Abstract
Context: Cipura paludosa Aubl. (Iridaceae) is widely used in folk medicine to treat several ailments. Experimental studies have confirmed its anti-inflammatory, antinociceptive, and neuroprotective effects.
Objective: This study evaluates the possible antiproliferative potential of the crude methanol extract and three isolated compounds from the bulbs of C. paludosa.
Materials and methods: Phytochemical analysis was carried out by conventional chromatographic techniques, and the resulting compounds were identified by NMR 1H and 13C. The antiproliferative activity was analysed using the sulforhodamine B assay.
Results: Crude methanol extract of C. paludosa bulbs showed GI50 values of between 1.6 and 30.8 μg/mL. The naphthoquinone derivatives (eleutherine, isoeleutherine, and eleutherol) isolated from the bulbs of C. paludosa exhibited promising cytotoxicity against several human tumour cell lines, especially the two main compounds, eleutherine and isoeleutherine, against glioma and breast cancer cell lines, with TGI values of between 2.6 and 13.8 μg/mL.
Conclusion: Cipura paludosa bulbs produce active principles with relevant antiproliferative potential, such as naphthoquinone derivatives, identified as eleutherine, isoeleutherine, and eleutherol. This is the first report indicating C. paludosa with antiproliferative potential.
Introduction
Plants provide a source of medicines for the treatment of a wide spectrum of diseases, including several kinds of cancer. They have a long history of use in the treatment of cancer, serving as a source of therapeutic agents for many centuries, many of which are still in use (Cragg and Newman Citation2013; Cragg et al. Citation2014).
Cipura paludosa Aubl. (Iridaceae), a native Brazilian plant found in the Amazon forest in the north of Brazil, is popularly known as “alho-do-mato”, “batata roxa”, and “cebolinha-do-campo” (Tessele et al. Citation2011).
This plant has been used in traditional medicine to treat several disorders, such as inflammation, infections, renal tract disorders, and pain (Lucena et al. Citation2013). Studies indicate a number of biological activities of C. paludosa, including anti-inflammatory, anti-nociceptive, and neuroprotective effects (Lucena et al. Citation2007, Citation2010; Silva Neto et al. Citation2014).
From the bulbs of this plant, four derivatives of naphthalene have been isolated and identified: three known compounds (eleutherine, isoeleutherine, and hongkonin) and a recently isolated new component (11-hydroxyeleutherine). It has been shown that the two main compounds of this plant (eleutherine and isoeleutherine) exhibited pronounced activity in different in vivo models of inflammation and hypernociception (Tessele et al. Citation2011).
In a screening with several Brazilian plants, as part of a project conducted by Network Ribecancer 212RT 0464 (CYTED/CNPq), it was verified that the extracts of C. paludosa bulbs exhibited promising cytotoxic profile. Thus, this study evaluates the antiproliferative activity of the crude methanol extract and three naphthoquinone derivatives (eleutherine, isoeleutherine, and eleutherol) isolated from C. paludosa bulbs.
Materials and methods
Plant material
Cipura paludosa was cultivated and collected in the town of Itajaí (SC, Brazil), in May 2013 and identified by Dr. Oscar B. Iza (University of Itajaí Valley). A voucher specimen was deposited at the Barbosa Rodrigues Herbarium (Itajaí-SC) under number VC Filho 108.
Extraction and isolation
Bulbs and roots of C. paludosa were macerated with methanol at room temperature for a period of 1 week. After filtration, the solvent was removed by evaporation under reduced pressure, affording the crude methanol extract CME. A screening test was conducted with these extracts to evaluate the possible antiproliferative activity. Since the bulbs exhibited the best promising biological activity, these were studied in more detail.
Fresh bulbs (1 kg) of C. paludosa were cut into small pieces and macerated with methanol at room temperature for approximately 7 d, providing the crude methanol extract (CME) after solvent evaporation (28 g; a yield of 2.8%).
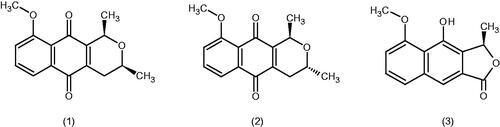
A part of CME (13 g) was fractionated over silica gel (60 g) on column chromatography, eluting with a 200 mL gradient of hexane and hexane:ethyl acetate (pure hexane, 90:10, 80:20, 70:30, 50:50, and pure ethyl acetate). Fractions of 10 mL were collected and evaluated by thin layer chromatography (TLC) profiles, using hexane:ethyl acetate (80:20) as the mobile phase. Spots were visualised by UV (254 nm) and reaction with sulphuric anisaldehyde heated at 100 °C. Fractions 16–27 (635 mg), which exhibited the same chromatography profile by TLC, were purified by another column chromatography, furnishing 23 mg of pure eleutherine (1) and 25 mg of iso-eleutherine (2), already isolated from the bulbs of C. paludosa (Tessele et al. Citation2011). Fractions 11–20 (83 mg) from the second column exhibited the same chromatography profile by TLC and were purified by flash chromatography, furnishing 14 mg of eleutherol (3), which was identified by spectroscopic data (NMR 1H and 13C) and comparison with of the literature (Hara et al. Citation1997).
Nuclear magnetic resonance
For structural elucidation of the isolated compounds, usual spectroscopic data were used, as Hydrogen Nuclear Magnetic Resonance (1H-NMR) and carbon 13 (13C -NMR). Both techniques were performed on BRUKER AV-300 and 300 MHz spectrometer (Bruker BioSpin Corporation, Billerica, MA), with tetramethylsilane (TMS) as an internal standard.
In vitro anticancer activity assay
Human tumour cell lines of U-251 (glioma), MCF-7 (breast), NCI/ADR-RES (ovary expressing multi-drug resistance phenotype), 786-0 (kidney), NCI-H460 (lung, non-small cells), HT-29 (colon), and K562 (leukemia) were kindly provided by the NCI, USA. Non-tumour cell line HaCat (human keratinocytes) was donated by Prof. Dr. Ricardo Della Coletta, FOP/UNICAMP. Stock cultures were grown in medium containing 5 mL RPMI 1640 (GIBCO BRL) supplemented with 5% fetal bovine serum. Penicillin:streptomycin (1000 lg/L: 1000 U/L, 1 mL/L) was added to the experimental cultures. Cells in 96-well plates (100 μL cells well−1) were exposed to sample concentrations in DMSO/RPMI (0.25, 2.5, 25, and 250 μg mL−1) at 37 °C, 5% CO2 in air for 48 h. The final DMSO concentration did not affect cell viability. Afterwards, cells were fixed with 50% trichloroacetic acid and cell growth was determined by spectrophotometry (540 nm) of cellular protein content using the sulforhodamine B assay (Monks et al. Citation1991). Doxorubicin chloridrate (0.1 mg/mg; Europharma) was used as a positive control. The concentration–response curve for each cell line and the total growth inhibition (TGI) values were determined through non-linear regression analysis, using the software ORIGIN 8.0 (OriginLab Corporation) (Shoemaker Citation2006).
Results
Although several experimental studies have confirmed the biological activities of C. paludosa, no reports were found for the antiproliferative activity.
The bulbs were the more active tested extract, demonstrating an interesting profile for all the human cancer cell lines tested, with selectivity for lung cancer cells. This extract showed GI50 (50% growth inhibition) values of between 1.6 and 30.8 μg/mL (). The roots also demonstrated some antiproliferative action, but not as much as for the bulbs, and for this reason, the studies were focused on the bulbs.
Table 1. Antiproliferative activity of doxorubicin (positive control) and the crude methanolic extract of bulbs and roots from C. paludosa against human cancer cell linesTable Footnotea. GI50 (μg/mL)Table Footnoteb.
After this initial analysis, a new collection of the bulbs of the plant was carried out for more detailed studies. Thus, a bio-guided phytochemical study was performed seeking to determine the active principles. Three pyranonaphthoquinone derivatives () were isolated and identified, eleutherine (1) and isoeleutherine (2), already isolated from the bulbs of this plant (Tessele et al. Citation2011), and others of the genus Eleutherine (Hong et al. Citation2008) and eleutherol (3), which was isolated from Eleutherine americana Aubl. (Iridaceae) bulbs (Hara et al. Citation1997), but reported here for the first time in C. paludosa.
Eleutherine and isoeleutherine demonstrated pronounced antiproliferative activity with cytocidal effect, especially against glioma (U251) and breast (MCF-7) cancer cell lines, with TGI values of between 2.6 and 13.8 μg/mL (). According to Fouche et al. (Citation2008), TGI values of between 6.25 and 15 μg/mL represent moderate activity, and values of less than 6.25 μg/mL indicate potent activity, demonstrating the pronounced activity of these two compounds
Table 2. Antiproliferative activity of doxorubicin (positive control), and the two main compounds from the bulbs of C. paludosa (eleutherine and isoeleutherine), against human cancer cell linesTable Footnotea. TGI (μg/mL)Table Footnoteb.
In general, eleutherine (1) was more active than isoeleutherine (2), especially against glioma (U251), being about four-fold more potent. These compounds are epimeric isomers and possess a naphtoquinone 1,4-moiety with only one structural difference, the methyl group β-methyl for 1 and α-methyl for 2. The results indicate that the better activity of 1 is related to the chirality at its pyran ring with the β-methyl group.
Eleutherol demonstrated cytostatic effect against all cancer cell lines testes, especially against glioma (U251), breast (MCF-7), and leukaemia (K562), inhibiting 50% of cell growth (values of GI50) ().
Table 3. Antiproliferative activity of doxorubicin (positive control) and eleutherol against human cancer cell linesTable Footnotea. GI50 (μg/mL)Table Footnoteb.
Discussion
The use of tumour cell lines allows investigators to test compounds under highly controlled and reproducible conditions. The availability of well-characterised cell lines enables parallel screening assays in which the effect of a drug candidate on proliferation of multiple tumour cell lines can be determined (HogenEsch and Nikitin Citation2012).
The National Cancer Institute (NCI) has identified the quinone group as an important pharmacologic element for cytotoxic activity, and naphthoquinones represent an important class of compounds with highlighted biological activities (Duchowicz et al. Citation2014). 1,4-Naphthoquinones are reported to have a variety of pharmacological properties, including anticancer activity (Kayashima et al. Citation2009; Kusuma et al. Citation2010; Bhasin et al. Citation2013).
The pyranonaphthoquinones 1 and 2 isolated from the bulbs of C. paludosa demonstrated previously anti-inflammatory and anti-hypernociceptive action against chemical and mechanical models of pain in mice (Tessele et al. Citation2011). It was also demonstrated that these two enhance short- and long-term memory in mice (Lucena et al. Citation2013) and have antifungal and immunomodulatory activities (Alves et al. Citation2003; Hong et al. Citation2008).
Eleutherine (1), the main isolated compound, also showed interesting inhibitory activity against human topoisomerase II, and compounds with this action can be considered as part of first-line chemotherapy for a wide variety of solid and hematological tumours (Hara et al. Citation1997; Kusuma et al. Citation2010). Eleutherol (3) has been isolated from the bulbs of Eleutherine bulbosa (Alves et al. Citation2003; Gallo et al. Citation2010). Some populations use this plant as a vermifuge (Schultes and Raffauf Citation1990), for intestinal disorders (Lin et al. Citation2002) and as an abortive and antifertility agent (Weniger et al. Citation1982). It is also the first time that this compound has been isolated from the bulbs of C. paludosa and, to the best of our knowledge, the first time its antiproliferative activity is reported.
This is the first report indicating C. paludosa with antiproliferative potential, showing that this species could be considered a potential source of compounds against cancer. Moreover, the naphthoquinone derivatives, eleutherine (1), isoeleutherine (2), and eleutherol (3) isolated from the bulbs of this plant, are the main active principles responsible for this antiproliferative activity. Studies are in progress to confirm these effects in other pharmacological and in vivo models.
Declaration of interest
The authors wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome. The authors are grateful to the Network Ribecancer 212RT 0464 (CYTED/CNPq), the National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq) and ProPPEC/UNIVALI for their financial support.
References
- Alves TM, Kloos H, Zani CL. 2003. Eleutherinone, a novel fungitoxic naphthoquinone from Eleutherine bulbosa (Iridaceae). Mem Inst Oswaldo Cruz. 98:709–712.
- Bhasin D, Chettiar SN, Etter JP, Mok M, Li PK. 2013. Anticancer activity and SAR studies of substituted 1,4-naphthoquinones. Bioorg Med Chem. 21:4662–4669.
- Cragg GM, Grothaus PG, Newman DJ. 2014. New horizons for old drugs and drug leads. J Nat Prod. 77:703–723.
- Cragg GM, Newman DJ. 2013. Natural products: a continuing source of novel drug leads. Biochim Biophys Acta 1830:3670–3695.
- Duchowicz PR, Bennardi DO, Bacelo DE, Bonifazi EL, Rios-Luci C, Padrón JM, Burton G, Misico RI. 2014. QSAR on antiproliferative naphthoquinones based on a conformation-independent approach. Eur J Med Chem. 77:176–184.
- Fouche G, Cragg GM, Pillay P, Kolesnikova N, Maharaj VJ, Senabe J. 2008. In vitro anticancer screening of South African plants. J Ethnopharmacol. 119:455–461.
- Gallo FR, Palazzino G, Federici E, Iurilli R, Galeffi C, Chifundera K, Nicoletti M. 2010. Polyketides from Eleutherine bulbosa. Nat Prod Res. 24:1578–1586.
- Hara H, Maruyama N, Yamashita S, Hayashi Y, Lee KH, Bastow KF, Chairul RM, Imakura Y. 1997. Elecanacin, a novel new naphthoquinone from the bulb of Eleutherine americana. Chem Pharm Bull. 45:1714–1716.
- Hogen Esch H, Nikitin AY. 2012. Challenges in pre-clinical testing of anti-cancer drugs in cell culture and in animal models. J Control Release. 164:183–186.
- Hong JH, Yu ES, Han AR, Nam JW, Seo EK, Hwang ES. 2008. Isoeleutherin and eleutherinol, naturally occurring selective modulators of Th cell-mediated immune responses. Biochem Biophys Res Commun. 371:278–282.
- Kayashima T, Mori M, Yoshida H, Mizushina Y, Matsubara K. 2009. 1,4-Naphthoquinone is a potent inhibitor of human cancer cell growth and angiogenesis. Cancer Lett. 278:34–40.
- Kusuma IW, Arung ET, Rosamah E, Purwatiningsih S, Kuspradini H, Syafrizal, Astuti J, Kim YU, Shimizu K. 2010. Antidermatophyte and antimelanogenesis compound from Eleutherine americana grown in Indonesia. J Nat Med. 64:223–226.
- Lin J, Puckree T, Mvelase TP. 2002. Anti-diarrhoeal evaluation of some medicinal plants used by Zulu traditional healers. J Ethnopharmacol. 79:53–56.
- Lucena GM, Gadotti VM, Maffi LC, Silva GS, Azevedo MS, Santos AR. 2007. Antinociceptive and anti-inflammatory properties from the bulbs of Cipura paludosa Aubl. J Ethnopharmacol. 112:19–25.
- Lucena GM, Matheus FC, Ferreira VM, Tessele PB, Azevedo MS, Cechinel-Filho V, Prediger RD. 2013. Effects of ethanolic extract and naphthoquinones obtained from the bulbs of Cipura paludosa on short-term and long-term memory: involvement of adenosine A1 and A2A receptors. Basic Clin Pharmacol Toxicol. 112:229–235.
- Lucena GM, Porto FA, Campos EG, Azevedo MS, Cechinel-Filho V, Prediger RD, Ferreira VM. 2010. Cipura paludosa attenuates long-term behavioral deficits in rats exposed to methylmercury during early development. Ecotoxicol Environ Saf. 73:1150–1158.
- Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, Hose C, Langley J, Cronise P, Vaigro-Wolff A, et al. 1991. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst. 83:757–766.
- Schultes RE, Raffauf RF. 1990. The healing forest: medicinal and toxic plants of the Northwest Amazonia. Portland, US: Dioscorides Press.
- Shoemaker RH. 2006. The NCI60 human tumour cell line anticancer drug screen. Nat Rev Cancer. 6:813–823.
- Silva Neto JAP, Menezes LD, Gomes GO, Cunha EMF, Azevedo MS, Ferreira VM, Da Silva MV 2014. Evaluation of antinociceptive and anti-inflammatory activities of the topical preparation of Cipura paludosa (Iridaceae). Acta Amaz. 44:263–270.
- Tessele PB, Delle Monache F, Quintão NLM, Da Silva GF, Rocha LW, Lucena GM, Ferreira VM, Prediger RD, Cechinel-Filho V 2011. A new naphthoquinone isolated from the bulbs of Cipura paludosa and pharmacological activity of two main constituents. Planta Med. 77:1035–1043.
- Weniger B, Haag-Berrurier M, Anton R. 1982. Plants of Haiti used as antifertility agents. J Ethnopharmacol. 6:67–84.