Abstract
Objectives. We assessed the effect of milrinone application timing after reperfusion against myocardial stunning as compared with levosimendan in swine. Furthermore, we examined the role of p38 mitogen-activated protein kinase (p38 MAPK) in the milrinone-induced cardioprotection. Design. All swine were subjected to 12-minutes ischemia followed by 90-minutes reperfusion to generate stunned myocardium. Milrinone or levosimendan was administered intravenously either for 20 minutes starting just after reperfusion or for 70 minutes starting 20 minutes after reperfusion. In another group, SB203580, a selective p38 MAPK inhibitor, was administered with and without milrinone. Regional myocardial contractility was assessed by percent segment shortening (%SS). Results. Milrinone starting just after reperfusion, but not starting 20 minutes after reperfusion, improved %SS at 30, 60, and 90 minutes after reperfusion compared with that in the control group. SB203580 abolished the beneficial effect of milrinone. On the other hand, levosimendan starting 20 minutes after reperfusion, but not for 20 minutes starting just after reperfusion, improved %SS at 60 and 90 minutes after reperfusion. Conclusions. Milrinone should be administered just after reperfusion to protect myocardial stunning through p38 MAPK, whereas levosimendan improvement of contractile function could be mainly dependent on its positive inotropic effect.
Introduction
Myocardial stunning is defined as reversible post-ischemic left ventricular (LV) dysfunction following a brief ischemic episode that does not result in necrosis (Citation1). In clinical situations, myocardial stunning occurs after percutaneous transluminal coronary angioplasty (PTCA) or cardiac surgery (Citation2,Citation3).
Milrinone, a phosphodiesterase III (PDE III) inhibitor, leads to sequential elevation of intracellular cyclic adenosine monophosphate (cAMP) and cAMP-dependent protein kinase (PKA) activation and exerts positive inotropic and vasodilatory effects. Milrinone has been clinically used for the treatment of acute heart failure or low-output syndrome caused by myocardial dysfunction after cardiopulmonary bypass (Citation4,Citation5). It has been shown that milrinone has both preconditioning (PreC) and postconditioning (PostC) properties against myocardial infarction (Citation6,Citation7). Milrinone also protects against myocardial stunning when administered before ischemia and just after reperfusion (Citation8). However, the underlying mechanisms involved in the milrinone-induced cardioprotective effect against myocardial stunning have not been fully clarified.
The clinical efficacy of levosimendan in patients with heart failure resulting from ischemic heart disease, dilated cardiomyopathy, and acute myocardial infarction has been well documented (Citation9,Citation10). Levosimendan produces PreC and PostC against myocardial infarction via activation of adenosine triphosphate (ATP)-sensitive potassium (KATP) channels (Citation11). It is also reported that levosimendan administered 10 minutes after PTCA improves contractile function in stunned myocardium (Citation12). In contrast, Brendt et al. (Citation13) reported that levosimendan exerted an anti-stunning effect when started before ischemia, but not when started after ischemia in isolated guinea pig hearts.
PostC is more likely than PreC to be feasible as a clinical application, and may be useful in unpredictable myocardial ischemia-reperfusion injury. The majority of the injury responsible for myocardial stunning and infarction develops during the early phase of reperfusion (Citation1). Our previous study also reported that fasudil administered just after reperfusion but not 30 minutes after reperfusion protects the stunned myocardium (Citation14). Therefore, a pharmacological cardioprotective effect depends on the application timing after reperfusion.
p38 mitogen-activated protein kinase (p38 MAPK) has been shown to play an important role in the cascade of not only PreC (Citation15) but also PostC (Citation16–18). Lemoine et al. (Citation17) showed that desflurane, a halogenated anesthetic, administered during reoxygenation activates p38 MAPK, and enhances the recovery of developed force in human atrial myocardium. Sanada et al. (Citation6) also showed that milrinone administered before ischemia reduce myocardial infarct size via cAMP-, PKA-, and p38 MAPK-dependent mechanisms.
The aims of this study were to clarify whether the administration timings of milrinone and levosimendan during reperfusion have critical influences on this compound-induced cardioprotection against myocardial stunning in anesthetized open-chest swine, and whether p38 MAPK is involved in the protective effect of milrinone.
Materials and methods
All experimental procedures used in this investigation were reviewed and approved by the Institutional Animal Care Committee of Nagasaki University. Date of issue and registration number is December 4 2007 (No. 712040634) and January 11 2011 (No. 1012270891). The experiments ended by euthanasia with a high dose of potassium inducing ventricular fibrillation (VF).
Surgical procedure
Seventy-six swine of either sex weighing 19–34 kg were sedated with 20 mg/kg intramuscular ketamine hydrochloride. Surgical preparation was performed as described previously (Citation8,Citation14,Citation19). Briefly, swine were anesthetized with 100 mg/kg intravenous α-chloralose and 10 g/kg fentanyl, followed by continuous infusion of 10 mg/kg/h α-chloralose and 5 g/kg/h fentanyl throughout the study period. Through a midline cervical incision, the trachea was intubated for connection to a Harvard respiratory pump (Harvard Apparatus Co., South Natick, MA, USA). Mechanical ventilation was facilitated by intermittent infusion of 0.2 mg/kg vecuronium, and adjusted to maintain the arterial carbon dioxide tension (PaCO2) at 35–40 mmHg and the arterial oxygen tension (PaO2) at 100–300 mmHg. The body temperature was maintained throughout the study period using a warming blanket and a heating lamp. A catheter was inserted into the right carotid vein to administer fluid and drugs. Lactated Ringer's solution was infused at a rate of 5 ml/kg/h. Systemic anticoagulation was achieved with sodium heparin, at 750 U/kg i.v., followed by continuous infusion at 250 U/kg/h. Sodium bicarbonate was administered to maintain base deficit within 5 mmol/l. Arterial blood glucose concentration was maintained at 70–140 mg/dl. A standard peripheral lead electrocardiogram was monitored continuously. Medial sternotomy was performed and the pericardium was opened to expose the heart. The left anterior descending coronary artery (LAD) distal to the first diagonal branch was cannulated with a stainless steel cannula and perfused with blood from the left carotid artery through an extracorporeal circuit. Coronary blood flow (CBF) of the perfused area of LAD was measured with an ultrasonic flow probe (ADP17; Crystal Biotech, Hopkinton, MA, USA) attached at the extracorporeal circuit. Pressure transducer-tipped catheters (PC500; Millar Instruments, Houston, TX, USA) were connected to the LV chamber cannula through an incision in the apex and right internal carotid artery cannula for continuous recording of left ventricular pressure (LVP) and arterial blood pressure, respectively. The peak rate of increase in LVP (LVdp/dt max) was determined by electric differentiation of the LV pressure waveform. Two ultrasonic segment length transducers were implanted 10–15 mm apart in the subendocardium of the perfused area of the extracorporeal circuit and aligned such that the intercrystal axis was parallel to the direction of myocardial fiber shortening. The regional contractile function was assessed by percent segment shortening (%SS). Segment length was monitored by ultrasonic amplifiers (VF-1; Crystal Biotech, Hopkinton, MA, USA). The end-systolic segment length (ESL) was determined to be 10 ms before maximum negative LVdp/dt, and the end-diastolic segment length (EDL) was determined to be 10 ms before the LVdP/dt max first exceeded 140 mmHg/s (immediately before the onset of LV isovolemic contraction). %SS was calculated using the following formula: %SS = (EDL –ESL) & 100 & 1/EDL. All hemodynamic data were continuously monitored on a polygraph and digitized through a computer interfaced with an analog-to-digital converter (HEM; Physio-Tech, Tokyo, Japan).
Experimental protocols
shows the experimental time course. Baseline systemic and coronary hemodynamics and %SS were recorded 30 minutes after instrumentation was completed. Seventy-six swine were randomly assigned to one of seven groups. If the pig was excluded before completion of the experiment, the next one was assigned to the same group. Each swine was subjected to 12-minutes ischemia with complete occlusion of the extracorporeal circuit followed by 90-minutes reperfusion. In large mammals, dogs, and pigs, myocardial stunning can be induced by a single completely reversible episode of regional ischemia lasting less than 20 minutes. We carried out preliminary study to determine the ischemic period. We aimed at about 50% recovery from baseline after 90-minutes reperfusion. Group A (n = 12) received intravenous infusion of saline after ischemia until the end of the reperfusion period. Group B (n = 11) received intravenous milrinone starting just after reperfusion at a rate of 5 μg/kg/min for 10 minutes followed by 0.5 μg/kg/min for 10 minutes. Group C (n = 12) received intravenous milrinone starting 20 minutes after reperfusion at a rate of 5 μg/kg/min for 10 minutes followed by 0.5 μg/kg/min until the end of reperfusion. Group D (n = 9) received intracoronary SB203580 starting just after reperfusion at a rate of 1.2 μg/kg/min for 20 minutes. Group E (n = 11) received a combination of intravenous milrinone at a rate of 5 μg/kg/min for 10 minutes followed by 0.5 μg/kg/min for 10 minutes and intracoronary SB203580 at a rate of 1.2 μg/kg/min for 20 minutes starting just after reperfusion. Group F (n = 10) received intravenous levosimendan starting just after reperfusion at a rate of 1.2 μg/kg/min for 10 minutes followed by 0.2 μg/kg/min for 10 minutes. Group G (n = 11) received intravenous levosimendan starting 20 minutes after reperfusion at a rate of 1.2 μg/kg/min for 10 minutes followed by 0.2 μg/kg/min until the end of reperfusion. The administration rates of intravenous milrinone and levosimendan were set on the basis of the clinical doses (Citation5,Citation10) and our previous study (Citation8). The administration rate of intracoronary SB203580 was set on the basis of a previous study (Citation6) and our preliminary study.
Figure 1. Time course of the experimental protocol. Hemodynamic and percent segment shortening (%SS) measurements were performed at the times indicated by triangles (▲) in the figure. P0: baseline, R−12: just before ischemia, R0: just before reperfusion, R5, R20, R30, R60, and R90: 5, 20, 30, 60, and 90 minutes after reperfusion, respectively.
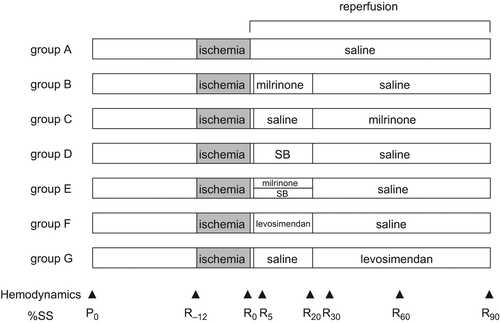
Hemodynamics and contractile function were monitored continuously throughout the experiment and recorded at the time points illustrated in (P0: baseline, R−12: just before ischemia, R0: just before reperfusion, R5, R20, R30, R60, and R90: 5, 20, 30, 60, and 90 minutes after reperfusion, respectively).
All swine received 2 mg/kg intravenous lidocaine at 1 minute before reperfusion. When five or more premature ventricular contractions per minute or multifocal premature ventricular contractions were observed after reperfusion, 1 mg/kg intravenous lidocaine was administered and repeatedly given if necessary. Swine with continuous VF or ventricular tachycardia (VT) after reperfusion were excluded from the study. The incidence of VF or VT and the total amount of lidocaine used for 10 minutes after reperfusion were compared among groups.
Statistics
All data are expressed as mean ± SD. One-way analysis of variance (ANOVA) for non-repeated measures followed by the Student-Newman-Keuls (SNK) post hoc test was used to test for differences in baseline hemodynamics, %SS, and total dose of lidocaine administered after reperfusion among groups. Data within groups were analyzed with one-way ANOVA for repeated measures, and data between groups were analyzed with two-way repeated measures ANOVA followed by the SNK post hoc test. The incidence of VF or VT was analyzed by the χ2 test. P values < 0.05 were considered statistically significant. Statistical analysis was performed using SPSS 15.0 software (SPSS Japan, Tokyo, Japan) or GraphPad Prism 5.0 (GraphPad Software, San Diego, CA).
Results
There were no significant differences in weight or sex among the groups. Arterial blood gas values and blood glucose were maintained within physiological ranges in all swine throughout the study period (data not shown).
Reperfusion-induced arrhythmias
summarizes the assessment of reperfusion-induced arrhythmias. Four swine in each of groups A and C, three swine in each of groups B, D, E, and G, and two swine in group F had continuous VF or VT after reperfusion and were excluded from further analysis. The incidence of VF or VT and the total amount of lidocaine used were not significantly different among groups.
Table I. Incidence of ventricular fibrillation (VF) or ventricular tachycardia (VT) and total amount of lidocaine used during the first 10 minutes after reperfusion.
Hemodynamics
shows the systemic and coronary hemodynamics throughout the time course of the study. There were no significant differences in any measured systemic or coronary hemodynamics at baseline among groups. There were no significant differences among groups at any measured point in terms of heart rate. Mean arterial pressure and LV systolic pressure in group F at R60 and R90, and LV end-diastolic pressure in group F at R0, and R5, were significantly increased from the baseline values. LVdp/dt max was significantly decreased in group F at R0. CBF increased significantly at R5 compared with that at baseline in all groups and returned thereafter, and there were no significant differences between groups at any measured point.
Table II. Systemic and coronary hemodynamics, and contractility.
Regional myocardial contractility
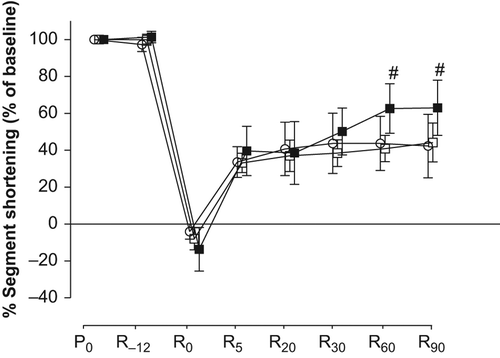
There were no significant differences in baseline values of %SS among groups (). The percent changes of %SS from baseline throughout the time course are shown in and . All swine showed negative %SS values at the end of the ischemic period (R0), which indicates bulging. The values of %SS at R30, R60, and R90 in group B (60 ± 7%, 72 ± 5%, and 79 ± 5% of baseline, respectively) were significantly higher than those in group A (38 ± 7%, 41 ± 7%, and 44 ± 11% of baseline, respectively). The values of %SS at R60 and R90 in group G (63 ± 13% and 63 ± 15% of baseline) were significantly higher than those in group A. However, there were no significant differences in the values of %SS in groups C, D, E, and F compared with those in group A at any measured point.
Figure 2. Effects of milrinone and/or SB203580 on percent segment shortening. Values are expressed as mean ± SD. #p < 0.05 vs. group A. ○: group A (n = 8), ▲: group B (n = 8), ▼: group C (n = 8), Δ: group D (n = 6), and ∇: group E (n = 8). P0: baseline, R−12: just before ischemia, R0: just before reperfusion, R5, R20, R30, R60, and R90: 5, 20, 30, 60, and 90 minutes after reperfusion, respectively.
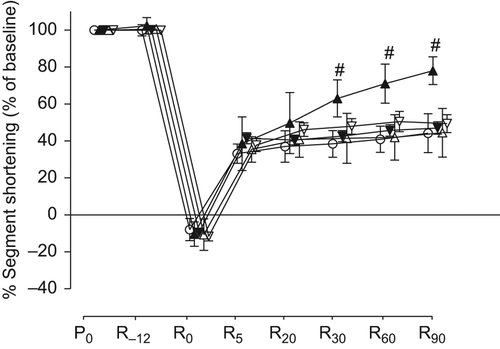
Figure 3. Effects of levosimendan on percent segment shortening. Values are expressed as mean ± SD. #; p < 0.05 vs. group A. ○: group A (n = 8), □: group F (n = 8), and ■: group G (n = 8). P0: baseline, R−12: just before ischemia, R0: just before reperfusion, R5, R20, R30, R60, and R90: 5, 20, 30, 60, and 90 minutes after reperfusion, respectively.
Discussion
The present results show that milrinone administered starting just after reperfusion, but not starting 20 minutes after reperfusion, improves myocardial stunning in anesthetized open-chest swine. The p38 MAPK inhibitor, SB203580, alone did not alter the extent of myocardial stunning, but it abolished the beneficial effect of milrinone administered just after reperfusion. In contrast, levosimendan administered intravenously starting just after reperfusion for 20 minutes did not improve myocardial stunning, whereas continuous infusion for 70 minutes starting 20 minutes after reperfusion could improve regional myocardial contractility.
Effects of milrinone
An important aspect of the pathophysiology of stunned myocardium is that the contraction can be restored when exposed to inotropic stimuli (Citation20). Therefore, milrinone administered after reperfusion might produce both inotropic and cardioprotective effects. However, in the present results, it is unlikely that milrinone administered just after reperfusion for 20 minutes in group B could have improved myocardial contractility through its direct inotropic action because it was reported that there is a positive correlation between the plasma concentration of milrinone and its inotropic effect, and that the plasma concentration of milrinone is undetectable 60 minutes after the administration of 60 μg/kg over 6 minutes (Citation21). Furthermore, the present results also show that milrinone administered starting 20 minutes after reperfusion until the end of reperfusion in group C could not have improved myocardial contractility, in spite of a probably higher plasma concentration than in group B. Thus, the beneficial effects of milrinone administered just after reperfusion would be exerted through its PostC effect against myocardial stunning, but not through its positive inotropic effect.
In the present results, milrinone administered just after reperfusion, but not 20 minutes after reperfusion, protects myocardial stunning, suggesting that treatment with milrinone should be applied just after reperfusion to protect myocardial stunning. Our previous study showed that a Rho kinase inhibitor, fasudil, administered just after reperfusion but not 30 minutes after reperfusion protects the stunned myocardium (Citation14). It is likely that a pharmacological cardioprotective effect would depend on the application timing after reperfusion.
Role of p38 MAPK
Several studies have suggested the involvement of p38 MAPK in the cascade of cellular events triggered during ischemia–reperfusion, and also following PreC (Citation15) and PostC (Citation16–18). Lemoine et al. (Citation17) showed that desflurane administered during the first 5 minutes of reoxygenation activates p38 MAPK and enhances the recovery of developed force in human atrial myocardium. They also showed that SB202190, a specific p38 MAPK inhibitor, abolished the enhanced recovery of contractile force resulting from desflurane-induced PostC. Liu et al. (Citation16) showed that hypoxic PostC attenuated ischemia/reperfusion-induced endoplasmic reticulum stress and apoptosis via p38 MAPK activation. We carried out preliminary study to clarify the role of p38 MAPK in the cardioprotective effect of milrinone administered before ischemia against myocardial stunning. Milrinone, administered intravenously at a rate of 5 μg/kg/min for 10 minutes followed by 0.5 μg/kg/min for 10 min until 30 minutes before ischemia, enhanced the functional recovery from myocardial stunning. Cotreatment with SB203580, administered by continuous intracoronary infusion at a rate of 1.2 μg/kg/min for 20 minutes until 30 minutes before ischemia, prevented this protective effect. Furthermore, our present results show that SB203580 abolished the beneficial effect of milrinone administered just after reperfusion, suggesting that milrinone administered before ischemia or just after reperfusion would exert the cardioprotective effect against myocardial stunning through activation of p38 MAPK.
Effects of levosimendan
Sonntag et al. (Citation12) reported that 24 μg/kg levosimendan administered intravenously 10 minutes after PTCA improves contractile function in stunned myocardium. In the present results, levosimendan administered intravenously starting 20 minutes after reperfusion (group G), but not starting just after reperfusion (group F), improved regional myocardial contractility at 60 and 90 minutes after reperfusion. Levosimendan is known to cause dose-dependent improvements in systemic and pulmonary hemodynamics and reduction in clinical symptoms in patients with heart failure (Citation22). The elimination half-life of levosimendan is 1.3 hours in patients who receive 0.2 μg/kg/min continuous infusion for 24 hours (Citation23). It is likely that the plasma concentration of levosimendan in group F would be insufficient to improve regional myocardial contractility at 60 and 90 minutes after reperfusion because these time points are 40 and 70 minutes after discontinuation of levosimendan. Brendt et al. (Citation13) reported that an anti- stunning effect was seen when levosimendan was started before ischemia, but not when administered for 10 minutes just after ischemia in isolated guinea pig hearts. They suggested that levosimendan administered after reperfusion could not produce cardioprotective effects against myocardial stunning. In the present results, the improvement of contractile function induced by levosimendan administered after reperfusion would be dependent on its positive inotropic effect, but not on the protective effect against myocardial stunning.
Conclusion
Both milrinone and levosimendan administrated after reperfusion improve contractile function in stunned myocardium. The effect of milrinone would be mediated by the activation of p38MAPK, excreted when milrinone is applied just after reperfusion. In contrast, the effect of levosimendan would be dependent on its positive inotropic effect, but not on the protective effect against myocardial stunning.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
The authors disclose no financial or personal relationships with other people or organizations that could inappropriately influence this work. This work was supported in part by Grants-in-Aid 22791440 (to Dr. Shibata), 22591738 (to Dr. Yoshitomi), 19591805 (to Dr. Cho), and 19390406 (to Dr. Sumikawa) for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. This work was presented in part at the 16th ASEAN Congress of Anesthesiologists, Koka Kinabalu, Sabah, Malaysia, July 2-5, 2009, the 13th Asian Australasian Congress of Anesthesiologists, Fukuoka, Japan, June 1-5, 2010, and the annual meeting of the American Society of Anesthesiologists, San Diego, California, October 16-20, 2010.
References
- Bolli R, Marban E. Molecular and cellular mechanisms of myocardial stunning. Physiol Rev. 1999;79:609–34.
- Kim SJ, Depre C, Vatner SF. Novel mechanisms mediating stunned myocardium. Heart Fail Rev. 1999;8:143–53.
- Ruiz-Bailen M, Aguayo de Hoyos E, Ruiz-Navarro S, Díaz-Castellanos MA, Rucabado-Aguilar L, Gómez-Jiménez FJ, . Reversible myocardial dysfunction after cardiopulmonary resuscitation. Resuscitation. 2005;66:175–81.
- Lobato EB, Florete O Jr, Bingham HL. A single dose of milrinone facilitates separation from cardiopulmonary bypass in patient with pre-existing left ventricular dysfunction. Br J Anaeth. 1998;81:782–4.
- Jebeli M, Ghazinoor M, Mandegar MH, Rasouli MR, Eghtesadi-Araghi P, Goodarzynejad H, . Effect of milrinone on short-term outcome of patients with myocardial dysfunction undergoing coronary artery bypass graft: A randomized controlled trial. Cardiol J. 2010;17:73–8.
- Sanada S, Kitakaze M, Papst PJ, Asanuma H, Node K, Takashima S, . Cardioprotective effect afforded by transient exposure to phosphodiesterase III inhibitors: the role of protein kinase A and p38 mitogen-activated protein kinase. Circulation. 2001;104:705–10.
- Matsumoto S, Cho S, Tosaka S, Higashijima U, Maekawa T, Hara T, . Hyperglycemia raises the threshold of levosimendan- but not milrinone-induced postconditioning in rat hearts. Cardiovasc Diabetol. 2012;11:4.
- Use T, Makita T, Ureshino H, Cho S, Yoshitomi O, Akiyama D, . Milrinone administered before ischemia or just after reperfusion, attenuates myocardial stunning in anesthetized swine. Cardiovasc Drugs Ther. 2006;20: 327–34.
- Pagel PS. Levosimendan in cardiac surgery: a unique drug for the treatment of perioperative left ventricular dysfunction or just another inodilators searching for a clinical application?Anesth Analg. 2007;104:759–61.
- Al-Shawaf E, Ayed A, Vislocky I, Radomir B, Dehrab N, Tarazi R. Levosimendan or milrinone in the type 2 diabetic patient with low ejection fraction undergoing elective coronary artery surgery. J Cardiothorac Vasc Anesth. 2006;20: 353–7.
- du Toit EF, Genis A, Opie LH, Pollesello P, Lochner A. A role for the RISK pathway and K(ATP) channels in pre- and post-conditioning induced by levosimendan in the isolated guinea pig heart. Br J Pharmacol. 2008;154:41–50.
- Sonntag S, Sundberg S, Lehtonen LA, Kleber FX. The calcium sensitizer levosimendan improves the function of stunned myocardium after percutaneous transluminal coronary angioplasty in acute myocardial ischemia. J Am Coll Cardiol. 2004;43:2177–82.
- Brendt P, Behrends M, Peters J. Myocardial stunning following no flow ischaemia is diminished by levosimendan or cariporide, without benefits of combined administration. Resuscitation. 2008;76:95–102.
- Shibata I, Yoshitomi O, Use T, Ureshino H, Cho S, Maekawa T, . Administration of the Rho-kinase inhibitor fasudil before ischemia or just after reperfusion, but not 30 min after reperfusion, protects the stunned myocardium in swine. Cardiovasc Drugs Ther. 2008;22:293–8.
- Hausenloy DJ, Yellon DM. Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc Res. 2006;70: 240–53.
- Liu XH, Zhang ZY, Sun S, Wu XD. Ischemic postconditioning protects myocardium from ischemia/reperfusion injury through attenuating endoplasmic reticulum stress. Shock. 2008;30:422–7.
- Lemoine S, Beauchef G, Zhu L, Renard E, Lepage O, Massetti M, . Signaling pathways involved in desflurane-induced postconditioning in human atrial myocardium in vitro. Anesthesiology. 2008;109:1036–44.
- Lemoine S, Puddu PE, Durand C, Lepage O, Babatasi G, Ivascau C, . Signaling pathways involved in postconditioning-induced cardioprotection of human myocardium, in vitro. Exp Biol Med (Maywood). 2010;235:768–76.
- Sakai K, Cho S, Shibata I, Yoshitomi O, Maekawa T, Sumikawa K. Inhalation of hygrogen gas protects against myocardial stunning and infarction in swine. Scand Cardiovasc J. 2012;46:183–9.
- Kloner RA, Jennings RB. Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 1. Circulation. 2001;104:2981–9.
- Alousi AA, Iwan T, Edelson J, Biddlecome C. Correlation of the hemodynamic and pharmacokinetic profile of intravenous milrinone in the anesthetized dog. Arch Int Pharmacodyn Ther. 1984;267:59–66.
- Slawsky MT, Colucci WS, Gottlieb SS, Greenberg BH, Haeusslein E, Hare J, . Acute hemodynamic and clinical effects of levosimendan in patient with severe heart failure. Circulation. 2000;102:2222–7.
- Kivikko M, Antila S, Eha J, Lehtonen L, Pentikainen PJ. Pharmacokinetics of levosimendan and its metabolites during and after a 24-hour continuous infusion in patients with severe heart failure. Int J Clin Pharmacol Ther. 2002; 40:465–71.