control and MPTP+ferulic acid-treated groups. †Significantly p < 0.05 differ from control, MPTP, and MPTP+ferulic acid (80 mg/kg bodyweight) groups. $Significantly p < 0.05 differ from control, MPTP, and MPTP+ferulic acid (20 mg/kg and 40 mg/kg bodyweight) groups.
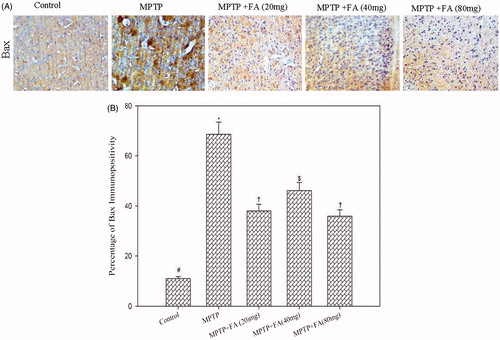
Figure 9. (A) Immunohistochemistry of Bcl-2 (20×). (B) Quantification of Bcl-2 expression. Values are given as mean ± SD for six mice in each group. Error bars sharing common symbol do not differ significantly at p < 0.05. #Significantly p < 0.05 differ from MPTP and MPTP + ferulic acid-treated groups. *Significantly p < 0.05 differ from control and MPTP + ferulic acid (40 mg/kg and 80 mg/kg body weight) groups. $Significantly p < 0.05 differ from control, MPTP, and MPTP+ferulic acid (20 mg/kg and 80 mg/kg body weight) groups. †Significantly p < 0.05 differ from control, MPTP, and MPTP+ferulic acid (20 mg/kg and 40 mg/kg body weight) groups.
Discussion
Epidemiological studies of human populations, and experiments in animal models of neurodegenerative disorders, have provided evidence that phytochemicals in fruits and vegetables can protect the nervous system against disease (Joseph et al., Citation2005; Liu, Citation2003). Oxidative stress has been implicated in mechanisms leading to neuronal cell injury in various pathological states of the brain, including PD. Hence phytochemical-based antioxidants may have neuroprotective (preventing apoptosis) and neuroregenerative roles by reducing or reversing cellular damage and by slowing progression of neuronal cell loss. Luo et al. (Citation2012) reported that FA a phenolic plant antioxidant could alleviate Vascular Dementia rats' learning and memory deficits, which might be due to antioxidation, the improvement of cholinergic system in brain, or the inhibitory of nerve injury by excitatory amino acids. In line with these findings, the present study explored the protective effect of FA against MPTP-induced neurotoxicity.
Behavior has been defined as the net sensorimotor and integrative processes occurring in the nervous system. An alteration in behavior might be a relatively sensitive indicator of exposure, since behavior reflects the coordinated function of a large portion of the neural network. Behavioral endpoints are generally non-invasive and can be used to assess subjects repeatedly during the course of an experiment (Haschek et al., Citation2009). Endpoints used commonly in MPTP neurotoxicity include motor activities as detected by OFT, RRT, FST, CAT, grip test, and pole test.
OFT is a widely used to measure exploratory behavior and general stimulant effects of compounds. Line crossing, central square entries, central square duration, rearing, stretch attend posture, grooming, freezing, urination, and defecation are among many measures that are reported. Thus, the OFT measures a number of facets of behavior beyond simple locomotion (Gould et al., Citation2009). In the present OFT, four parameters namely frequencies of line crossings and rearings (as measures of locomotor activity), grooming (as a measure of displacement response), and central square entries (as a measure of exploratory behavior) were evaluated. RRT evaluates the global motor coordination in PD models (Stachowiak et al., Citation1984) and may be dependent on coordinated action of both intact and non-lesioned nigrostriatal pathways. Significantly decreased frequencies in OFT parameters and decreased time to remain on the accelerating rotarod were observed in MPTP alone-administered mice. Oral supplementation of FA at different doses to MPTP-administered mice improved these motor deficits and induced a mild to moderate but significantly improved the performance in OFT and RRT specifically at a dose of 40 mg/kg body weight. MPTP by generating MPP+ could elicite nigral DA neuron loss and thereby slow down motor function. As a potent antioxidant, FA could counteract the neurotoxicity by attenuating the formation of MPP+. Our present findings are in agreement with the earlier reports that DA level and motor deficits in Parkinsonian mice have been attenuated by antioxidant supplementation (Chung et al., Citation2011; Khan et al., Citation2010; Moon et al., Citation2009).
FST is a test of forced motor behavior developed to predict the efficacy of antidepressant drugs (Lucki, Citation1997). When mice are forced to swim in a deep cylinder with tepid water, they become nearly immobile and cease trying to escape. Significant increase in immobility time observed in MPTP alone-administered mice reflects behavioral depression. Significantly increased score of active behaviors like swimming and climbing in FA-supplemented MPTP mice clearly showed its influence on the neurotransmitter system that controls the active behaviors (Cryan et al., Citation2005). Zeni et al. (Citation2012) demonstrated that FA exerts antidepressant-like effect in the FST and tail suspension test (TST) in mice through modulation of the serotonergic system. CAT is a state of behavioral immobility characterized by muscle rigidity and failure to correct an externally imposed posture for a prolonged period of time. CAT is of important clinical relevance because of its similarity to behaviors seen in human disorders such as PD and schizophrenia. Increased cataleptic (postural immobility) time in MPTP-treated mice also reflects the hypofunctioning of the nigrostriatal dopaminergic system. The Grip test examines the abnormalities in balance and grip strengths. Significantly reduced score obtained by the MPTP-injected mice showed significant loss in motor coordination and neuromuscular strength which was improved by FA supplementation.
To confirm motor deficits due to nigrostriatal dysfunction, the pole test was conducted. This test monitors the forepaw dexterity of mice and is reported to be highly correlated with striatal DA content (Matsuura et al., Citation1997). MPTP alone-injected mice took significantly more time to descend than the control which could be due to decreased striatal DA content. FA supplementation improved MPTP-induced bradykinesia reflecting its ability to maintain the striatal DA content by attenuating MPTP-induced neurotoxicity. Hence, in the present study, pre-treatment of FA a polyphenolic antioxidant could prevent the formation of MPP+ and thereby offer neuronal protection against MPTP. Our reports are in consistent with Mamiya et al. (Citation2008) who has shown that FA (5 mg/kg subcutaneously for 6 d) reduced brain damage from oxidative stress. But still whether FA supplementation potentiates the levels of endogenous antioxidants in MPTP administered mice has to be investigated.
Activated microglia has been shown to play an essential role in MPTP-induced neurotoxicity and microglial activation is usually triggered by NADPH-oxidase activity as a consequence of ROS production (Gao et al., Citation2002; Wu et al., Citation2003). To substantiate the protective effect of FA against MPTP-induced neurotoxicity, coronal sections were stained for glial cell activation by LFB-CV staining. Appearance of pyknosis (i.e., irreversible condensation of chromatin in the nucleus of the cells undergoing necrosis and apoptosis) and increased number of microglial cells in the SNpc region of MPTP-intoxicated mice reflect neural damage and inflammation. The density of these glial cells was dramatically decreased in the SNpc of FA-supplemented MPTP-administered mice. Thus FA inhibited microglial activation and thereby protected DA neurons against MPTP-induced neurotoxicity.
Another downstream result of oxidative stress is apoptosis which has also been implicated in the pathophysiology of PD (Varçin et al., Citation2012). Components of signaling pathways that initiate cell death are highly concentrated in vulnerable substantia nigra (SN) neurons and may, therefore, contribute to the relentless demise of DA cells (Horowitz et al., Citation2003). Preemptive treatment with antioxidants to antagonise inflammation and/or oxidative stress is a way to minimize signs of apoptosis and neuronal loss in degenerative conditions. In line with this, the findings of the present study showed that FA supplementation enhanced Bcl2 expression and reduced Bax expression with a concomitant decrease in Bax/Bcl2 ratio favoring anitapoptotic mechanisms in the SNpc region substantiating its neuroprotective action. Although the exact reason behind the differential apoptotic response to different doses of FA remains obscure, it would still be speculated that the higher concentration of FA (80 mg/kg body weight) might have resulted in the production of by-products that are interfering with the free radical scavenging activity and thereby its efficacy. The mechanism by which FA interacts with signaling components of apoptosis was demonstrated by Yang et al. (Citation2007) stating that mitochondrial release of cytochrome c is an initiating step in the apoptotic pathway, and this initiating activity is reportedly decreased when bound to stabilized FA. However, whether FA exerts its effect on mitochondrial release of cytochrome c in MPTP neurotoxicity model has to be explored. Of the three tested doses, 20 mg/kg body weight does not show much protective effect which might be due insufficient concentration of FA to exert the desired protection. Although FA exerted significant neuroprotective effect both at 40 and at 80 mg/kg body weight, the effect was more pronounced at 40 mg/kg body weight rather than 80 mg/kg body weight. This is because higher concentration of FA (80 mg/kg body weight) might have resulted in the production of by-products that are interfering with the antioxidant activity, and consequently, decreasing its effect on MPTP detoxification and neuroprotection.
Conclusion
To conclude, our findings demonstrated that FA attenuated the formation of MPP+ and thereby altered Bax/Bcl2 ratio exerting antiapoptotic effect in the SNpc region. These synergistic antioxidant and antiapoptotic effects of FA improve motor balance and coordination in mice challenged with MPTP. Our findings provide elementary evidence for the neuroprotective action of FA against MPTP-induced PD in mice and represent FA as a promising candidate for further elaborative preclinical research in order to develop it as a potent neuroprotective agent in PD patients.
Acknowledgements
The authors thank Dr. S. Swaminathan (Director, CeNTAB), Dr. S. Panchapekesan (Cooridinator, Central Animal Facility) and Dr. P. Brinda (Dean, CARISM) of SASTRA University for extending their great support to perform the study.
Declaration of interest
This study is supported by the T. R. Rajagopalan seed money grant of SASTRA University, Thanjavur, Tamil Nadu, India.
References
- Ardiansyah A, Ohsaki Y, Shirakawa H, et al. (2008). Novel effects of a single administration of ferulic acid on the regulation of blood pressure and the hepatic lipid metabolic profile in stroke-prone spontaneously hypertensive rats. J Agric Food Chem 56:2825–30
- Benetti F, Gustincich S, Legname G. (2012). Gene expression profiling and therapeutic interventions in neurodegenerative diseases: A comprehensive study on potentiality and limits. Expert Opin Drug Discov 7:245–59
- Brown RE, Corey SC, Moore AK. (1999). Differences in measures of exploration and fear in MHC-congenic C57BL/6J and B6-H-2K mice. Behav Genet 29:263–71
- Chung YC, Kim SR, Park JY, et al. (2011). Fluoxetine prevents MPTP-induced loss of dopaminergic neurons by inhibiting microglial activation. Neuropharmacology 60:963–74
- Cryan JF, Valentino RJ, Lucki I. (2005). Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev 29:547–69
- Dauer W, Przedborski S. (2003). Parkinson's disease: Mechanisms and models. Neuron 39:889–909
- Fahn S. (2003). Description of Parkinson's disease as a clinical syndrome. Ann N Y Acad Sci 991:1–14
- Ferré S, Guix T, Prat G, et al. (1990). Is experimental catalepsy properly measured? Pharmacol Biochem Behav 35:753–7
- Gao HM, Jiang J, Wilson B, et al. (2002). Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: Relevance to Parkinson's disease. J Neurochem 81:1285–97
- Gould TD, Dao DT, Kovacsics CE. (2009). The open field test. In: Gould TD, ed. Mood and Anxiety Related Phenotypes in Mice. Neuromethods. New York: Humana Press, 1–20
- Haschek WM, Rousseaux CG, Wallig WA. (2009). Fundamentals of Toxicologic Pathology, 2nd ed. San Diego: Academic Press
- Horowitz JM, Pastor DM, Goyal A, et al. (2003). BAX protein-immunoreactivity in midbrain neurons of Parkinson's disease patients. Brain Res Bull 62:55–61
- Jackson-Lewis V, Przedborski S. (2007). Protocol for the MPTP mouse model of Parkinson's disease. Nat Protoc 2:141–51
- Joseph JA, Bartus RT, Clody DE. (2005). Reversing the deleterious effects of aging on neuronal communication and behavior: Beneficial properties of fruit polyphenolic compounds. Am J Clin Nutr 81:313S–16
- Khan MM, Hoda MN, Ishrat T, et al. (2010). Amelioration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced behavioural dysfunction and oxidative stress by Pycnogenol in mouse model of Parkinson's disease. Behav Pharmacol 21:563–71
- Khuwaja G, Khan MM, Ishrat T, et al. (2011). Neuroprotective effects of curcumin on 6-hydroxydopamine-induced Parkinsonism in rats: Behavioral, neurochemical and immunohistochemical studies. Brain Res 1368:254–63
- Kim HS, Cho JY, Kim DH, et al. (2004). Inhibitory effects of long-term administration of ferulic acid on microglial activation induced by intracerebroventricular injection of beta-amyloid peptide (1-42) in mice. Biol Pharm Bull 27:120–1
- Liu RH. (2003). Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr 78:517S–20
- Lucki I. (1997). The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol 8:523–32
- Luo Y, Zhao HP, Zhang J, et al. (2012). Effect of ferulic acid on learning and memory impairments of vascular dementia rats and its mechanism of action. Yao Xue Xue Bao 47:256–60
- Mamiya T, Kise M, Morikawa K. (2008). Ferulic acid attenuated cognitive deficits and increase in carbonyl proteins induced by buthionine-sulfoximine in mice. Neurosci Lett 430:115–18
- Mancuso C, Santangelo R. (2014). Ferulic acid: Pharmacological and toxicological aspects. Food Chem Toxicol 65:185–95
- Martínez-Banaclocha MA. (2012). N-acetyl-cysteine in the treatment of Parkinson's disease. What are we waiting for? Med Hypotheses 79:8–12
- Matsuura K, Kabuto H, Makino H, Ogawa N. (1997). Pole test is a useful method for evaluating the mouse movement disorder caused by striatal dopamine depletion. J Neurosci Methods 73:45–8
- Moon M, Kim HG, Hwang L, et al. (2009). Neuroprotective effect of ghrelin in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease by blocking microglial activation. Neurotox Res 15:332–47
- Nakamura K, Bindokas VP, Marks JD, et al. (2000). The selective toxicity of 1-methyl-4-phenylpyridinium to dopaminergic neurons: The role of mitochondrial complex I and reactive oxygen species revisited. Mol Pharmacol 58:271–8
- Ogawa N, Hirose Y, Ohara S, et al. (1985). A simple quantitative bradykinesia test in MPTP-treated mice. Res Commun Chem Pathol Pharmacol 50:435–41
- Porsolt RD, Bertin A, Jalfre M. (1977). Behavioral despair in mice: A primary screening test for antidepressants. Arch Int Pharmacodyn Ther 229:327–36
- Pothakos K, Kurz MJ, Lau YS. (2009). Restorative effect of endurance exercise on behavioral deficits in the chronic mouse model of Parkinson's disease with severe neurodegeneration. BMC Neurosci 10:6
- Prabhakar PK, Prasad R, Ali S, Doble M. (2013). Synergistic interaction of ferulic acid with commercial hypoglycemic drugs in streptozotocin induced diabetic rats. Phytomedicine 20:488–94
- Rappold PM, Tieu K. (2010). Astrocytes and therapeutics for Parkinson's disease. Neurotherapeutics 7:413–23
- Rozas G, López-Martín E, Guerra MJ, Labandeira-García JL. (1998). The overall rod performance test in the MPTP-treated-mouse model of Parkinsonism. J Neurosci Methods 83:165–75
- Sedelis M, Schwarting RK, Huston JP. (2001). Behavioral phenotyping of the MPTP mouse model of Parkinson's disease. Behav Brain Res 125:109–25
- Singh S, Singh K, Patel DK, et al. (2009). The expression of CYP2D22, an ortholog of human CYP2D6, in mouse striatum and its modulation in 1-methyl 4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson's disease phenotype and nicotine-mediated neuroprotection. Rejuvenation Res 12:185–97
- Stachowiak MK, Bruno JP, Snyder AM, et al. (1984). Apparent sprouting of striatal serotonergic terminals after dopamine-depleting brain lesions in neonatal rats. Brain Res 291:164–7
- Stefanis L. (2012). α-Synuclein in Parkinson's disease. Cold Spring Harb Perspect Med 2:a009399
- Sultana R. (2012). Ferulic acid ethyl ester as a potential therapy in neurodegenerative disorders. Biochim Biophys Acta 1822:748–52
- Trombino S, Cassano R, Ferrarelli T, et al. (2013). Trans-ferulic acid-based solid lipid nanoparticles and their antioxidant effect in rat brain microsomes. Colloids Surf B Biointerfaces 109:273–9
- Varçin M, Bentea E, Michotte Y, Sarre S. (2012). Oxidative stress in genetic mouse models of Parkinson's disease. Oxid Med Cell Longev 2012:624925
- Wu DC, Teismann P, Tieu K, et al. (2003). NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. Proc Natl Acad Sci USA 100:6145–50
- Yan JJ, Jung JS, Kim TK, et al. (2013). Protective effects of ferulic acid in amyloid precursor protein plus presenilin-1 transgenic mouse model of Alzheimer disease. Biol Pharm Bull 36:140–3
- Yang F, Zhou BR, Zhang P, et al. (2007). Binding of ferulic acid to cytochrome c enhances stability of the protein at physiological pH and inhibits cytochrome c-induced apoptosis. Chem Biol Interact 170:231–43
- Yasuda T, Nakata Y, Mochizuki H. (2013). α-Synuclein and neuronal cell death. Mol Neurobiol 47:466–83
- Zeni AL, Zomkowski AD, Maraschin M, et al. (2012). Ferulic acid exerts antidepressant-like effect in the tail suspension test in mice: Evidence for the involvement of the serotonergic system. Eur J Pharmacol 679:68–74
