ABSTRACT
Objective Interpretation of the electrocardiogram (ECG) during exercise is not easy in patients with right bundle branch block (RBBB). Also, the value of exercise echocardiography (ExE) for predicting outcome in them has not been addressed. We sought to assess its prognostic value in patients with RBBB and known/suspected coronary disease. Design Retrospective analysis of data on 703 patients with RBBB who were submitted to a clinically-indicated ExE. The end points were overall mortality and combined myocardial infarction and cardiovascular mortality. Results During follow-up (4.1 ± 4.5 years) there were 130 deaths and 108 combined events. Independent predictors of combined events were history of coronary artery disease (hazard ratio [HR] = 2.37, 95% Confidence Interval [CI] = 1.24–4.52, p = 0.009) resting wall motion score index (HR = 2.14, 95% CI = 1.12–4.10, p = 0.02), metabolic equivalents (HR = 0.89, 95% CI = 0.93–0.97, p = 0.007), Δ in double product with exercise (HR = 0.96, 95% CI = 0.92–1.00, p = 0.036) and Δ in left ventricular ejection fraction (LVEF) with exercise (HR = 0.97, 95% CI = 0.94–0.99, p = 0.01). Neither positive clinical nor ECG exercise testing was predictive. Combined event rates were 3.3% in patients with ΔLVEF > 5%, 4.7% in those with ΔLVEF between 1–5% and 8.2% in those with no increase (Δ < 1%). Conclusions A decrease in LVEF during exercise is predictive of serious events in patients with RBBB.
Introduction
Right bundle branch block (RBBB) is a common electrocardiographic abnormality whose prognostic implications depend on the presence of underlying cardiac disease. While subjects with isolated RBBB have a good prognosis,[Citation1] the presence of this finding in patients with coronary artery disease (CAD) portends a worse outcome.[Citation2–5] Although exercise ECG is commonly used as the initial diagnostic technique in these patients [Citation6] the diagnosis of exercise-induced ST-segment depression may be challenging in patients with RBBB.[Citation7] Therefore the need for imaging techniques in these patients remains controversial. Previous studies have established the prognostic value of pharmacological stress echocardiography in patients with RBBB.[Citation8,Citation9] However, despite exercise should be the stress of choice whenever possible,[Citation10] the value of exercise echocardiography (ExE) for predicting outcomes in these patients has not been evaluated. Thus, we sought to assess whether ExE yields additional information for the prediction of events beyond clinical data and exercise ECG results in patients with RBBB and known or suspected CAD.
Methods
Patients
Between March 1995 and July 2013, 13 442 patients with known or suspected CAD under-went a first treadmill ExE at our institution. Of them, 703 patients (5.2%) with complete RBBB were identified.
Demographic data, clinical characteristics and stress testing results were entered in our prospective database at the time of the tests. Complete RBBB was defined by the following criteria: (1) QRS duration ≥120 ms; (2) rsr’, rsR’ or rSR’ patterns in leads V1-V2; and (3) wide S waves in leads I, V5, V6 with S wave duration > R wave.[Citation11]
Patients referred for evaluation of chest pain were classified as having typical angina, probable angina or non-ischemic chest pain.[Citation6] A history of CAD was defined as previous myocardial infarction, previous coronary revascularization, or previous angiography demonstrating CAD no suitable for revascularization. All patients gave informed consent before testing, and the study was approved by our local research ethics committee.
Exercise echocardiography
Blood pressure, heart rate, and a 12-lead ECG were obtained at baseline and at each stage of the exercise protocol. Patients underwent a treadmill exercise test until they reached an end point, including physical exhaustion, severe angina, significant arrhythmia, severe hypertension (systolic blood pressure >240 mm Hg or diastolic blood pressure >110 mm Hg), or severe hypotensive response (decrease >20 mm Hg in systolic blood pressure from baseline). ECG changes were considered ischemic if a ST-segment shift of ≥0.1 mV from baseline at 80 ms after the J point occurred in leads V5 and V6 in the absence of Q waves.[Citation12] ECG changes were not considered as criteria for test positivity in the absence of wall motion abnormalities. Exercise protocols were the standard Bruce protocol in 612 patients (87%), and other protocols in 91 patients (13%).
Echocardiographic imaging was performed from the apical long-axis, apical four- and two-chambers and parasternal long- and short-axis views, at rest, at peak exercise and immediately after exercise. The methodology employed for imaging acquisition at peak exercise has been previously described.[Citation13–15] Briefly, images were acquired with the patient still exercising, when signs of exhaustion were present or an end point was achieved. When necessary, patients were asked to walk quickly rather than run, to decrease body movements. The transducer was firmly placed on the apical and parasternal areas and pressure was applied to the patient’s back with the left hand to decrease the relative movement between the transducer and the patient’s body. Exercise images were obtained with a continuous imaging capture system. The best-quality images corresponding to each view were selected for comparison with those acquired at rest. Echocardiographic 2-dimensional analysis was performed on a digital quad-screen display system. Wall motion abnormalities (WMAs) were matched to a 16-segment model of the left ventricle,[Citation16] and each segment was assigned a score, with 1 being normal, 2 being hypokinetic, 3 being akinetic, and 4 being dyskinetic. Wall motion score index (WMSI) was calculated at rest and at exercise as the sum of the scores divided by the number of segments. Ischemia was defined as the development of new or worsening WMAs with exercise, except worsening from akinesia to dyskinesia, and isolated hypokinesia of the inferobasal segment.[Citation17] A fixed WMAs or necrosis was considered when there were WMAs that did not impair with exercise. Abnormal ExE was defined as an ExE showing ischemia or fixed WMAs, independently of the clinical history or any other characteristics of the test. Resting and exercise left ventricular ejection fraction (LVEF) were estimated visually or by the biplane Simpson’s method.[Citation18] Variability in the assessment of LVEF at rest and at exercise was calculated in 30 randomized patients. Whenever possible, beta-blocker therapy was discontinued for at least 48 h before testing. However, 7.8% of the patients were still under the influence of beta-blocker drugs at the time of their tests.
Outcomes
Follow-up data were retrieved from healthcare databases, medical records and death certificates, as well as by telephone interviews when necessary. The end-points were a combined event of myocardial infarction and cardiovascular mortality and also overall mortality to avoid ascertainment of the cause of death.[Citation19]
Statistical analysis
Categorical variables were reported as percentages and comparison between groups based on the chi-square test. Continuous variables were reported as mean ± standard deviation and differences were assessed with the unpaired t-test or Mann–Whitney U test as appropriate.
Survival free of the end point of interest was estimated by the Kaplan–Meier method, and survival curves were compared with the log-rank test. For the analysis of combined events, patients were censored at the time of any revascularization previous to the end point. For the analysis of overall mortality, patients were not censored. Univariable and multivariable associations of clinical, exercise and echocardiographic variables with overall mortality were assessed with Cox’s proportional hazard models. Hazard ratios (HR) with 95% confidence intervals (CI) were estimated. In case of variables with similar meaning, continuous variables were chosen instead of dichotomous variables. In case of continuous variables highly correlated (for example WMSI and LVEF), those having higher area under the curve in the receiver operator curve analysis were used. Variability in the assessment of LVEF at rest and at exercise and in the assignation of the Δ in LVEF from rest to exercise to one of three categories (<1, 1–5, >5) or to one of two categories (<1 or ≥1) was performed by interclass correlation coefficients and k analysis, respectively. The Statistical Package for Social Sciences software, version 15.0 (SPSS, Chicago, IL) was used for all analyses.
Results
Inter- and intra-observer variability for the assessment of LVEF at rest and at exercise
LVEF was visually estimated for most of the patients (94.3%). The assessment by the biplane Simpson’s method was only used in a minority (5.7%). Variability for the assessment of LVEF at rest and at exercise by interclass correlation coefficients is shown in . Variability for the classification of the Δ in LVEF in the three different categories (<1, 1–5, >5) was moderate, with k=0.66 when performed by the same observer and k=0.53 when performed by a different observer (both p < 0.001). Kappa values for variability of Δ in LVEF in two categories (<1 or ≥1) were k=0.73 when performed by the same observer (p < 0.001) and k=0.63 when another observer was used (p = 0.001).
Table 1. Intraclass correlation coefficients.
Baseline characteristics and ExE results
Clinical baseline characteristics, exercise ECG and exercise echocardiography results are depicted in and . shows graphically how the percentage of patients with ischemia and abnormal ExE results was higher as the Δ in LVEF was lower.
Figure 1. Percentage of patients with ischemia and abnormal ExE results among those with Δ in ejection fraction > 5%, Δ in ejection fraction between 1 and 5%, and no increase (Δ < 1%).
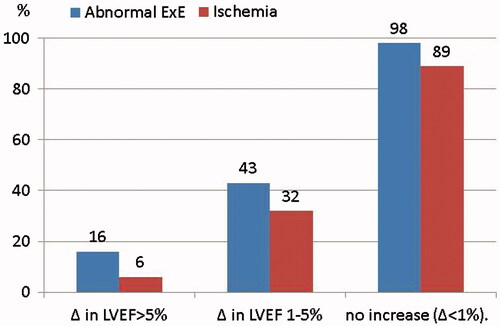
Table 2. Baseline characteristics of 703 patients with right bundle branch block.
Table 3. Exercise ECG and exercise echocardiography data of the 703 patients.
Outcomes
During a mean follow-up of 4.1 years (range 17.4 years), 130 patients died and 108 had a combined event. Univariate and multivariate associations with overall mortality and with combined events are listed in and , respectively. In the multivariate analysis, Δ in LVEF remained an independent predictor for either overall mortality and for the prediction of combined events. Whether peak WMSI was introduced in the model instead of Δ in LVEF, it also resulted an independent predictor of overall mortality (hazard ratio 1.75, 95% confidence interval 1.04–2.92, p = 0.03).
The annualized combined event rates were 3.3% in patients with ΔLVEF > 5%, 4.7% in those with ΔLVEF between 1–5% and 8.2% in those with no increase in LVEF (p < 0.001). The respectively corresponding figures for mortality were 3, 4.3 and 7%. Annualized combined event and mortality rates in patients with and without ischemia were 6.3% and 3.8%% (p = 0.009), and 6% and 3.7% (p = 0.006), respectively. Annualized combined event and mortality rates were also higher in patients with abnormal than with normal ExE (8.4% versus 3.6%, and 5.7% versus 3.5%, both p = 0.01). and show the survival curves and the combined event free survival curves in patients subdivided according to their increases in LVEF and according to the presence or absence of ischemia, respectively.
Figure 2. Overall mortality curves (A) and combined events curves (B) for patients with increases in LVEF > 5%, between 1-5% and with no increase.
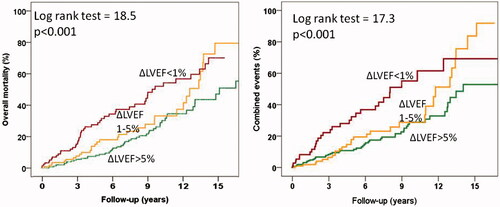
Figure 3. Overall mortality curves (A) and combined events curves (B) for patients with and without ischemia during exercise echocardiography.
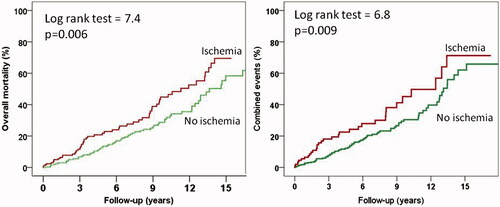
Table 4. Univariate and multivariate predictors of overall mortality.
Table 5. Univariate and multivariate predictors of combined myocardial infarction and cardiovascular mortality.
Discussion
This study demonstrates that ExE provides significant prognostic information for the prediction of adverse events in patients with RBBB. To our knowledge, this study is the first to assess the prognostic value of ExE in these patients. RBBB was found in 5% of patients referred for exercise echocardiography to our institution, which is concordant with the 3% of patients with RBBB found among those referred for a nuclear perfusion study.[Citation20] Although a change in the ST segment may have diagnostic value in these patients, up to 40% of the patients included in our study were deemed to have non-diagnostic ECGs, due to baseline ST abnormalities that reached the lateral leads. This issue is noteworthy because current guidelines consider that the ECG is in general interpretable in patients with right bundle branch block.[Citation6]
The available data on the prognostic value of stress echocardiography in patients with RBBB is limited to pharmacological stress echocardiography.[Citation8,Citation9] Our study complements and expands those findings by addressing the prognostic value of ExE in this subgroup of patients. This is important because exercise rather than pharmacological stress is the recommended type of stress whenever the patients are able to exercise,[Citation10,Citation21] not only because the former is safer, but also because it provides additional prognostic information,[Citation22] such as exercise capacity, that cannot be obtained with pharmacological stress. As in other studies that have assessed the incremental prognostic value of exercise ECG and exercise echocardiography over clinical characteristics in different groups of patients,[Citation14,Citation23–25] it is noteworthy that the highest incremental value corresponds to the exercise testing, thus emphasizing the superiority of ExE over pharmacological stress echocardiography for predicting outcome. Although patients with abnormal findings on ExE, particularly those with higher peak WMSI or decreases in LVEF had worse outcome, it is clear from our results than even patients without ischemia or without decreases in LVEF had increased annualized mortalities up to ≈3–4%. This figure contrast with the <1% events found in patients with a normal stress echocardiogram [Citation25,Citation26] but is similar to the % of overall mortality found in patients with LBBB and absence of ischemia on ExE (5-year mortality rate of 12.6%).[Citation27] It has also been previously reported among patients referred for myocardial perfusion imaging that the RBBB was as strong an independent predictor of mortality as LBBB.[Citation20] We speculate that some of these patients might already have an initial LV cardiomyopathy, as it happens with patients with LBBB. The addition of CAD would increase furthermore their risk.
Although the superiority of ExE over other variables for the prediction of events was statistically demonstrated, the increase in risk found by an abnormal ExE result was not overwhelming and likely should be judged in a clinical consistent context, except for those with the more severe ExE results. For instance, the risk of mortality and of combined events was almost triple in patients with a fall in LVEF in comparison to those in whom the LVEF increased with exercise by more than five points.
Our study has several limitations. This was an observational retrospective study and, as such, it may be susceptible to unmeasured confounding. The results of the tests were available to treating physicians, so patients with ischemic results on ExE may have been more likely to undergo coronary revascularization; therefore, the actual prognostic value of ExE may have been significantly underestimated, particularly in patients with extensive ischemia. In addition, the medication change after testing and its effect on outcome were not assessed. Finally, we performed imaging acquisition at peak exercise because it has higher sensitivity for detecting exercise-induced wall motion abnormalities [Citation13,Citation14] and higher prognostic value than post-exercise imaging.[Citation15] However, this technique is not widely employed, and our results might have differed if we had only performed post-treadmill exercise echocardiography.
Conclusions
Patients with RBBB referred for ExE had annualized mortality rates exceeding 3%, despite normal tests. ExE provides significant prognostic information for predicting outcome in them. Patients with no increase in LVEF or with wall motion abnormalities during ExE have a significantly higher risk of events.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Fahy GJ, Pinski SL, Miller DP, et al. Natural history of isolated bundle branch block. Am J Cardiol. 1996;77:1185–1190.
- Ricou F, Nicod P, Gilpin E, et al. Influence of right bundle branch block on short- and long-term survival after acute anterior myocardial infarction. J Am Coll Cardiol. 1991;17:858–863.
- Sgarbossa EB, Pinski SL, Topol EJ, et al. Acute myocardial infarction and complete bundle branch block at hospital admission: clinical characteristics and outcome in the thrombolytic era. J Am Coll Cardiol. 1998;31:105–110.
- Go AS1, Barron HV, Rundle AC, et al. Bundle-branch block and in-hospital mortality in acute myocardial infarction. National Registry of Myocardial Infarction 2 Investigators. Ann Intern Med. 1998;129:690–697.
- Melgarejo-Moreno A1, Galcerá-Tomás J, Garciá-Alberola A, et al. Incidence, clinical characteristics, and prognostic significance of right bundle-branch block in acute myocardial infarction: a study in the thrombolytic era. Circulation 1997;96:1139–1144.
- Gibbons RJ, Balady GJ, Bricker JT, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Committee to Update the 1997 Exercise Testing Guidelines. ACC/AHA 2002 guideline update for exercise testing:summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). J Am Coll Cardiol. 2002;40:1531–1540.
- Yen RS, Miranda C, Froelicher VF. Diagnostic and prognostic accuracy of the exercise electrocardiogram in patients with preexisting right bundle branch block. Am Heart J. 1994;127:1521–1525.
- Biagini E, Schinkel AF, Rizzello V, et al. Prognostic stratification of patients with right bundle branch block using dobutamine stress echocardiography. Am J Cardiol. 2004;94:954–957.
- Cortigiani L, Bigi R, Gigli G, et al. Prediction of mortality in patients with right bundle branch block referred for pharmacologic stress echocardiography. Am J Cardiol. 2003;92:1429–1433.
- Pellikka PA, Nagueh SF, Elhendy AA, et al. American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr. 2007;20:1021–1041.
- Agarwal AK, Venugopalan P. Right bundle branch block: varying electrocardiographic patterns. Aetiological correlation, mechanisms and electrophysiology. Int J Cardiol. 1999;71:33–39.
- Chaitman BR. Exercise stress testing. In Braunwald E. Zipes DP, Libby P, eds. Heart disease: a textbook of cardiovascular medicine. 6th ed. Philadelphia, PA: WB Saunders Co; 2001. p.:129–159.
- Peteiro J, Fabregas R, Montserrat L, et al. Comparison of treadmill exercise echocardiography before and after exercise in the evaluation of patients with known or suspected coronary artery disease. J Am Soc Echocardiogr. 1999;12:1073–1079.
- Peteiro J, Garrido I, Monserrat L, et al. Comparison of peak and postexercise treadmill echocardiography with the use of continuous harmonic imaging acquisition. J Am Soc Echocardiogr. 2004;17:1044–1049.
- Peteiro J, Bouzas-Mosquera A, Broullón FJ, et al. Prognostic value of peak and post-exercise treadmill exercise echocardiography in patients with known or suspected coronary artery disease. Eur Heart J. 2010;31:187–195.
- Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–67.
- Hoffmann R, Lethen H, Marwick T, et al. Standardized guidelines for the interpretation of dobutamine echocardiography reduce interinstitutional variance in interpretation. Am J Cardiol. 1998;82:1520–1524.
- Stamm RB, Carabello BA, Mayers DL, et al. Two-dimensional echocardiographic measurement of left ventricular ejection fraction: prospective analysis of what constitutes an adequate determination. Am Heart J. 1982;104:136–144.
- Lauer MS, Blackstone EH, Young JB, et al. Cause of death in clinical research: time for a reassessment? J Am Coll Cardiol. 1999;34:618–620.
- Hesse B, Diaz LA, Snader CE, et al. Complete bundle branch block as an independent predictor of all-cause mortality: report of 7,073 patients referred for nuclear exercise testing. Am J Med 2001;110: 253–9.
- Wolk MJ, Bailey SR, Doherty JU, et al. American College of Cardiology Foundation Appropriate Use Criteria Task Force. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS: 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;63:380–406.
- Lauer MS. The “exercise” part of exercise echocardiography. J Am Coll Cardiol 2002;39:1353–1355.
- Bouzas-Mosquera A, Peteiro J, Alvarez-Garcia N, et al. Prediction of mortality and major cardiac events by exercise echocardiography in patients with normal exercise electrocardiographic testing. J Am Coll Cardiol. 2009;53:1981–1990.
- Peteiro J, Monserrrat L, Pineiro M, et al. Comparison of exercise echocardiography and the Duke treadmill score for risk stratification in patients with known or suspected coronary artery disease and normal resting electrocardiogram. Am Heart J. 2006;151:1324.e1–10.
- Peteiro J, Bouzas-Mosquera A, Broullón F, et al. Value of an exercise workload ≥10 metabolic equivalents for predicting inducible myocardial ischemia. Circ Cardiovasc Imaging. 2013;6:899–907.
- Metz LD, Beattie M, Hom R, et al. The prognostic value of normal exercise myocardial perfusion imaging and exercise echocardiography: a meta-analysis. J Am Coll Cardiol. 2007;49:227–237.
- Bouzas-Mosquera A, Peteiro J, Alvarez-García N, et al. Prognostic value of exercise echocardiography in patients with left bundle branch block. JACC Cardiovasc Imaging. 2009;2:251–259.
