Abstract
A series of biologically active oxovanadium(IV) complexes of triazole derived Schiff bases L1–L5 have been synthesized and characterized by their physical, analytical, and spectral data. The synthesized ligands potentially act as bidentate, in which the oxygen of furfural and nitrogen of azomethine coordinate with the oxovanadium atom to give a stoichiometry of vanadyl complexes 1:2 (M:L) in a square-pyramidal geometry. In vitro antibacterial and antifungal activities on different species of pathogenic bacteria (E. coli, S. flexneri, P. aeruginosa, S. typhi, S. aureus, and B. subtilis) and fungi (T. longifusus, C. albicans, A. flavus, M. canis, F. solani, and C. glabrata) have been studied. All compounds showed moderate to significant antibacterial activity against one or more bacterial strains and good antifungal activity against most of the fungal strains. The brine shrimp bioassay was also carried out to check the cytotoxicity of coordinated and uncoordinated synthesized compounds.
Introduction
Diabetes mellitus (DM) is a lethal metabolic disease, which is caused by an increased blood sugar level. Chronic diabetes mellitus becomes a source of various secondary diseases such as retinopathy, microangiopathy, renal dysfunction, atherosclerosis, and ocular and cardiac disordersCitation1. Many drug therapies are in practice to address this severe emerging issue. Amongst them, vanadium-based metallotherapy has provedCitation2,Citation3 to be one of the most effective clinical remedies. Vanadium is an important trace transition metalloelement that exists in variable oxidation states, and many reports prove its insulin mimetic activityCitation4,Citation5. It interacts mostly with those biomolecules that have negatively charged oxygen donor groups such as carboxylate, phenolate, phosphate, phosphonate, and hydroxamateCitation6. Apart from the potential insulin mimetic activity, the structural behavior of various vanadyl compounds is also a subject of great interest. The mechanism involved in the insulin-like effects of vanadium compounds is not yet clearly understoodCitation7.
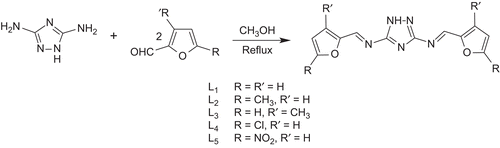
Triazoles are extensively used as potential ligands in various bioinorganic syntheses. They coordinate to the metal ion in many ways, depending upon the nature of the functional groupsCitation8–11. Studies have indicated that many triazole derived Schiff bases show antibacterialCitation12–14, antifungalCitation15,Citation16, antimicrobialCitation17–20, anticancerCitation21,Citation22, analgesicCitation23, anticonvulsantCitation24, antitumorCitation25–27, antitubercularCitation28–31, insecticidal, herbicidal, and plant growth regulatoryCitation32–34 activities. Due to increased interest in the chemistry of vanadyl compounds and the potentially bioactive nature of triazoles, we have made an effort to combine the chemistry of vanadium(IV) with newly synthesized triazole Schiff bases L1–L5 (). The vanadyl(IV) Schiff base compounds were formed with a stoichiometric ratio of M:L (1:2) where M = V(IV) and L = L1–L5. The synthesized compounds have been characterized by their physical, analytical, and spectral data. In order to evaluate the effect of vanadium(IV) metal on the antibacterial and antifungal activity of the newly synthesized compounds, the synthesized Schiff bases and their vanadyl(IV) complexes have been subjected to in vitro antibacterial activity testing against Escherichia coli, Shigella flexneri, Pseudomonas aeruginosa, Salmonella typhi, Staphylococcus aureus, and Bacillus subtilis and antifungal activity testing against Trichophyton longifusus, Candida albicans, Aspergillus flavus, Microsporum canis, Fusarium solani, and C. glabrata. The in vitro brine shrimp bioassay has also been carried out to study the cytotoxic properties of these compounds.
Materials and methods
All reagents and solvents used were of analytical grade. Infrared (IR) spectra were recorded on a Shimadzu FT-IR spectrophotometer. Elemental analysis was carried out on a PerkinElmer analyzer. 1H and 13C nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Spectrospin Avance DPX-400 spectrometer using tetramethylsilane (TMS) as an internal standard and dimethylsulfoxide (DMSO)-d6 as a solvent. Electron impact mass spectra (EIMS) were recorded on a Jeol MS Route instrument. In vitro antibacterial, antifungal, and cytotoxic properties were studied at the HEJ Research Institute of Chemistry, International Center for Chemical Sciences, University of Karachi, Pakistan.
Synthesis of Schiff base ligands
General procedure
N,N’-Bis[(E)-furan-2-ylmethylidene]-1H-1,2,4-triazole-3,5-diamine (L1) To a hot, magnetically stirred methanol (40 mL) solution of 3,5-diamino-1,2,4-triazole (0.99 g, 0.01 M), furan-2-carboxaldehyde (1.66 mL, 0.02 M) in methanol (20 mL) was added with constant stirring. The solution was refluxed for 4 h, during which a precipitated product was formed. The solution was refluxed for another 1 h. It was then cooled to room temperature, filtered, washed with methanol (3 × 5 mL) then with diethyl ether (2 × 5 mL), and dried. The same method was applied for the preparation of all other ligands L2–L5.
Physical, analytical, and spectral data of the ligands
N,N’-Bis[(E)-furan-2-ylmethylidene]-1H-1,2,4-triazole-3,5-diamine (L1)
Yield (1.90 g, 74%); m.p. 172°C; IR (KBr, cm−1): 3188 (NH), 1020 (N-N), 1627 (HC=N), 1610 (C=N, triazole), 1579, 1560 (C=C); 1H NMR (DMSO-d6): δ 7.1 (dd, 2H, furanyl), 7.39 (d, 2H, furanyl), 7.93 (d, 2H, furanyl), 8.75 (s, 2H, N=CH), 11.92 (s, 1H, NH); 13C NMR (DMSO-d6): δ 122.73, 126.15, 138.26, 146.39 (furanyl), 155.19, (2 × C, triazole), 166.2 (C=N, azomethine); EIMS (70 eV) m/z (%): 255 ([M]+, 23), 228 (98), 177 (100), 161 (8), 134 (11), 121 (73), 120 (29), 108 (10), 97 (10), 78 (20), 52 (18); Anal. Calcd. for C12H9N5O2 (255.23): C, 56.47; H, 3.55; N, 27.44; Found: C, 56.43; H, 3.53; N, 27.38%.
N,N’-Bis[(E)-(5-methylfuran-2-yl)methylidene]-1H-1,2,4-triazole-3,5-diamine (L2)
Yield (1.16 g, 81%); m.p. 236°C; IR (KBr, cm−1): 3195 (NH), 1019 (N-N), 1626 (HC=N), 1609 (C=N, triazole), 1581, 1563 (C=C); 1H NMR (DMSO-d6): δ 2.36 (s, 6H, CH3), 7.07 (d, 2H, furanyl), 7.23 (d, 2H, furanyl), 8.64 (s, 2H, N=CH), 11.84 (s, 1H, NH); 13C NMR (DMSO-d6): δ 14.5 (CH3-furanyl), 121.33, 124.92, 141.67, 147.71 (furanyl), 155.12 (2 × C, triazole), 164.82 (C=N, azomethine); EIMS (70 eV) m/z (%): 283 ([M]+, 36), 268 (8), 191 (100), 176 (16), 134 (23), 120 (22), 78 (19), 63 (13); Anal. Calcd. for C14H13N5O2 (283.28): C, 59.36; H, 4.63; N, 24.72; Found: C, 59.32; H, 4.60; N, 24.65%.
N,N’-Bis[(E)-(3-methylfuran-2-yl)methylidene]-1H-1,2,4-triazole-3,5-diamine (L3)
Yield (0.96 g, 67%); m.p. 242°C; IR (KBr, cm−1): 3192 (NH), 1018 (N-N), 1628 (HC=N), 1608 (C=N, triazole), 1583, 1565 (C=C); 1H NMR (DMSO-d6): δ 2.26 (s, 6H, CH3), 7.11 (d, 2H, furanyl), 7.74 (d, 2H, furanyl), 8.68 (s, 2H, N=CH), 11.89 (s, 1H, NH); 13C NMR (DMSO-d6): δ 11.3 (CH3-furanyl), 119.86, 125.11, 139.3, 146.63 (furanyl), 155.95 (2 × C, triazole), 164.44 (C=N, azomethine); EIMS (70 eV) m/z (%): 283 ([M]+, 16), 268 (100), 253 (35), 191 (21), 176 (34) 134 (13), 108 (48), 81 (14), 53 (10); Anal. Calcd. for C14H13N5O2 (283.28): C, 59.36; H, 4.63; N, 24.72; Found: C, 59.34; H, 4.59; N, 24.78%.
N,N’-Bis[(E)-(5-chlorofuran-2-yl)methylidene]-1H-1,2,4-triazole-3,5-diamine (L4)
Yield (1.64 g, 72%); m.p. 227°C; IR (KBr, cm−1): 3185 (NH), 1021 (N-N), 1630 (HC=N), 1612 (C=N, triazole), 1576, 1557 (C=C), 810 (C-Cl); 1H NMR (DMSO-d6): δ 7.32 (d, 2H, furanyl), 7.39 (d, 2H, furanyl), 8.78 (s, 2H, N=CH), 11.95 (s, 1H, NH); 13C NMR (DMSO-d6): δ 123.81, 128.35, 136.61, 148.23 (furanyl), 157.65 (2 × C, triazole), 165.18 (C=N, azomethine); EIMS (70 eV) m/z (%): 324 ([M]+, 22), 289 (100), 253 (15), 176 (8), 155 (16), 140 (14), 134 (24), 129 (19), 112 (16), 76 (11); Anal. Calcd. for C12H7Cl2N5O2 (324.12): C, 44.47; H, 2.18.; N, 21.61; Found: C, 44.43; H, 2.12; N, 21.57%.
N,N’-Bis[(E)-(5-nitrofuran-2-yl)methylidene]-1H-1,2,4-triazole-3,5-diamine (L5)
Yield (1.96 g, 80%); m.p. 198°C; IR (KBr, cm−1): 3180 (NH), 1023 (N-N), 1632 (HC=N), 1613 (C=N, triazole), 1570, 1555 (C=C), 1355 (NO2); 1H NMR (DMSO-d6): δ 7.84 (d, 2H, furanyl), 7.99 (d, 2H, furanyl), 8.86 (s, 2H, N=CH), 12.1 (s, 1H, NH); 13C NMR (DMSO-d6): δ 121.12, 127.34, 142.71, 149.65 (furanyl), 158.1 (2 × C, triazole), 166.12 (C=N, azomethine); EIMS (70 eV) m/z (%): 345 ([M]+, 6), 299 (12), 222 (100), 176 (22), 166 (11), 152 (24), 134 (62), 110 (13), 79 (53), 68 (9), 57 (25), 51 (26); Anal. Calcd. for C12H7N7O6 (345.22): C, 41.75; H, 2.04; N, 28.40; Found: C, 41.79; H, 2.08; N, 28.38%.
Synthesis of vanadyl(IV) complexes
General procedure
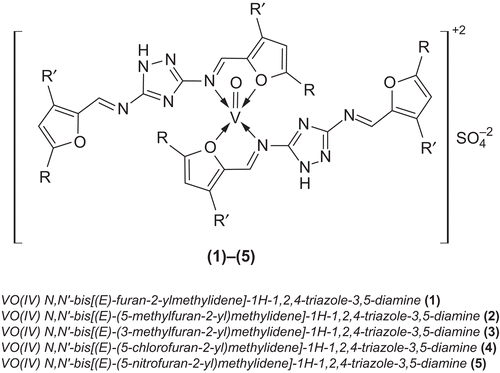
Oxovanadium(IV) complex of N,N’-bis[(E)-furan-2-ylmethylidene]-1H-1,2,4-triazole-3,5-diamine (1) To a hot, magnetically stirred 1,4-dioxane (50 mL) solution of L1 (1.02 g, 0.002 mol), a methanolic solution (20 mL) of vanadyl sulfate (0.163 g, 0.001 mol) was added. The mixture was refluxed for 3 h during which precipitated product was formed. It was then cooled to room temperature. The precipitates thus formed were filtered, washed with methanol (3 × 5 mL) then with diethyl ether, and dried. All other complexes (2)–(5) were prepared following the same method using the same vanadyl salt with different ligands, respectively ().
Antibacterial studies
All the synthesized compounds L1–L5 and their oxovanadium(IV) complexes (1)–(5) were screened in vitro for their antibacterial activity against four Gram-negative (E. coli, S. flexneri, P. aeruginosa, S. typhi) and two Gram-positive (S. aureus, B. subtilis) bacterial strains by the agar-well diffusion methodCitation35,Citation36. The wells (6 mm in diameter) were dug in the media with the help of a sterile metallic borer with centers at least 24 mm apart. Two to eight hours old bacterial inocula containing approximately 104–106 colony-forming units (CFU/mL) were spread on the surface of the nutrient agar with the help of a sterile cotton swab. The recommended concentration of test sample (1 mg/mL in DMSO) was introduced in the respective wells. Other wells supplemented with DMSO and reference antibacterial drug, imipenem, served as negative and positive controls, respectively. The plates were incubated at 37°C for 24 h. Activity was determined by measuring the diameter of the zone showing complete inhibition (mm). In order to clarify any participating role of DMSO in the biological screening, separate studies were carried out with solutions of DMSO alone and showed no activity against any bacterial strain.
Antifungal activity (in vitro)
Antifungal activities of all compounds were studiedCitation37 against six fungal strains (T. longifusus, C. albicans, A. flavus, M. canis, F. solani, and C. glabrata). Sabouraud dextrose agar (Oxoid, Hampshire, England) was seeded with 105 CFU/mL fungal spore suspensions and transferred to Petri plates. Disks soaked in 20 mL (200 µg/mL in DMSO) of the compounds were placed at different positions on the agar surface. The plates were incubated at 32°C for 7 days. The results were recorded as percentage inhibition and compared with standard drugs miconazole and amphotericin B.
Minimum inhibitory concentration
Compounds containing high antibacterial activity were selected for minimum inhibitory concentration (MIC) studies. The minimum inhibitory concentration was determined using the disk diffusion techniqueCitation38 by preparing disks containing 10, 25, 50, and 100 μg/mL of the compounds and applying the protocolCitation39.
Cytotoxicity (in vitro)
Brine shrimp (Artemia salina Leach) eggs were hatched in a shallow rectangular plastic dish (22 × 32 cm) filled with artificial seawater, which was prepared with a commercial salt mixture and double distilled water. An unequal partition was made in the plastic dish with the help of a perforated device. Approximately 50 mg of eggs were sprinkled into the large compartment, which was darkened, while the other compartment was opened to ordinary light. After 2 days a pipette collected nauplii from the light side. A sample of the test compound was prepared by dissolving 20 mg of each compound in 2 mL of dimethyl formamide (DMF). From this stock solution, 500, 50, and 5 µg/mL were transferred to nine vials (three for each dilution were used for each test sample, and LD50 is the mean of three values) and one vial was kept as a control, having 2 mL of DMF only. The solvent was allowed to evaporate overnight. After 2 days, when shrimp larvae were ready, 1 mL of seawater and 10 shrimps were added to each vial (30 shrimps/dilution) and the volume was adjusted with seawater to 5 mL per vial. After 24 h the number of survivors was counted. Data were analyzed by a Finney computer program to determine the LD50 valuesCitation40,Citation41.
Results and discussion
Chemistry
The triazole-derived Schiff bases L1–L5 were prepared as shown in . All ligands were only soluble in dioxane, DMF, and DMSO, but not in common organic solvents. The composition of the ligands is consistent with their microanalytical data. The oxovanadium(IV) complexes (1)–(5) were prepared according to the following equations:
Physical measurements and micronalytical data for complexes (1)–(5) are given in .
Table 1. Micronalytical data of the oxovanadium(IV) complexes.
Physical measurements (conductance and magnetic susceptibility)
The molar conductance values (in DMF) of complexes (1)–(5) fall within the range 80–89 Ω−1 cm2 mol−1 (), showing their electrolyticCitation42 nature. The room temperature magnetic moment values of the complexes are given in . The observed magnetic moment (1.70–1.78 BM) is consistent with half-spin (S = 1/2) square-pyramidal geometry of the oxovanadium(IV) complexesCitation43.
Table 2. Physical, spectral, and IR data of oxovanadium(IV) complexes.
IR spectra
IR spectra of the Schiff bases showed the absence of bands at 1735 and 3325 cm−1, originally assigned to carbonyl ν(C=O) and ν(NH2) stretching vibrations. The appearance of a strong new band at 1626–1632 cm−1 gave a clue of condensation of the carbonyl ν(C=O) group of furfuraldehyde with the amino groups of triazole to develop an azomethine ν(HC=N) linkageCitation44. IR peaks at 1610–1613 cm−1 and 1555–1583 cm−1 were assigned to ν(C=N) and ν(C=C), respectively. Comparison of the IR spectra of the Schiff bases with their vanadyl complexes indicated that the Schiff bases were principally coordinated to the metal ion bidentately through oxygen of furfural and nitrogen of triazole. The band appearing at 1616–1623 cm−1 in the spectra of the vanadyl complexes due to the azomethine vibration was shifted to a lower frequency by 10–15 cm−1, indicatingCitation45 coordination of the azomethine nitrogen to the vanadyl metal atom. Furthermore, the coordination of these Schiff bases with the vanadyl metal ion was confirmed by the appearance of weak low-frequency new bands at 455–466 and 485–496 cm−1, assigned to ν(V-N) and ν(V-O), respectively (). These new bands were only assignable to the spectra of the vanadyl complexes and not to the spectra of the Schiff bases. This, in turn, supported the evidence of the participation of heteroatoms, O and N, in the coordination. The presence of a band at 3180–3195, 1605–1611, and 1018–1023 cm−1, due to NH, C=N, and N-N vibrations of triazole, remained unchanged in all the ligands, indicating that NH, C=N, and N-N of triazole were not taking part in the complexation, respectively. In all the vanadyl complexes, bands appearing at 978–982 and 1085–1088 cm−1 were assigned to ν(V=O)Citation43 and attributed due to SO4Citation46. All this evidence corresponds with formation of the vanadyl(IV) complexes with the prepared Schiff bases.
1H NMR spectra
1H NMR spectra of the free ligands were recorded in DMSO-d6. The 1H NMR spectral data along with the possible assignments are recorded above in “Physical, analytical, and spectral data.” All the protons due to heteroaromatic/aromatic groups were found to be in their expected regionCitation47. 1H NMR spectra of the compounds L1–L5 displayed a characteristic azomethine (CH=N) peak at δ 8.64–8.86 as a singlet, and protons of the furanyl ring fell in the region of δ 7.07–7.99 as doublets, except for the H-3 proton of L1 that was found at δ 7.1 as a double doublet. A singlet at δ 11.84–12.1 was observed for the NH proton in all ligands. Ligands L2 and L3 displayed a singlet at around δ 2.26–2.36, attributable to the methyl group. Furthermore, the number of protons calculated from the integration curvesCitation48 and those obtained from the values of the expected, CHN analysis agreed well with each other.
13C NMR spectra
13C NMR spectra of the free ligands were also recorded in DMSO-d6. The 13C NMR spectral data along with the possible assignments are recorded above in “Physical, analytical, and spectral data.” The conclusions drawn from these studies present further support to the modes of bonding discussed above for their IR and 1H NMR spectra. The 13C NMR spectra of the ligands L1–L5 showed an azomethine carbon (CH=N) at δ 164.44–166.2. The furanyl carbons were present in the region of δ 119.86–149.65. Triazole carbons appeared in the region of δ 155.12–158.1 in all ligands. In L2–L3, (CH3 × 4) appeared in the region of δ 11.84–11.89. Moreover, the numbers of carbons present were well in agreement with the expected values.
Mass spectra
The electron impact mass spectral (EIMS) compositions were: C12H9N5O2, 255 (calcd. 255.23); C14H13N5O2, 283 (283.28); C14H13N5O2, 283 (283.28); C12H7Cl2N5O2, 324 (324.12); C12H7N7O6, 345 (345.22). L1 showed a base peak at 177 of fragment [C7H7N5O]+; for L2 this was observed at 267 of fragment [C13H9N5O2]+; for L3 at 267 of fragment [C13H9N5O2]+; for L4 at 288 of fragment [C12H7ClN5O2]+; and for L5 at 299 of fragment [C12H7N6O4]+; these are the most expected stable fragments. The most likely fragmentation pattern followed the cleavage of C=N (exocyclic as well as endocyclic), C=C, C-C, C-Cl, and C-NO2 bonds.
Electronic spectra
The electronic spectra of the oxovanadium(IV) complexes in DMF exhibited three distinct low-intensity bands (labeled as ν1, ν2 and ν3) which were assigned to b2 (dxy) → eπ(dxz, dyz), b2 (dxy) → b1 (dx2–y2), and b2 (dxy) → a1 (dz2) transitions, respectivelyCitation49. The first band at 13,250–13,410 cm−1 can be assigned to b2 → eπ d-d transitions. The second band at 18,790–18,910 cm−1 can be attributed to b2 → b1, and the band at 29,850–29,970 cm−1can be assigned to transitions b2 → a1. These observations correspond with the square-pyramidal geometry of the oxovanadium(IV) complexesCitation50–52.
Biological activity
Antibacterial bioassay (in vitro)
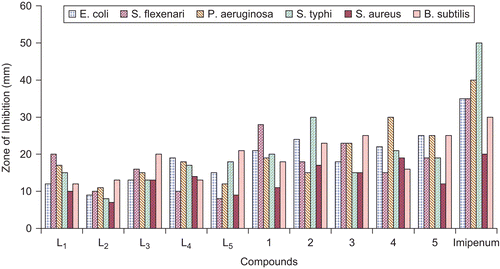
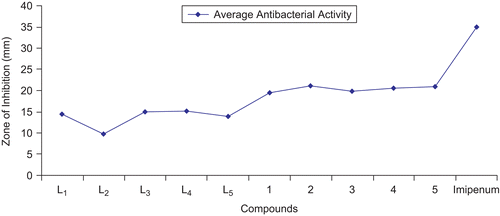
The in vitro antibacterial results are summarized in and and . The antibacterial studies revealed that all the triazole derived Schiff bases and their oxovanadium(IV) complexes contributed significantly toward enhancing the biological activity. It is evident that coordination made the ligands more strongly antibacterial and inhibited the growth of bacteria more, compared with the parent ligandCitation53,Citation54. All compounds were tested against four Gram-negative (E. coli, S. flexneri, P. aeruginosa, S. typhi) and two Gram-positive (S. aureus, B. subtilis) bacterial strains () according to literature protocolsCitation35,Citation36. The results were compared with those of the standard drug imipenem (). The percentage of activity was compared with the activity of the standard drug, considering its activity as 100%. All ligands and their vanadyl complexes possessed good biological activity against all Gram-negative and Gram-positive bacterial strains. L1 showed significant (57%) activity against S. flexneri, (b), and a weaker (30%) activity against S. typhi, (d). A greater (42.5%) activity for P. aeruginosa, (c), was found than for (d) and less than for (b). The ligand L2 possessed good (43.33%) activity against B. subtilis, (f), and weaker (25.71%) against E. coli, (a). L3 exhibited maximum (66.66%) activity against (f), weakest (26%) against (d), and moderate (65%) activity against S. aureus, (e). Similarly, L4 possessed maximum (70%) activity against (e), weakest (28.57%) against (b), and (45%) against (c). L5 showed significant (70%) activity against (e), weaker (23%) against (b), and moderate (30%) against (c). Compounds (1)–(5) exhibited overall a significant activity against (a)–(f), except (b). The results of these studies indicate that antibacterial activity was overall enhanced upon complexation (). On comparison, the metal complex (1) showed significant (80%) activity against (b) and (d). Compound (2) had maximum (85%) activity against (e) and strong (83.5%) against (c). Similarly, compound (3) had significant (83.33%) activity against (f). Compound (4) exhibited maximum (95%) activity against (e) and weak (42.85%) against (b). Compound (5) showed strong (83.33%) activity against (f).
Table 3. Antibacterial activity (concentration used 1 mg/mL of DMSO) of triazole derived Schiff bases and oxovanadium(IV) complexes.
Antifungal bioassay (in vitro)
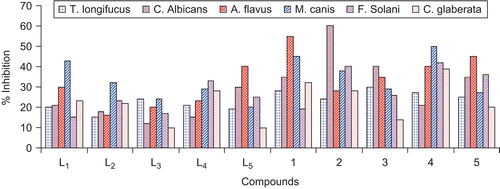
The antifungal screening of all compounds was carried out against T. longifusus, C. albicans, A. flavus, M. canis, F. solani, and C. glabrata fungal strains () according to the literature protocolCitation37. All synthesized ligands and their vanadyl(IV) complexes showed good antifungal activity against different fungal strains. The results of inhibition were compared with the results for standard drugs, miconazole and amphotericin B ( and ). The ligand L1 possessed maximum (43%) activity against M. canis, (d), and showed weaker (15%) activity against F. solani, (e). L2 similarly showed good (32%) activity against (d) as compared to all other fungal strains, but minimum (15%) activity against T. longifusus, (a). Compound L3 possessed significant (24%) activity against (a) and (d), but weaker (10%) against C. glabrata, (f). L4 showed maximum (33%) activity against (e) and weaker (15%) against C. albicans, (b). L5 possessed significant (40%) activity against A. flavus, (c), and weaker (10%) against (a). The vanadyl complexes (1) and (5) showed good (45–55%) activity against strain (f); similarly, compounds (2) and (3) also possessed good (40–60%) activity against (f). Compound (4) showed significant (50%) activity against (d), and (1) and (2) weaker (19 and 24%) against (e) and (a), respectively. Compounds (3) and (5) similarly showed weaker (14–20%) activity against (f), and the same weaker (21%) activity was observed for compound (4) against (b)Citation55–57.
Table 4. Antifungal activity (concentration used 200 µg/mL) of triazole derived Schiff bases and oxovanadium(IV) complexes.
Minimum inhibitory concentration for antibacterial activity
The results of preliminary antibacterial screening concluded that compounds (2), (3), and (4) were the most (above 80%) active. These compounds were therefore selected for antibacterial minimum inhibitory concentration (MIC) studies (). The MIC values of compounds (2), (3), and (4) were found to be in the range 5.476 × 10−7 to 2.303 × 10−5 M. However, compound (2) was found to be the most active, showing inhibition of 5.476 × 10−7 M against bacterial species B. subtilis.
Table 5. Minimum inhibitory concentration (M/mL) of selected compounds (2), (3), and (4) against selected bacteria.
Cytotoxic bioassay (in vitro)
All the synthesized compounds were screened for their cytotoxicity (brine shrimp bioassay) activityCitation41. From the data recorded in , it is evident that the three compounds (2), (4), and (5) exhibited potent cytotoxic activity against Artemia salina, while all others were inactive for this assay. Compound (2) possessed activity (LD50) of 5.358 × 10−3 M/mL, (4) of 8.213 × 10−4 M/mL, and (5) of 6.819 × 10−3 M/mL in the present series of compounds. It was interesting to note that only the oxovanadium complexes showed potent cytotoxicity. This activity relationship may help to serve as a basis for future research pursuits in the design/development of cytotoxic agents used for clinical applicationsCitation58,Citation59.
Table 6. Brine shrimp bioassay data of triazole derived Schiff bases L1–L5 and oxovanadium(IV) complexes (1)–(5).
Conclusion
The synthesized ligands L1–L5 acted bidentately via coordination of the azomethine nitrogen and furanyl oxygen to the oxovanadium metal ion. The binding of ligands to the vanadium metal atom was confirmed by their physical, analytical, and spectral data. The results of antibacterial and antifungal studies confirm that all ligands are biologically active and their vanadyl complexes show pronounced activity against one or more bacterial and/or fungal strains. All these observations lead us to the conclusion that those compounds that are not biologically active become biologically active, and those that are biologically active become more active, upon coordination/chelation with the metal ions.
Acknowledgement
One of the authors (S.H.S.) is thankful to the Higher Education Commission (HEC), Government of Pakistan, for an award to carry out this research. We are also indebted to the HEJ Research Institute of Chemistry, University of Karachi, Pakistan, for providing assistance in recording NMR and mass spectra, and for help in carrying out antibacterial, antifungal, and brine shrimp bioassays.
Declaration of interest
The authors report no conflict of interest and are responsible for the contents and writing of the paper.
References
- Sakurai H. A new concept: the use of vanadium complexes in the treatment of diabetes mellitus. Chem Rec 2002;2:237–48.
- Thompson KH, McNeill JH, Orvig C. Vanadium compounds as insulin mimics. Chem Rev 1999;99:2561–72.
- Takino T, Yasui H, Yoshitake A, Hamajima Y, Matsushita R, Takada J, et al. A new halogenated antidiabetic vanadyl complex, bis(5-iodopicolinato)oxovanadium (IV): in vitro and in vivo insulinomimetic evaluations and metallokinetic analysis. J Bio Inorg Chem 2001;6:133–42.
- Butler A, Carrano CJ. Coordination chemistry of vanadium in biological systems. Coord Chem Rev 1991;109:61–105.
- Sakurai H, Kojitane Y, Yoshikawa Y, Kawabe K, Yasui H. Antidiabetic vanadium (IV) and zinc (II) complexes. Coord Chem Rev 2002;226:187–98.
- Xing YH, Aoki K, Bai FY. A new insulin-like vanadyl complex: synthesis and structure of V(IV)O(H2O)2 (2,6-pyridinedicarboxylate) E2H2O. J Coord Chem 2004;57:157–65.
- Thompson KH, Orvig C. Coordination chemistry of vanadium in metallopharmaceutical candidate compounds. Coord Chem Rev 2001;219–21:1033–53.
- Haasnoot JG. Mononuclear, oligonuclear and polynuclear metal coordination compounds with 1,2,4-triazole derivatives as ligands. Coord Chem Rev 2000;200–2:131–85.
- Avaji PG, Patil SA, Badami PS. Synthesis, spectral, thermal, solid-state DC electrical conductivity and biological studies of Co(II) complexes with Schiff bases derived from 3-substituted-4-amino-5-hydrazino-1,2,4-triazole and substituted salicylaldehydes. Transition Met Chem 2008;33:275–83.
- Singh K, Barwa MS, Tyagi P. Synthesis, characterization and biological studies of Co(II), Ni(II), Cu(II) and Zn(II) complexes with bidentate Schiff bases derived by heterocyclic ketone. Eur J Med Chem 2006;41:147–53.
- Badwaik VB, Aswar AS. Synthesis, characterization and biological studies of some Schiff base complexes. Russ J Coord Chem 2007;33:755–60.
- Gulerman NN, Dogan HN, Rollas S, Johansson C, Celik C. Synthesis and structure elucidation of some new thioether derivatives of 1,2,4-triazoline-3-thiones and their antimicrobial activities. Farmaco 2001;56:953–8.
- Singh K, Singh DP, Barwa MS, Tyagi P. Some bivalent metal complexes of Schiff bases containing N and S donor atoms. J Enzyme Inhib Med Chem 2006;21:749–55.
- Bagihalli GB, Avaji PG, Patil SA, Badami PS. Synthesis, spectral characterization, in vitro antibacterial, antifungal and cytotoxic activities of Co(II), Ni(II) and Cu(II) complexes with 1,2,4-triazole Schiff bases. Eur J Med Chem 2008;43:2639–49.
- Rezaei Z, Khabnadideh S, Pakshir K, Hossaini Z, Amiri F, Assadpour E. Design, synthesis and antifungal activity of triazole and benzotriazole derivatives. Eur J Med Chem 2008;xxx:1–4.
- Reddy V, Patil S, Reddy T, Angadi SD. Synthesis, characterization and biological activities of Cu(II), Co(II), Ni(II), Mn(II) and Fe(III) complexes with Schiff base derived from 3-(4-chlorophenoxymethyl)-4-amino-5-mercapto-1,2,4-triazole. E-J Chem 2008;5:529–38.
- Serdar M, Gumrukcuoglu N, Karaoglu SA, Demirbas N. Synthesis of some novel 3,5-diaryl-1,2,4-triazole derivatives and investigation of their antimicrobial activities. Turk J Chem 2007;31:315–26.
- Ulusoy N, Gursoy A, Otuk G. Synthesis and antimicrobial activity of some 1,2,4-triazole-3-mercaptoacetic acid derivatives. Farmaco 2001;56:947–52.
- Demirba NS, Demirba AS, Alpay-Karaoglu Elif, EC. Synthesis and antimicrobial activities of some new [1,2,4]triazolo [3,4-b]thiadiazoles and [1,2,4]triazole [3,4-b][1,3,4]thiadiazines. ARKIVOC 2005;(i):75–91.
- Demirbas N, Demirbas A, Karaoglu SA. Synthesis and biological activities of new 1,2,4-triazol-3-one derivatives. Russi J Bioorg Chem 2005;31:387–97.
- Bekircan O, Kahveci B, Kucuk M. Synthesis and anticancer evaluation of some new unsymmetrical 3,5-diaryl-4H-1,2,4-triazole derivatives. Turk J Chem 2006;30:29–40.
- Holla BS, Veerendra B, Shivanada MK, Poojary B. Synthesis, characterization and anticancer activity studies on some Mannich bases derived from 1,2,4-triazoles. Eur J Med Chem 2003;38:759–67.
- Turan-Zitouni G, Kaplancikli ZA, Erol K, Kilic FS. Synthesis and analgesic activity of some triazoles and triazolothiadiazines. Farmaco 1999;54:218–23.
- Chen J, Sun XY, Chai KY, Lee JS, Song MS, Quan ZS. Synthesis and anticonvulsant evaluation of 4-(4-alkoxylphenyl)-3-ethyl-4H-1,2,4-triazoles as open-chain analogues of 7-alkoxyl-4,5-dihydro[1,2,4]triazolo[4,3-a]quinolines. Bioorg Med Chem 2007;15:6775–81.
- Guo-Qiang H, Li-Li H, Song-Qiang X, Wen-Long H. Design, synthesis and antitumor activity of asymmetric bis(s-triazole Schiff-bases) bearing functionalized side-chain. Chin J Chem 2008;26:1145–9.
- Invidiata FP, Grimaudo S, Giammanco P, Giammanco L. Synthesis and pharmacological properties of 6-substituted 3-(pyridine-4-yl)-1,2,4-triazole [3,4-b][1,3,4]thiadiazoles. Farmaco 1991;46:1489–95.
- Bekircan O, Gumrukcuoglu N. Synthesis of some 3,5-diphenyl-4H-1,2,4-triazole derivatives as antitumor agents. Indian J Chem 2005;44B:2107–13.
- Monika W, Swatko-Ossor M, Mazur L, Rzaczynska Z, Siwek A. Synthesis, structure and investigations of tuberculosis inhibition activities of new 4-methyl-1-substituted-1H-1,2,4-triazoles-5(4H)-thione. J Hetrocyclic Chem 2008;45:1893–6.
- Kucukguzel I, Kucukguzel SG, Rollas S, Kirazb M. Some 3-thioxo/alkylthio-1,2,4-triazoles with a substituted thiourea moiety as possible antimycobacterials. Biorg Med Chem Lett 2001;11:1703–7.
- Klimesova V, Zahajska L, Waisser K, Kaustova J, Mollmann U. Synthesis and antimycobacterial activity of 1,2,4-triazole 3-benzylsulfanyl derivatives. Farmaco 2004;59:279–88.
- Dabak K, Sezer O, Akar A, Anac O. Synthesis and investigation of tuberculosis inhibition activities of some 1,2,3-triazole derivatives. Eur J Med Chem 2003;38:215–/18.
- Chu CH, Sun XW, Sun L, Zhang ZY, Li ZC, Liao RA. Synthesis and biological activity of omega-(5-aryl-1,3,4-oxadiazol-2-thio)- and mega-(5-aryl-l,3,4-oxadiazol-2-thioacetoxyl)-omega-(1-H-1,2,4-triazol-1-yl)acetophenones. J Chin Chem Soc 1999;46:229–32.
- Czollner L, Sxilagli G, Janaky J. Synthesis of new 1,5-diphenyl-3-1H-1,2,4-triazoles substituted with H-, alkyl, or carboxyl groups at C-3. Arch Pharmazie 1990;323:225–51.
- Izumi K, Yamaguchi I, Wada A, Oshio Takahashi, HN. Effects of a new plant-growth retardant (E)-1-(4-chlorophenyl)-4,4 dimethyl-2-(1,2,4-triazol-1-yl)-1-penten-3-ol (s-3307) on the growth and gibberellin content of rice plants. Plant Cell Physiol 1984;25:611–15.
- Chohan ZH, Supuran CT. In-vitro antibacterial and cytotoxic activity of cobalt (II), copper (II), nickel (II) and zinc (II) complexes of the antibiotic drug cephalothin (keflin). J Enzyme Inhib Med Chem 2005;20:463–8.
- Chohan ZH, Arif M, Shafiq Z, Yaqub M, Supuran CT. In vitro antibacterial, antifungal and cytotoxic activity of some isonicotinoylhydrazide Schiff bases and their cobalt (II), copper (II), nickel (II) and zinc (II) complexes. J Enzyme Inhib Med Chem 2006;21:95–103.
- McLaughlin JL, Chang C-J, Smith DL. “Bench-top” bioassays for the discovery of bioactive natural products: an update. In: Atta-ur-Rahman, ed. Studies in Natural Products Chemistry. Amsterdam: Elsevier Science, 1991;9:383–409.
- Atta-ur-Rahman Choudhary, MI, Thomsen WJ. Bioassay Techniques for Drug Development. Amsterdam: Harwood Academic Publishers, 2001:22.
- Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 1966;45:493.
- Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL. Brine shrimp: a convenient general bioassay for active plant constituents. Planta Med 1982;45:31–4.
- Finney DJ. Probit Analysis, 3rd ed. Cambridge: Cambridge University Press, 1971.
- Geary WJ. The use of conductivity measurements in organic solvents for the characterization of coordination compounds. Coord Chem Rev 1971;7:81–121.
- Pandey OP. Oxovanadium(IV) complexes of carbohydrazones and thiocarbohydrazones. Polyhedron 1986;5:1587–91.
- Yadawe MS, Patil SA. Synthesis, characterization and biological studies of cobalt(II) and nickel(II) complexes with new Schiff bases. Transition Met Chem 1997;22;220–4.
- Bagihalli GB, Badami PS, Patil SA. Synthesis, spectral characterization and in vitro biological studies of Co(II), Ni(II) and Cu(II) complexes with 1,2,4-triazole Schiff bases. J Enzyme Inhib Med Chem 2008;23:1–14.
- Stoilova D, Georgiev M, Marinova D. Infrared study of the vibrational behavior of SO42_ guest ions matrix-isolated in metal (II) chromates (Me = Ca, Sr, Ba). Vib Spectrosc 2005;39:46.
- Simmons WW. The Sadtler Handbook of Proton NMR Spectra. Philadelphia, PA: Sadtler Research Laboratories, 1978.
- Pasto DJ. Organic Structure Determination. London: Prentice Hall International, 1969.
- Ballhausen CJ, Gray HB. The electronic structure of the vanadyl ion. Inorg Chem 1962;1:111–22.
- Selbin J. The chemistry of oxovanadium (IV). Chem Rev 1965;65:153–75.
- Selbin J. Oxovanadium (IV) complexes. Coord Chem Rev 1966;1:293–314.
- Selbin J, Morpurgo L. Spectral studies of low symmetry oxovanadium(IV) complexes. J Inorg Nucl Chem 1965;27:673–678.
- Chohan ZH. Synthesis and biological properties of Cu(II) complexes with 1,10-disubstituted ferrocenes. Synth React Inorgan Met Organ Chem 2004;34:833–46.
- Chohan ZH, Supuran CT. Structural and biological properties of first row d-transition metal complexes with N-substituted sulfonamides. J Enzyme Inhib Med Chem 2008;23:240–51.
- Chohan ZH, Shad HA. Structural elucidation and biological significance of 2-hydroxy-1-naphthaldehyde derived sulfonamides and their first row d-transition metal chelates. J Enzyme Inhib Med Chem 2008;23:369–79.
- Chohan ZH. Metal-based sulfonamides: their preparation, characterization and in vitro antibacterial, antifungal and cytotoxic properties. X-ray structure of 4-[(2-hydroxybenzylidene)amino]benzosulfonamide. J Enzyme Inhib Med Chem 2008;23:120–30.
- Chohan ZH. Metal-based antibacterial and antifungal sulfonamides: synthesis, characterization and biological properties. Transition Met Chem 2009;34:153–61.
- Chohan ZH. Synthesis of organometallic-based biologically active compounds: in vitro antibacterial, antifungal and cytotoxic properties of some sulfonamide incorporated ferrocenes. J Enzyme Inhib Med Chem 2009;24:169–75.
- Chohan ZH, Shad HA, Nasim FH. Synthesis, charcterization and biological properties of sulfonamide-derived compounds and their transition metal complexes. Appl Organomet Chem 2009;23:319–28.