Abstract
Objectives. Plasma hyaluronan and syndecan-1 levels represent shedding of the endothelium glycocalyx during ischemia and edema. Diazoxide, a KATP-channel opener, has been shown to decrease myocardial edema during coronary artery bypass grafting (CABG). We evaluated whether Diazoxide exerts an impact on plasma hyaluronan and syndecan-1 levels during CABG. Design. Representative blood samples for hyaluronan and syndecan-1, before, during and after surgery, were obtained in 13 out of 16 patients that had a history of stable coronary artery disease undergoing CABG with or without Diazoxide. Electron microscopy from biopsies procured from the right atrium in 9 patients was performed to confirm ultrastructural differences among patients before and during CABG. Results. Ultrastructural differences were apparent between individual patients already before operation at base line reflecting differences in the severity of myocardial ischemia and edema. A significant decrease of hyaluronan and syndecan-1 values was observed in patients with Diazoxide after surgery (p < 0.04). Significant correlation of Plasma hyaluronan and syndecan-1 levels was observed in patients with Diazoxide but not in controls (p < 0.005, Spearman rank rho). Conclusion. Diazoxide may have an impact on levels of peripheral plasma hyaluronan and syndecan-1 after CABG, suggesting decreased shedding of the endothelial glycocalyx layer.
Introduction
Attempts to verify the beneficial effect of Diazoxide in protecting myocardial tissue after coronary artery bypass grafting (CABG) have not yet been confirmed clinically. Diazoxide, a mitochondrial adenosine triphosphate-sensitive potassium (KATP) channel opener, has nevertheless been shown in a few experimental studies to alleviate ischemia reperfusion injury and edema (Citation1,Citation2). Under clinic circumstances, patient heterogeneity before surgery makes it difficult to compare the outcome after CABG with or without Diazoxide, since base line tissue edema depending on the individual severity of ischemia confounds comparison among patients (Citation1,Citation3).
Endothelial glycocalyx shedding has emerged as a novel concept reflecting tissue injury after ischemia. The healthy vascular endothelium is covered by an endothelial glycocalyx layer mainly consisting of hyaluronan and syndecan glycoproteins, the shedding of which indicates vascular permeability resulting in tissue edema (Citation4). In contrast, preserving the endothelial glycocalyx and sustaining the vascular barrier reduces interstitial edema. The amount of shedded peripheral plasma hyaluronan and syndecan-1 values therefore mirrors endothelial glycocalyx integrity. The evaluation of these parameters may prove clinically practicable to interpret the state of endothelial glycocalyx after CABG (Citation5,Citation6).
We hypothesized that Diazoxide may preserve endothelial glycocalyx integrity during ischemia reperfusion and CABG. As we have shown earlier that Diazoxide alleviates edema in relation to histopathology, we wanted to confirm the mitochondrial state as interpreted by electron microscopy (EM) before and during surgery in order to understand the heterogeneity of patients depending on atrial tissue ischemia and edema preoperatively. We intended to investigate whether peripheral hyaluronan and syndecan-1 levels may differ after CABG in patients with Diazoxide as compared with nontreated controls.
Material and methods
Study population
After institutional approval by the Tampere University Hospital Ethics Committee, the protocol for this prospective randomized, double blind, placebo-controlled study was reviewed by the National Agency for Medicines, Finland. Sixteen patients offered their informed consent. The patients were scheduled for elective CABG using on pump cardiopulmonary bypass technique (CPB) and were divided into two groups: patients either received Diazoxide 50 μg/l injected into the aortic root at the onset of cross-clamping, or served as controls without additional medication (Citation7). The inclusion criteria included stable myocardial coronary artery disease eligible for elective CABG, but with a history of a recent less than one month old myocardial infarction and detection of elevated Troponin T (TNT > 0.01 μg/l) or Creatinin kinase (CK-MB > 1 U/l) release. Anesthesia was induced with propofol (0.5–1.0 mg/kg), sufentanil (0.6–0.8 μg/kg) and cis-atracurium, and Sufentanil infusion was continued with a rate of 0.03–0.05 μg/kg/min. Sevoflurane was used as the main anesthetic agent throughout the operation, which included standardized surgical techniques in every case.
Hemodynamic measurements
Technically, surgery was uneventful for all patients. As previously reported, there were no differences in either preoperative or postoperative hemodynamic parameters up to the following day after surgery (Citation7). These included measurement of preoperative and postoperative heart rate, mean arterial pressure, cardiac output, and calculated cardiac index.
Electron microscopy (EM)
For 9 patients (4 patients with Diazoxide and 5 controls), tissue harvesting for EM included 2 samples of right atrium. The first sample was obtained at cannulation of right atrium. The second sample was taken from the same location before CPB was terminated and decannulation. Muscle specimens were fixed in 2% glutaraldehyde in 0.1 M phosphate buffer, washed in 0.1 M phosphate buffer, dehydrated and embedded in Epoxy resin. The plastic embedded samples were sectioned for ultrathin sections, stained with Uranyl acetate and lead citrate, and examined with an Olympus-sis Morada digital camera (Olympus Soft Imaging Solutions, Munster, Germany). The state of tissue damage before and after CABG was verified blinded to the study protocol (SH, FZ) and technically unclear slides were rejected. The following signs of injury were noted separately: the presence of mitochondrial swelling indicating injury and the presence of preserved mitochondrial integrity before and during surgery.
Sample collection and Elisa for hyaluronan and syndecan-1
For evaluation of hyaluronan and syndecan-1, blood was collected at induction of anesthesia (Citation1), immediately after aortic clamp removal (Citation2), and 1 hour after surgery and closure of sternal skin wound (Citation3). All 3 samples at different time-points were available in 13 patients (6 controls and 7 in Diazoxide group). Plasma hyaluronan and syndecan-1 values were determined by enzyme linked immunosorbent assay (ELISA) by using reagents from R&D Systems Europe Ltd (Abingdon, UK). Detection limits were 123 pg/ml for hyaluronan and 62.5 pg/ml for syndecan-1.
Statistical analysis
Data is presented as median ± standard error of the mean (sem). Hyaluronan and syndecan-1 values were adjusted to the base line value for correlation by Spearman rank rho. Nonparametric data were analyzed with Kruskal-Wallis for various time points among groups, and Mann Whitney was used for comparison of two groups for each time points. Statistical significance was attributed to p-values lower than 0.05. Statistical analyses were performed with commercial statistical software (SPSS 19.0, SPSS Inc, Chicago, IL). Power calculation was set to display the 95% confidence interval and performed with statistical software (Power And Precision 4.0, Biostat, Englewood, NJ).
Results
EM
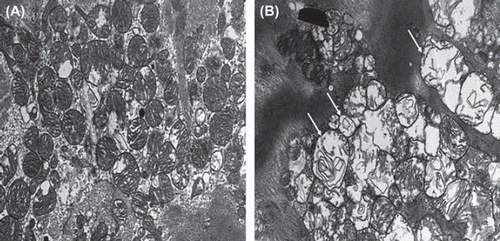
Before and during operation, edema was apparent in both Diazoxide-treated patients and in controls. The cardiomyocytes were often swollen with varying degrees of myofibrillar loss and secondary glycogen accumulates, as well as focal areas with contraction band degeneration. Swelling also involved the capillary endothelial cells. Marked mitochondrial changes due to the ischemic injury were observed in both the earlier and later obtained samples. However, these changes were more advanced in the later samples with most of the mitochondria in the fibres being swollen or damaged (). Major differences in these parameters of tissue edema between patients were observed already before operation at the base line and during operation, reflecting the severity of myocardial ischemia.
Hyaluronan and syndecan-1
For time points 1, 2 and 3, respectively, syndecan-1 values for controls were 6.5 ± 1.0 ng/ml, 8.5 ± 2.2 ng/ml and 5.6 ± 0.9 ng/ml, and for Diazoxide-treated patients 3.5 ± 0.7 ng/ml, 7.6 ± 0.7 ng/ml, and 4.2 ± 0.6 ng/ml. Hyaluronan values for controls were 16.2 ± 6.6 ng/ml, 48.5 ± 13.3 ng/ml and 56.1 ± 15.4 ng/ml, and for Diazoxide-treated patients 18.3 ± 3.5 ng/ml, 117.2 ± 22.6 ng/ml and 64.3 ± 7.9 ng/ml. Statistically, neither the values of syndecan-1 or hyaluronan differed at any time points 1, 2 or 3 between the patient groups.
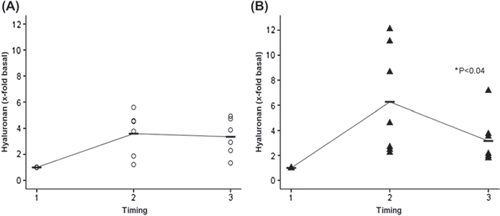
Neither the base line median for hyaluronan (16.2 ± 6.6 ng/ml and 18.3 ± 3.5 ng/ml) or the syndecan-1 (6.5 ± 1.0 ng/ml and 3.5 ± 0.7 ng/ml) values at induction of anesthesia (time point 1) differed in the controls as compared with Diazoxide-treated patients, respectively. At time point 2 (onset of reperfusion), both hyaluronan (48.5 ± 13.3 ng/ml and 117.2 ± 22.6 ng/ml) and syndecan-1 (8.5 ± 2.2 ng/ml and 7.6 ± 0.7 ng/ml) values increased in both groups as compared with base line values. However, hyaluronan (64.3 ± 7.9 ng/ml and 56.1 ± 15.4 ng/ml) and syndecan-1 (4.2 ± 0.6 ng/ml and 5.6 ± 0.9 ng/ml) decreased significantly (p < 0.04 and p < 0.04, respectively) from time points 2 to 3 (termination of surgery) in Diazoxide treated patients only as compared with the controls, respectively. and show perioperative changes of syndecan-1 and hyaluronan levels according to values adjusted to fold change base line values at time point 1.
Figure 2. Syndecan-1 fold change concentrations adjusted to base line before coronary artery bypass grafting (Citation1), immediately after aortic declamping (Citation2) and after operation (Citation3) in controls (A, open circles) and in patients with Diazoxide (B, black triangles). Median is shown in each group with a horizontal line. An interpolation line has been added between median markers. Note significant increase (p < 0.004) and decrease (p < 0.04) from base line to time points 2 and 3, respectively, in patients with Diazoxide (B).
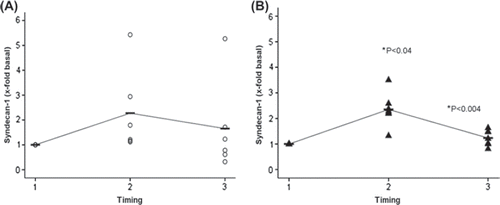
Figure 3. Hyaluronan fold change concentrations adjusted to base line before coronary artery bypass grafting (Citation1), immediately after aortic declamping (Citation2) and after operation (Citation3) in controls (A, open circles) and in patients with Diazoxide (B, black triangles). Median is shown in each group with a horizontal line. An interpolation line has been added between median markers. In both controls and patients with Diazoxide, an increase of Hyaluronan was noted at time point 2. Note significant decrease (p < 0.04) from time point 2 to 3 in patients with Diazoxide (B) but not controls (A).
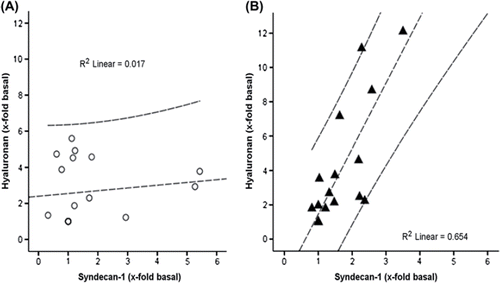
Correlation of hyaluronan and syndecan-1 plasma levels
Hyaluronan and syndecan-1 plasma levels correlated in Diazoxide (R2 linear = 0.654, p < 0.005), but not in controls (R2 linear = 0.017, ns) ().
Discussion
This pilot study shows that Diazoxide administered during CABG decreases levels of both peripheral plasma hyaluronan and syndecan-1 in patients undergoing CABG despite major ultra structural differences before surgery among patients. Consistent decreased hyaluronan and syndecan-1 levels correlate reflecting decreased degradation of the endothelial glycocalyx layer after ischemia reperfusion injury and edema (Citation5,Citation6).
As previously observed, Diazoxide was associated with decrease of tissue edema after CABG (Citation7). While opening KATP-channels, Diazoxide may also directly or indirectly ameliorate cellular electrolyte balance after ischemia-reperfusion. In experimental models of acute myocardial infarction, Diazoxide decreases edema and attenuates mitochondrial damage (Citation8,Citation9). We encountered several observations with EM. The randomly distributed changes included a wide variety of severely swollen and injured to intact mitochondria and scattered swollen endothelial changes, together with nonspecific degeneration of myocytes with disturbed sarcomers (Citation10). EM histology of individual patients is most heterogeneous, not to mention outcome after surgery. The EM images confirm the heterogeneity of the heart before and after surgery, with or without treatment. Though the patients were carefully selected according to preoperative demographics for this study, important variances of tissue response before and after surgery were observed. This is in line with earlier experimental reports demonstrating that mitochondrial, endothelial and edema changes already before surgery make it inconsistent to quantify the effect of treatment in a clinical setting based on outcome alone (Citation5,Citation6).
Attempts to limit endothelial glycocalyx degradation seem to enhance recovery after ischemia reperfusion. Attenuation of tissue edema and inflammation after ischemia reperfusion was achieved by hydrocortisone that protected the glycocalyx layer from shedding (Citation11). Sevoflurane induced a preconditioning and postconditioning effect against the glycocalyx barrier (Citation12). Antithrombin reduces endothelial glycocalyx shedding after reperfusion (Citation13). Interestingly, in decreasing edema with albumin augmentation in an experimental swine model for ischemia reperfusion injury, it was shown that vascular endothelium was protected from glycocalyx shedding and subsequent inflammatory endothelial leukocyte adhesion (Citation14). In all these studies, the net target of treatment is the endothelium and specifically its glycocalyx layer that reacts after ischemia reperfusion injury. As syndecan-1 and hyaluronan are important constituents of the glycocalyx layer, increased amounts of these molecules are observed peripherally during shedding of the glycocalyx. Here, both increases of syndecan-1 and hyaluronan were detected after aortic declamping, confirming the ischemia reperfusion related endothelial injury after CABG (Citation6). Importantly, however, both increases of peripheral syndecan-1 and hyaluronan levels decreased in Diazoxide patients in contrast to controls. According to the manufacturer, the Elisa kit identifies specifically syndecan-1 and not other members of the syndecan family such as syndecan-2, -3, -4, whereas the kit for hyaluronan identifies non-specifically low- (15–40 kDa), medium- (90–150 kDa) and high-( > 950 kDa) molecular-weight hyaluronans. We therefore tested the correlation of releases of hyaluronan associated with syndecan-1; in patients with Diazoxide, hyaluronan increase correlates with syndecan-1, whereas an uncontrolled release of these glycocalyx constituents is eminent in controls (). As acute attenuation of endothelial glycocalyx increases coronary blood volume and tissue edema (Citation15), it is tempting to deduce that controls are susceptible to uncontrolled endothelial injury with shedding of the glycocalyx barrier lasting at least till the end of surgery, as compared with patients treated with Diazoxide. Diazoxide may stabilize the glycocalyx layer and prevent uncontrolled degradation, including various components of the layer.
Therefore, a simple future bed side test, such as evaluation of peripheral plasma syndecan-1 and hyaluronan levels, to verify the beneficial outcome of the patient endothelial glycocalyx shedding after CABG (Citation6) may prove to be most welcome for clinicians in order to predict tissue outcome after ischemia reperfusion. As previously shown, degradation of glycocalyx components occurs during septic shock and trauma; increased levels of glycosaminoglycans predicted mortality during septic shock (Citation16), while enhanced shock after trauma, sympathoadrenal activation, tissue damage and coagulopathy were associated with disruption of the peripheral endothelial layer (Citation17). As a marker of endothelial glycocalyx degradation, increased level of syndecan-1 is associated with inflammation, protein C depletion, fibrinolysis, and mortality in trauma patients (Citation18). By means of evaluating peripheral glycocalyx components, it was feasible to identify different coagulopathic states, threby facilitating specific treatment strategies (Citation19).
Limitations of this study include the small number of patients. In addition, early preoperative application of Diazoxide could even be more advantageous as compared with intraoperative Diazoxide treatment; preoperative Diazoxide via early opening of the KATP-channel through nitric oxide metabolism mimicked the effect of ischemic preconditioning (Citation9). The surgical technique may also be important, as it was previously shown that avoiding cardiopulmonary bypass and cardioplegic arrest during off-pump CABG resulted in differences in the profile of peripheral syndecan-1 and heparin sulphate concentrations (Citation20). While the uniform endothelial glycocalyx layer of healthy vascular endothelium is shedded during trauma such as CABG, ischemia reperfusion injury alone does not appear to be the sole trigger for disruption (Citation20). However, one may be able to control endothelial glycocalyx shedding and, the extent of subsequent tissue destruction.
In summary, we link Diazoxide with controlled decrease of hyaluronan and syndecan-1 after CABG as compared with the controls. It remains to be shown whether this indicates that Diazoxide treatment may be associated with preserving heart energy supplies and functional capacity after CABG.
Acknowledgements
Dr Mennander is the recipient of the Ingegeerd and Viking O. Bjork award for cardiothoracic surgery in 2011.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This study was financially supported by the Competitive Research Funding of the Tampere University Hospital, the Finnish Cultural Foundation and the Tampere Tuberculosis Foundation.
References
- Al-Dadah AS, Voeller R, Shuessler RB, Damiano R, Lawton JS. Maintenance of myocyte volume homeostasis during stress by Diazoxide is cardioprotective. Ann Thorac Surg. 2007;84:857–62.
- Mizutani S, Al-Dadah AS, Bloch JB, Prasad SM, Diodato MD, Shuessler RB, . Hyperkalemic cardioplegia-induced myocyte swelling and contractile dysfunction: Prevention by Diazoxide. Ann Thorac Surg. 2006;81: 154–9.
- Broadhead MW, Kharbanda RK, Peters MJ, MacAllister RJ. KATP channel activation induces ischemic preconditioning of the endothelium in humans in vivo. Circulation. 2004;110: 2077–82.
- Van den Berg BM, Vink H, Spaan JAE. The endothelial glycocalyx protects against myocardial edema. Circulation Res. 2003;4:592–4.
- Rehm M, Bruegger D, Christ F, Conzen P, Thiel M, Jacob M, . Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation. 2007;116:1896–906.
- Svennevig K, Hoel TN, Thiara AS, Kolset SO, Castelheim A, Mollnes TE, . Syndecan-1 plasma levels during coronary artery bypass surgery with and without cardiopulmonary bypass. Perfusion. 2008;23:165–71.
- Shalaby A, Mennander A, Rinne T, Oksala N, Aanismaa R, Narkilahti S, . Aquaporin-7 expression during coronary artery bypass grafting with Diazoxide. Scand Cardiovasc J. 2001;45:354–9.
- McCully JD, Wakiyama H, Cowan DB, Federman M, Parker RA, Levitsky S. Diazoxide amelioration of myocardial injury and mitochondrial damage during cardiac surgery. Ann Thorac Surg. 2002;74:2138–46.
- Beresewicz A, Maczewski M, Duda M. Effect of classic preconditioning and Diazoxide on endothelial function and O2 and NO generation in the post-ischemic guinea-pig heart. Cardiovasc Res. 2004;63:118–29.
- Deja MA, Malinowski M, Golba K, Kajor M, Lebda-Wyborny T, Hudziak D, . Diazoxide protects myocardial mitochondria, metabolism, and function during cardiac surgery: A double-blind randomized feasibility study of diazoxide-supplemented cardioplegia. J Thorac Cardiovasc Surg. 2009;137:997–1004.
- Chappell D, Jacob M, Hofmann-Kiefer K, Bruegger D, Rehm M, Conzen P, . Hydrocortisone preserves the vascular barrier by protecting the endothelial glycocalyx. Anesthesiology. 2007;107:776–84.
- Annecke T, Chappell D, Chen C, Jacob M, Welsch U, Sommerhoff CP, . Sevoflurane preserves the endothelial glycocalyx against ischaemia-reperfusion injury. Br J Anaesth. 2010;104:414–21.
- Chappell D, Jacob M, Hofmann-Kiefer K, Rehm M, Welsch U, Conzen P, . Antithrombin reduces shedding of the endothelial glycocalyx following ischaemia/reperfusion. Cardiovasc Res. 2009;83:388–96.
- Jacob M, Paul O, Mahringer L, Chappell D, Rehm M, Welsch U, . Albumin augmentation improves condition of guinea pig hearts after 4 hr of cold ischemia. Transplantation. 2009;87:956–65.
- Brands J, Spaan JAE, Van den Berg BM, Vink H, VanTeeffelen JWGE. Acute attenuation of glycocalyx barrier properties increases coronary blood volume independently of coronary flow reserve. Am J Physiol Heart Circ Physiol. 2010;298:H515–23.
- Nelson A, Berkestedt I, Schmidtchen A, Ljunggren L, Bodelsson M. Increased levels of Glycosaminoglycans during septic shock: Relation to mortality and the antibacterial actions of plasma. Shock. 2008;30:623–7.
- Johansson PI, Sorensen AM, Perner A, Welling KL, Wanscher M, Larsen CF, . High sCD40L levels early after trauma are associated with enhanced shock, sympathoadrenal activation, tissue and endothelial damage, coagulopathy and mortality. J Thromb Haemost. 2012;10: 207–16.
- Johansson PI, Stensballe J, Rasmussen LS, Ostrowski SR. A high admission Syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann Surg. 2011;254:194–200.
- Johansson P, Sorensen AM, Perner A, Welling KL, Wanscher M, Larsen CF, . Disseminated intravascular coagulation or acute coagulopathy of trauma shock early after trauma? An observational study. Critical Care. 2011;15:272–82.
- Bruegger D, Rehm M, Abicht J, Paul JO, Stoeckelhuber M, Pfirrmann M, . Shedding of the endothelial glycocalyx during cardiac surgery: On-pump versus off-pump coronary artery bypass graft surgery. J Thorac Cardiovasc Surg. 2009;138:1445–7.