Abstract
Objectives. Cardiac Resynchronization Therapy (CRT) for heart-failure patients has a well-documented positive effect, but the overall mortality in this group remains high. This study aimed to explore whether additional information from the device post-implant (occurrence of ventricular high-rate episodes), could add prognostic value for patients on CRT-pacemaker (CRT-P) treatment. Design. Clinical data and device-interrogation data were retrospectively gathered from the medical records of 220 patients treated with CRT-P. Ventricular high-rate (VHR) episodes were defined as a ventricular rate ≥ 180 beats per minute. The primary outcome was 5-year mortality. Results. During follow-up, 132 patients (60%) died or underwent heart transplant. Overall, the 5-year mortality rate was 52%; 77% for patients with VHR during the first year of follow-up and 48% for patients without VHR during the first year of follow-up (p = 0.001). In a multivariate model, the occurrence of VHR episodes was an independent predictor of 5-year mortality (HR 9.96, p = 0.022). The most common cause of death was heart failure, and death from arrhythmia did not differ between groups (p = 0.065). Conclusions. In heart-failure patients with CRT-P therapy, occurrence of VHR episodes within the first year post-implant was an independent predictor of higher 5-year mortality and inferior long-term survival, but not of death from malignant arrhythmia.
Introduction
Cardiac Resynchronization Therapy (CRT) has a well-known effect on mortality and morbidity in patients with symptomatic heart failure, depressed left ventricular ejection fraction (LVEF), and wide QRS complex, on an electrocardiogram (ECG) (Citation1,Citation2). Similarly, primary prophylactic implantable defibrillators have been shown to reduce mortality in patients with symptomatic heart failure and depressed LVEF, with or without inducible arrhythmias or previous episodes of non-sustained ventricular tachycardia (NsVT) (Citation3–5). In the COMPANION study, it was shown that both CRT-P and CRT-defibrillators (CRT-D) are superior to optimal medical therapy with regard to morbidity, but only CRT-D showed a significant reduction of mortality (Citation2). This important finding, in combination with the knowledge derived from the primary preventive defibrillator studies, has led to the widespread clinical use of primary preventive CRT-Ds. However, recent publications have highlighted the need for active risk stratification in patients treated with CRT, when it comes to determining whether the patient really needs a CRT-D or whether a CRT-P is the better choice (Citation6–8). If more patients are to receive a CRT-P as the initial treatment device (instead of a CRT-D), then a critically important consideration will be to accurately determine whether a patient with a CRT-P has developed an increased risk for malignant arrhythmias during the course of treatment, and is therefore in need of an upgrade to a CRT-D. Recent studies suggest that diagnostic data from ICD devices carry information that may help to improve management and clinical outcomes in HF patients (Citation9). The prognostic impact of episodes of ventricular tachycardia or ventricular fibrillation (VT/VF) and appropriate shocks in ICD patients is well described (Citation10), and in analogy to this we hypothesized that episodes with detected ventricular high-rates in CRT-P-treated patients may be a valuable marker in the context of monitoring heart failure monitoring and predicting risk.
The present study aimed to explore if device information regarding occurrence of “ventricular high-rate episodes” (VHR episodes), representing episodes of NsVT, could add prognostic value for patients on CRT-pacemaker treatment. We also aimed to explore if there is a correlation between early post-implant occurrence of NsVT episodes and a higher risk of cardiac arrest within the first 5 years.
Material and methods
Study population and data collection
We retrospectively screened the medical records of all consecutive patients receiving CRT-P implants from 1999 through 2008 at a University Hospital in Sweden, where the pacemaker department serves a population of 1.7 million people. Consecutive patients with a CRT-P device and with at least 5 years of possible follow-up until the cross-validation with the National Swedish Death Register was performed on the 25th of May 2013, were included. Patients aged less than 18 years, and procedures with unsuccessful left ventricular lead implant or immediate explant (within 2 months of implant), were excluded. The method has been previously described (Citation11). Clinical data known at the time of implant were collected in a standardized fashion from the medical records. Patients received devices primarily from Medtronic, and in a minority of cases from St. Jude Medical or Biotronic. The pacemakers were factory-programmed, and the VHR was defined as an episode with a regular ventricular rate of ≥ 175–180 beats per minute and ≥ 5 cycles. Follow-up for survival started after implant, and data from device interrogations were analyzed from this point, including data regarding biventricular pacing (BivP) in percent values, and VHR episodes. Episodes were adjudicated by device electrocardiograms if possible, and only definite NsVT episodes were considered as VHR episodes, whereas atrial tachycardia (AT) and episodes with rapidly conducted atrial fibrillation (AF) were excluded. Only VHR episodes occurring within the first 12 months post-implant were considered for the primary outcome analyses. No quantification of the total number of VHR episodes was made, only the occurrence of any such episode within the first 12 months post-implant was used to evaluate the clinical outcome. Survival analyses and logistic regression analyses were done as landmark-analyses from the date of ICD-follow-up interrogation within 1 year post-implant, thus not including patients who died within the first year without any additional ICD interrogation having been performed (not counting the postoperative 1-month control). Data on the causes of death were gathered from the National Swedish Death Register. Left ventricular lead position was determined from chest X-rays, according to the method proposed by Wilton et al. (Citation12). The value of LVEF was determined qualitatively by eye-balling by an experienced echocardiographer. The primary endpoint variable was five-year all-cause mortality, and the secondary endpoint was death from any cause during the entire follow-up period. The mode of death was compared, between patients with VHR versus patients with no VHR. The local ethics committee approved the study.
Statistical methods
Categorical data have been presented as numbers and percentages, and differences were tested with the Pearson chi-square test. Non-Gaussian distributed variables were tested with the Mann-Whitney U-Test or the Kruskal-Wallis test, as appropriate, and Gaussian distributed with the independent samples t-test. A univariate as well as a multivariate logistic-regression analysis was used to evaluate the predictors of 5-year survival. Baseline variables with p values < 0.15 in the univariate analysis were used in the multivariate analysis, in order to identify independent predictors of mortality. Bivariate correlation analysis was performed to exclude one or more internally correlated variables from the multivariate logistic-regression analysis (in case of an internal correlation coefficient of > 0.3, only the most clinically relevant variable was entered into the final multivariate model). Kaplan-Meier plots and the log rank test were used to compare survival over time between groups. A patient was considered to have survived when neither death nor heart-transplant had occurred. Percentages compared in the results paragraph were obtained from simple count. The statistical analyses were performed using SPSS Statistics (release 22.0, IBM, Chicago, IL).
Results
A total of 494 patients who had an attempted CRT-P implant between 1999 and 2012, were identified. Out of these, 321 had a potential follow-up duration of at least 5 years. After having excluded 27 patients lost to follow-up (their pacemaker interrogations were made elsewhere), 21 patients who died before second device-control (and therefore made it impossible to detect a possible VHR episode), 19 unconventionally implanted (RVOT), 16 upgraded to CRT-D during follow-up, 13 with no left ventricular lead, 2 under the age of 18, 2 with no CRT-indication, and 1 patient with unsuccessful implantation, a total of 220 patients remained, and these comprised the study cohort. In this study, CRT-Ps from three different manufacturers were used. Thirty-five patients received a pacemaker from St. Jude Medical, 39 patients received a pacemaker from Biotronic, and 145 patients received a pacemaker from Medtronic. The manufacturer's data for the CRT-P was missing in 1 patient. There were no significant differences regarding the percentage of VHR episodes diagnosed with the different brands of devices. The median follow-up duration was 46 months (interquartile range 63 months). The median duration of follow-up was less than 5 years, due to a high mortality during the first 5 years of follow-up. A flowchart of included and excluded patients is presented in . shows baseline characteristics, medical treatments, and ECG and echocardiographic parameters of the cohort, with patients stratified for occurrence or absence of VHRs.
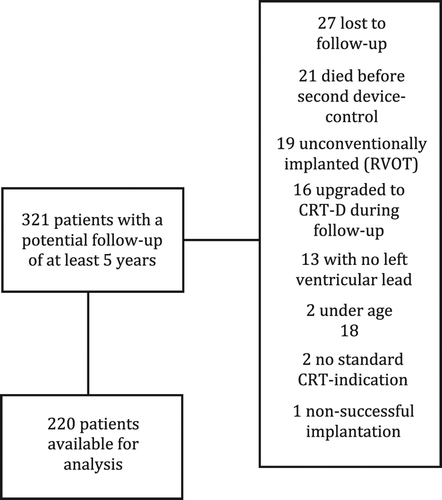
Table I. Baseline characteristics comparing patients with and without VHR episodes during first year of follow-up.
Long-term prognosis
During the first year post-implant, 13.6% (n = 30) of the patients had one or several episodes of VHR episodes. Over the entire follow-up period, 132 patients (60%) died, or had undergone a heart transplant (129 deaths and 3 heart transplants, ). The overall 5-year mortality was 52%; 77% for patients with VHR during first year of follow-up and 48% for patients without VHR during first year of follow-up (p = 0.001). The mode of death is presented in , stratified for occurrence of VHR episodes during the first year post-implant. There was a trend for higher risk of death from heart failure in patients who had early VHR episodes, compared to those who did not have early VHR episodes (60% vs 43%, p = 0.138), and a strong trend for increased risk of death from cardiac arrest in the group of patients without VHR episodes (19.2% vs 4.0%, p = 0.065).
Table II. Mode of death.
In , univariate and multivariate predictors of mortality are presented. The odds ratio for death within 5 years was 9.96 (p = 0.022) for patients with VHR episodes within the first 12 months post-implant. In addition to VHR episodes, other well-known clinical variables were associated with higher mortality; non-LBBB morphology on ECG, higher age, previous myocardial infarction, previous history of AF, lower LVEF, use of loop-diuretics, lower hemoglobin, and higher creatinine. Furthermore, the results showed that in addition to having an independent impact on the odds ratio for 5-year survival, the occurrence of VHR episodes was also associated with a higher mortality during the entire follow-up period, as presented in the Kaplan Meier curve in (log rank test p = 0.001 for difference between groups).
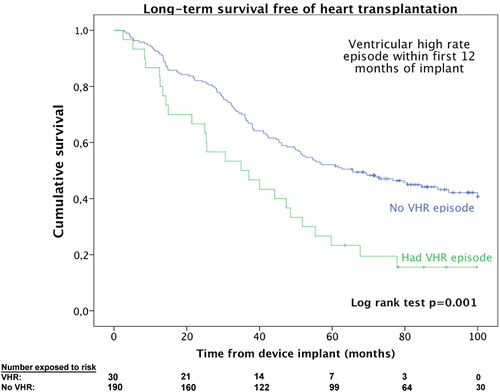
Table IIIa. Univariate logistic regression analysis of 5-year mortality.
Table IIIb. Multivariate logistic regression analysis for 5-year mortality.
Discussion
We present a cohort of consecutive CRT-P treated patients with long-time follow-up data on mortality. In this cohort, we show that the occurrence of VHR-episode(s) during the first year post-implant was an independent predictor of increased 5-year mortality and an inferior very-long-term prognosis. Other independent predictors of mortality were lower values of LVEF and higher levels of creatinine.
The mode of death in the patients with early VHR episodes was most commonly heart failure (60%), which implies that VHR episodes may be an independent marker of more advanced disease in this patient group, rather than a marker for increased risk of malignant arrhythmias. Using data from device interrogation, in addition to clinical data, may help physicians to risk-stratify the patients, and a more vigilant monitoring for worsening heart failure in patients with documented VHR episodes during the first year post-implant may be warranted, knowing that the 5-year mortality odds ratio is increased by a factor of 9.96 in this group of patients. However, the 95% confidence interval was wide (1.4–71.6), and the exact odds ratio must be interpreted with caution.
Impact of VHR episodes
Previous studies have shown that sustained VT within the year after CRT-P implant is associated with adverse short-term outcomes, especially SCD (Citation13), but the significance of NsVT is more uncertain. NsVT is found in a wide range of conditions, and its prognostic significance varies depending on the underlying condition(Citation14). In patients with ischemic heart disease and a value of LVEF < 40%, NsVT has been shown to have an adverse prognostic significance, but in patients with dilated cardiomyopathy, the prognostic significance is not established(Citation15). In a previous study, VHR, defined as a ventricular rate > 162 bpm, has been observed as being an independent predictor of hospitalization for cardiovascular symptoms in patients with AF, and therefore the monitoring of such episodes has been suggested as a tool to identify high-risk subgroups of CRT-patients with AF (Citation16). To our knowledge, however, there are no previous studies suggesting VHR during follow-up as a predictor of mortality in patients with CRT-P.
In our material, VHR episodes were indeed an independent predictor of outcome, and there were no major differences in other patient demographics preoperatively (when stratifying for later VHR episodes); the only significant difference was that patients with VHR during the first year of treatment were somewhat older. Increasing age was not an independent predictor of 5-year survival in the multivariate logistic regression analysis, although increasing age had a significant impact on mortality in the univariate analysis. Previously published risk scores for patients with ICDs have mostly focused on assessment of pre-existing comorbidities as risk factors for high mortality (Citation17–19). However, in addition to the other clinical variables, it is appealing to use the device-derived data from the interrogations at regular follow-up visits, and this extra information may possibly help to identify the patients that will develop malignant arrhythmias. The intention of this study was to further investigate if this data could predict not only mortality, but also cardiac arrest. However, our results do not indicate that VHR episodes are associated with a higher risk of cardiac arrest (4% vs 19%, p = 0.065); on the contrary, there was a trend for higher risk of death from heart failure in this group compared to patients without VHR episodes (60% vs 43%, p = 0.138). The data on cause of death were based on the Swedish National Death Register, and the exact cause of death was not adjudicated by autopsy or post-mortem device interrogation. This allows for a degree of uncertainty regarding the exact cause of death, and although the most plausible interpretation is that early VHR episodes may be a marker of more advanced and more rapidly progressing heart failure disease, the possibility that these patients could also be at a higher risk of developing malignant arrhythmias cannot be excluded. It should also be acknowledged that risk of mortality cannot be evaluated based on the occurrence of early VHR episodes alone, since the sensitivity will be too low for that. Rather, the information regarding VHR has to be added and combined with other clinical data, in order to achieve a complete evaluation of the individual patient.
This was an observational, retrospective study, and therefore, no causal relationship can be detected. The cohort consisted of consecutive patients treated with CRT-P at the same hospital, with long-term follow-up data available. All included patients were from the same distinct geographical area in Sweden, and were covered by the Swedish state health insurance. The patient characteristics compare well with those of the CRT-P treated patients in the Care-HF trial, except that the patients in the present cohort were slightly older (72 vs 66 years), more often had an etiology of ischemic heart failure, and more often used loop-diuretics. Even though a number of patients were excluded in order to allow for a homogenous population with good follow-up data, we believe that the patients in this study are representative of the “standard type” of CRT patients in Sweden, outside of clinical trials. Data collection could have been influenced by the fact that there was no standardized method in which physicians recorded episodes of VHR in the medical records during CRT device follow-up, but device interrogation printouts were typically scanned into the electronic medical records and could be reviewed appropriately. We wanted to examine the impact of VHR episodes according to the previously described definition, and care was taken not to include episodes with rapidly conducted AF. However, it may sometimes be difficult to distinguish between those episodes and VHR according to our definition, and a possible over-estimation of true VHR episodes cannot be excluded. The possibility that physicians did not note VHR episodes in the charts among patients with a low risk profile cannot be excluded, thereby introducing a bias in the material. All pacemakers used were in “factory-mode” and the definition of VHR was similar among the three different manufacturers.
The data on the mode of death was obtained from the validated well-known Swedish Cause of Death Registry, but not individually adjudicated by hospital records or pacemaker interrogation. Differentiating between AMI and malignant arrhythmia post-mortem may be difficult or impossible without an autopsy. However, we did merge all cases of “cardiovascular mortality” into one group for statistical analyses, in order to account for this, and there was still no significant difference between groups. The study was relatively small, which is reflected by the large confidence intervals in the hazard ratios. Relatively few early VHR episodes were registered, suggesting that even slight changes in the number of VHR episodes may have a significant impact on the results. The study should be regarded as hypothesis-generating, and further studies are needed to verify the results.
Conclusion
In heart-failure patients with CRT-P therapy, occurrence of VHR episodes within the first year post-implant was an independent predictor of higher 5-year mortality. The most common cause of death was heart failure, and there was no evidence that these patients had a higher risk of cardiac arrest. Vigilant monitoring for worsening heart failure symptoms may be warranted, to improve prognosis for this high-risk subgroup of patients.
Funding sources
Dr. Platonov and Dr. Borgquist were supported by governmental funding of clinical research within the Swedish National Health Service. Dr Platonov was also supported by a grant from the Swedish Heart and Lung Foundation.
Declaration of interest: None.
References
- Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L; Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49.
- Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–50.
- Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83.
- Lee KL, Hafley G, Fisher JD, Gold MR, Prystowsky EN, Talajic M, et al. Effect of implantable defibrillators on arrhythmic events and mortality in the multicenter unsustained tachycardia trial. Circulation. 2002;106:233–8.
- Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335: 1933–40.
- Daubert JC, Donal E, Linde C. A plea for the wider use of CRT-P in candidates for cardiac resynchronisation therapy. Heart Fail Rev. 2012;17:767–75.
- Kramer DB, Buxton AE, Zimetbaum PJ. Time for a change–a new approach to ICD replacement. N Engl J Med. 2012; 366:291–3.
- Estes NA III. Is it time for a new approach to implantable cardioverter-defibrillator replacement? J Am Coll Cardiol. 2014;63:2395–7.
- Hindricks G, Taborsky M, Glikson M, Heinrich U, Schumacher B, Katz A, et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. Lancet. 2014;384:583–90.
- Bencardino G, A DIM, Rio T, Frontera A, Santangeli P, Leo M, et al. The Association Between ICD Interventions and Mortality is Independent of their Modality: Clinical Implications. J Cardiovasc Electrophysiol. 2014;25:1363–7.
- Reitan C, Bakos Z, Platonov PG, Hoijer CJ, Brandt J, Wang L, Borgquist R. Patient-assessed short-term positive response to cardiac resynchronization therapy is an independent predictor of long-term mortality. Europace. 2014; 16:1603–9.
- Wilton SB, Exner DV, Healey JS, Birnie D, Arnold MO, Sapp JL, et al. Left ventricular lead position and outcomes in the resynchronization-defibrillation for Ambulatory Heart Failure Trial (RAFT). Can J Cardiol. 2013.
- Boveda S, Marijon E, Jacob S, Defaye P, Winter JB, Bulava A, et al. Incidence and prognostic significance of sustained ventricular tachycardias in heart failure patients implanted with biventricular pacemakers without a back-up defibrillator: results from the prospective, multicentre, Mona Lisa cohort study. Eur Heart J. 2009;30:1237–44.
- Katritsis DG, Zareba W, Camm AJ. Nonsustained ventricular tachycardia. J Am Coll Cardiol. 2012;60:1993–2004.
- Katritsis DG, Camm AJ. Nonsustained ventricular tachycardia: where do we stand? Eur Heart J. 2004;25:1093–9.
- Willems R, Morck ML, Exner DV, Rose SM, Gillis AM. Ventricular high-rate episodes in pacemaker diagnostics identify a high-risk subgroup of patients with tachy-brady syndrome. Heart Rhythm. 2004;1:414–21.
- Goldenberg I, Vyas AK, Hall WJ, Moss AJ, Wang H, He H, et al. Risk stratification for primary implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol. 2008;51:288–96.
- Chan PS, Nallamothu BK, Spertus JA, Masoudi FA, Bartone C, Kereiakes DJ, Chow T. Impact of age and medical comorbidity on the effectiveness of implantable cardioverter-defibrillators for primary prevention. Circ Cardiovasc Qual Outcomes. 2009;2:16–24.
- Barsheshet A, Moss AJ, Huang DT, McNitt S, Zareba W, Goldenberg I. Applicability of a risk score for prediction of the long-term (8-year) benefit of the implantable cardioverter- defibrillator. J Am Coll Cardiol. 2012;59:2075–9.
