Abstract
This study aimed to investigate the acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitory activity of the essential oils from Pinus nigra subsp. nigra, P. nigra var. calabrica, and P. heldreichii subsp. leucodermis. This activity is relevant to the treatment of Alzheimer’s disease (AD), since cholinesterase drugs are currently the only drugs available to treat AD. P. heldreichii subsp. leucodermis exhibited the most promising activity, with IC50 values of 51.1 and 80.6 μg/mL against AChE and BChE, respectively. An interesting activity against AChE was also observed with P. nigra subsp. nigra essential oil, with an IC50 value of 94.4 μg/mL. Essential oils were analyzed by GC and GC-MS with the purpose of investigating their relationships with the observed activities. Among the identified constituents, terpinolene, β-phellandrene, linalyl acetate, trans-caryophyllene, and terpinen-4-ol were tested. trans-Caryophyllene and terpinen-4-ol inhibited BChE with IC50 values of 78.6 and 107.6 μg/mL, respectively. β-Phellandrene was selective against AChE (IC50 value of 120.2 μg/mL).
Introduction
Neurodegenerative disease is a generic term applied to a variety of conditions arising from a chronic breakdown and deterioration of the central nervous system (CNS) neurons. Among the numerous variants of degenerative dementia, Alzheimer’s disease (AD) is by far the most prevalent, affecting more than 20 million people worldwide. In spite of the multifactorial nature of AD, only one therapeutic approach is currently followed. This strategy is based on the so-called cholinergic hypothesis of cognitive dysfunctionCitation1. This hypothesis postulates that at least some of the cognitive decline experienced by patients of AD results from a deficiency in neurotransmitter acetylcholine (ACh) and thus in cholinergic neurotransmission in brain cortical or hippocampal regions, which seems to play a fundamental role in memoryCitation2. Moreover, in the late AD stage, levels of acetylcholinesterase (AChE) have declined by up to 85%, and butyrylcholinesterase (BChE) represents the predominant cholinesterase in the brainCitation3. BChE, primarily associated with glial cells but also with specific neuronal pathways, cleaves ACh in a manner similar to AChE to terminate its physiological actionCitation4,Citation5. Such studies have targeted BChE as a new approach to intercede in the progression of ADCitation6.
Thus, restoring the level of ACh through inhibition of both major forms of cholinesterase, AChE and BChE, continues to be a therapeutic useful approach to treat not only AD but also other forms of dementia.
The potential use of natural products has been successfully demonstrated in the field of ADCitation7,Citation8. Among natural sources, essential oils are attracting special attention. Indeed, the results of different recent studies indicate that several essential oils from food cropsCitation9, medicinal plantsCitation10–13, and tea tree oilsCitation14,Citation15 show significant anti-cholinesterase inhibitory activity. The effects of Salvia lavandulaefolia essential oil and some of its constituents on acetylcholinesterase have been reported in vitro and in vivoCitation16,Citation17. In our previous investigations, the inhibition of acetylcholinesterase and butyrylcholinesterase enzymes by S. leriifolia, Origanum ehrenbergii, and O. syriacum essential oils was reportedCitation18–20. Various essential oil components have been investigated for their effects on AChE. It has been found that the majority of AChE inhibitors identified in the essential oils are terpenoidsCitation15,Citation20–23. Due to their small molecular size and lipophilicity, volatile constituents of essential oils are likely to readily cross the blood–brain barrierCitation24.
As a part of our efforts to find new anti-cholinesterase active compoundsCitation18–20,Citation25, in this study we aimed to evaluate the AChE and BChE inhibitory activity of essential oils from three Pinus species, i.e. Pinus nigra subsp. nigra, P. nigra var. calabrica, and P. heldreichii subsp. Leucodermis, and some identified components by screening them using the microplate assay method. The composition profiles of the essential oils were characterized by gas chromatography-mass spectrometry (GC-MS) analysis, and the relationships between the chemical components and the AChE and BChE inhibitory activity were outlined.
Materials and methods
General
Methanol and dichloromethane were purchased from VWR International (Milan, Italy). Acetylcholinesterase (AChE) from Electrophorus electricus (EC 3.1.1.7, Type VI-S) and butyrylcholinesterase (BChE) from equine serum (EC 3.1.1.8), physostigmine, galanthamine hydrobromide, 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB), acetylthiocholine iodide (ATCI), butyrylthiocholine iodide (BTCI), α-pinene, β-pinene, limonene, sabinene, α-terpinene, γ-terpinene, terpinolene, linalool, linalyl acetate, trans-caryophyllene, α-humulene, δ-cadinene, palmitic acid, myristic acid, palmitic acid methyl ester, tricosane, heneicosane, and pentacosane were purchased from Sigma-Aldrich (Milan, Italy).
Plant material
The needles of Pinus nigra Arnold subsp. nigra, P. nigra Arnold var. calabrica C. K. Scheid, and P. heldreichii Christ subsp. leucodermis (Antoine) E. Murray were collected at the full flowering stage from plants growing in Calabria, Italy (P. heldreichii subsp. leucodermis and P. nigra subsp. nigra on the Parco Nazionale del Pollino, P. nigra var. calabrica on Sila) and authenticated by Dr. N. G. Passalacqua of the Botany Department at the University of Calabria (Italy). A voucher specimen has been retained at the Herbarium of the University of Calabria (CLU).
Essential oil isolation
The essential oils from the needles of P. nigra subsp. nigra, P. nigra var. calabrica, and P. heldreichii subsp. leucodermis were obtained by hydrodistillation for 3 h, using a Clevenger-type apparatusCitation26. The oils were dried (anh. Na2SO4) and stored under N2 at +4°C in brown bottles until analyzed and tested. The yield of the oils was 0.3% (v/w) for P. nigra subsp. nigra, 0.2% (v/w) for P. nigra var. calabrica, and 0.2% (v/w) for P. heldreichii subsp. leucodermis.
Gas chromatography-mass spectrometry (GC-MS) analysis
Analytical gas chromatography was carried out on a Hewlett-Packard 6890 gas chromatograph equipped with an SE30 capillary column (30 m length, 0.25 mm i.d., 0.25 μm film thickness) and interfaced with a Hewlett-Packard 5973 mass selective detector. Ionization of the sample components was performed in electron impact mode (EI, 70 eV), with helium as the carrier gas. The analytical conditions were: oven temperature 5 min isothermal at 50°C, then 50–250°C at a rate of 13°C/min, then held isothermal for 10 min. Injector and detector were maintained at 250°C and 280°C, respectively. Analyses were also run by a HP-Innowax capillary column (30 m length, 0.25 mm i.d., 0.25 μm film thickness). Gas chromatographic conditions were as given.
Gas chromatography (GC) analyses
Essential oils were analyzed by a Shimadzu GC17A gas chromatograph system. An SE30 capillary column (30 m with an internal diameter of 0.25 mm and a film thickness of 0.25 μm) was used with nitrogen as the carrier gas. GC oven temperature and conditions were as described above. Quantification of the components was performed on the basis of their GC peak areas and percentages of the characterized essential oil components were as given in .
Table 1. Chemical composition of the essential oils from P. nigra var. calabrica (P1), P. heldreichii subsp. leucodermis (P2), and P. nigra subsp. nigra (P3).
Qualitative and quantitative analyses
Identification of the compounds was achieved through retention indices (I), against those from the literatureCitation27,Citation28 or those of authentic compounds available in our laboratory. The retention indices were determined in relation to a homologous series of n-alkanes (C8–C24) under the same operating conditions. Further identification was made by comparison of their mass spectra on both columns with those stored in Wiley 138, Wiley 275, or NIST 98 libraries or with mass spectra from the literatureCitation28. Component relative concentrations were calculated based on GC peak areas without using correction factors.
Microtiter cholinesterase inhibition assay
Acetylcholinesterase and butyrylcholinesterase inhibiting activities were measured by slightly modifying the spectrophotometric method previously developed by Ellman et al.Citation29, which is based on the reaction of released thiocholine to give a colored product with a chromogenic reagent. Electrophorus electricus (EC 3.1.1.7, Type VI-S) AChE and equine serum (EC 3.1.1.8) BChE were used, while acetylthiocholine iodide and butyrylthiocholine iodide, respectively, were used as substrates of the reaction. 5,5′-Dithio-bis(2-nitrobenzoic-acid) (DTNB) was used for measurement of the cholinesterase activity. In this procedure, AChE or BChE (0.20 U/mL in buffer, pH 8) plus Pinus essential oils at final concentrations in the test solution ranging from 20 to 500 μg/mL (20 μL) and pure compounds at final concentrations ranging from 10 to 200 μg/mL (20 μL) were added to 2 mL of buffer, pH 8, and pre-incubated in an ice bath at 4°C for 30 min. Tested essential oils and control were dissolved in 5% MeOH. Duplicate tubes were also treated this way, with 20 μL of positive control (0.1 mM), to allow interference of the test substances in the assay to be assessed, and to control for any hydrolysis of acetylcholine or butyrylcholine not due to enzyme activity. The reaction was started by adding DNTB solution (20 μL of 0.05 mM in buffer, pH 7) and acetylthiocholine iodide (ATCI) or butyrylthiocholine iodide (BTCI) (20 μL of 0.018 mM in buffer, pH 7) and tubes were kept in a water bath for 20 min at 37°C. The reaction was halted by placing the assay solution tubes in an ice bath and adding physostigmine (20 μL of 0.018 mM in buffer, pH 7). Blanks were used of reagents without essential oils, and the positive control physostigmine (20 μL of 0.018 mM in buffer, pH 7) was added. The hydrolysis of acetylthiocholine and butyrylthiocholine was monitored by formation of the yellow 5-thio-2-nitrobenzoate anion, as the result of the reaction of DTNB with thiocholine, released during enzymatic hydrolysis; this was immediately recorded on a spectrophotometer (Jenway 6300) at 405 nm and the percentage inhibition was calculated. The samples and positive controls were dissolved in 5% methanol, which was used for the control. All the reactions were performed in triplicate. The inhibition rate (%) was calculated by equation:
Statistical analysis
Differences were evaluated by one-way analysis of variance (ANOVA) test completed by a multicomparison Dunnett’s test. Differences were considered significant at p < 0.01. The 50% inhibitory concentration (IC50) was calculated from a dose–response curve obtained by plotting the percentage of inhibition versus concentration with the use of GraphPad Prism 4.0 software.
Results and discussion
Essential oils composition
In order to identify putative active compounds present within the essential oils, gas chromatography systems were employed. The chemical composition of the oils is reported in . P. nigra var. calabrica essential oil was characterized by 53 constituents (93.4% of the total oil), in which the dominant components were α-pinene (24.6%), β-pinene (10.9%), γ-cadinene (9.9%), β-phellandrene (6.3%), manoyl oxide (6.2%), and trans-caryophyllene (4.2%). P. nigra subsp. nigra essential oil was characterized by 41 components, representing 95.7% of the total oil. The main components were α-pinene (25.3%), limonene (22.6%), sabinene (12.8%), α-terpineol (8.3%), β-pinene (4.8%), and terpinolene (4.5%). Many terpenoid compounds are typical constituents of conifer resin, and have been reported from P. nigra foliage beforeCitation30–33. Regardless of how the pine twigs were treated, α-pinene, β-pinene, β-myrcene, limonene, and β-phellandrene were the major monoterpenes. The components (E)-β-caryophyllene and germacrene D were the predominating sesquiterpenes, confirming former studiesCitation30–33. Macchioni et al.Citation30 analyzed essential oils from needles, branches without needles, and cones of P. nigra separately. They detected the components 1,8-cineole, pinocamphone, and myrtenal in branches and cones only, but not in needle tissue. On the other hand, α-terpinyl acetate, β-cubebene, (E)-β-farnesene, and α-humulene were exclusively detected in needle tissueCitation30.
A total of 50 compounds (91.3% of the total oil) were identified in P. heldreichii subsp. leucodermis essential oil. α-Pinene (24.2%) and β-pinene (8.4%), limonene (7.8%), α-cubebene (7.6%), terpinolene (5.9%), and trans-caryophyllene (4.5%) were the major constituents. A recent studyCitation34 reported the essential oil compositions of P. heldreichii from Montenegro and Serbia. In the pine-needle terpene profile from three populations from Montenegro, and one from Serbia, the dominant constituents were limonene (26.3%), α-pinene (17.5%), germacrene D (13.5%), and β-caryophyllene (10.4%), constituting ∼67.7% of the essential oil. Medium-to-high contents (0.5–10%) of the following 16 additional components were found: β-pinene, β-myrcene, α-humulene, δ-cadinene, α-muurolene, (E)-hex-2-enal, β-gurjunene, γ-muurolene, isopimarol, camphene, γ-cadinene, aromadendrene, β-bisabolene, trans-β-farnesene, α-cadinene, and (Z)-hex-3-en-1-ol.
The analyzed essential oils showed marked differences, especially from the quantitative point of view of the most abundant components. In particular, in comparison to the other oils, P. nigra subsp. nigra essential oil was characterized by a high content of sabinene (12.8%), limonene (22.6%), and α-terpineol (8.3). Manoyl oxide (6.2%) and γ-cadinene (9.9%) were present in high quantity only in P. nigra var. calabrica essential oil. Interestingly, β-phellandrene (6.3%) and α-bergamotene (1.0%) were detected only in P. nigra var. calabrica oil, and linalyl acetate (3.6%) only in P. heldreichii subsp. leucodermis essential oil.
Cholinesterase activity
In spite of the multifactorial nature of Alzheimer’s disease, most current agents follow one therapeutic approach, based on the so-called cholinergic hypothesis of cognitive dysfunction. Currently, cholinesterase inhibition is the major treatment for the symptoms of AD, and inhibition of AChE and BChE are therapeutic targets for improving the cholinergic deficit.
The in vitro cholinesterase inhibitory activity of three Pinus species growing in Calabria (Italy) essential oils and some identified constituents was evaluated by Ellman’s spectrophotometric method using an enzyme-linked immunosorbent assay (ELISA) microplate reader. All studied essential oils were able to inhibit in vitro enzymes in a concentration-dependent manner (). IC50 values were calculated and are reported in . P. heldreichii subsp. leucodermis exhibited the most promising activity, with IC50 values of 51.1 and 80.6 µg/mL, against AChE and BChE, respectively. Interesting activity against AChE was observed also with P. nigra var. calabrica and P. nigra subsp. nigra, with IC50 values of 101.5 and 94.4 µg/mL, respectively. BChE appeared less sensitive to P. nigra var. calabrica and P. nigra subsp. nigra essential oil (IC50 values of 128.0 and 162.5 µg/mL, respectively). AChE plays an important role in the central nervous system. It is one of the fastest known enzymes, and catalyzes the cleavage of acetylcholine in the synaptic cleft after depolarization. Inhibitors of AChE, such as galanthamine, are used frequently in the pharmacotherapy of AD. The less specific BChE has recently been a focus of research, because BChE concentration stays the same, or is even up-regulated, while AChE is dramatically down-regulated in the brains of patients suffering from ADCitation36. It was previously reported that essential oils rich in terpenes exhibit a strong inhibitory activity against both enzymesCitation35,Citation37,Citation38. Among the Pinus essential oil components, five compounds, namely β-phellandrene, terpinolene, terpinen-4-ol, linalyl acetate, and trans-caryophyllene, were tested for the first time in this study to evaluate their potential activity (). The dose-dependent inhibitory activity of terpinolene and β-phellandrene against AChE is reported in . β-Phellandrene showed a selective activity against AChE, with an IC50 value of 120.2 µg/mL, while terpinolene inhibited both AChE and BChE enzymes with IC50 values of 156.4 µg/mL and 147.1 µg/mL, respectively. Except β-phellandrene, all tested terpenes showed BChE inhibitory activity (, ), with IC50 values ranging from 78.6 µg/mL to 168.7 µg/mL for trans-caryophyllene and linalyl acetate, respectively. Perusal of the literature revealed a promising agreement between our results and the reported activities of a number of terpenes. Indeed, an impressive body of information exists on the anti-cholinesterase activity of plant terpenes, and in particular of monoterpenes. Many works have concentrated on the action of compounds identified in Pinus essential oils and on the synergistic effect of a mixture of some terpenes. Perry et al.Citation16 reported the AChE inhibitory activity of α-pinene with an IC50 of 0.63 mM. The AChE inhibitory property was also reported for several monoterpene hydrocarbons such as α-terpinene and limoneneCitation21. In contrast a weak AChE inhibitory activity was found for linalyl acetate that inhibited the enzyme with a percentage of 38% at 82 µg/mLCitation39. β-Pinene inhibited AChE with a percentage of 48.5% at 1 mMCitation22. Cholinesterase inhibitory activity was described also for 3-carene that reached 50% inhibition of BChE at a final concentration of 2 mM during the 5 min pre-incubation period. Incubation time affected the inhibitory activity; in fact the IC50 values were significantly decreased from 2 mM in 5 min to 0.7 mM in 60 min. In contrast to the inhibition of BChE, the human anti-AChE activity of β-caryophyllene, 3-carene, α-pinene, and β-pinene was apparent. β-Caryophyllene, at a final concentration of 0.06 mM, gave 32% of AChE inhibition, whereas 3-carene, at a final concentration of 0.08 mM, gave 33% of AChE inhibition. Limonene and sabinene were recently tested to evaluate their potential anti-cholinesterase activityCitation20. Sabinene exhibited the highest activity against AChE and BchE, with IC50 values of 176.5 µg/mL and 218.6 µg/mL, respectively.
Figure 1. Dose-dependent inhibitory activity of Pinus essential oils against AChE (a) and BChE (b). Data are given as mean ± SD (n = 3).
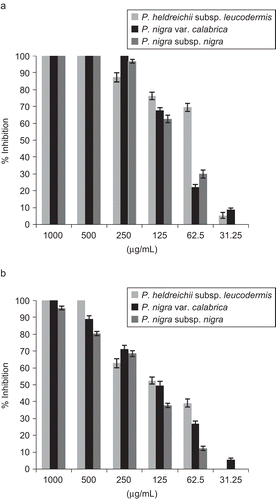
Figure 2. Dose-dependent inhibitory activity of terpinolene and β-phellandrene against AChE. Data are given as mean ± SD (n = 3).
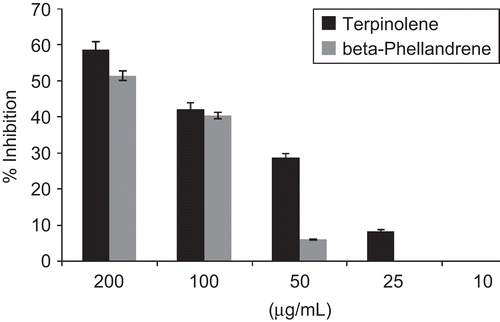
Figure 3. Dose-dependent inhibitory activity of terpinolene, β-phellandrene, terpinen-4-ol, linalyl acetate, and trans-caryophyllene against BChE. Data are given as mean ± SD (n = 3).
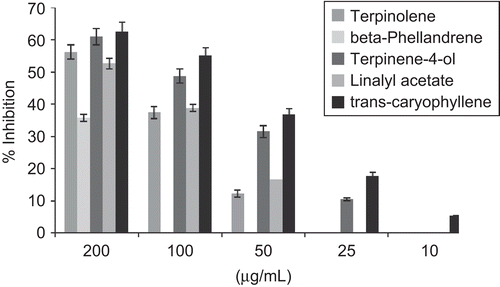
Table 2. Cholinesterase inhibitory activity of essential oils and identified constituents from Pinus species (IC50, μg/mL).
The synergistic effect of different mixtures of terpenes has also been evaluatedCitation15,Citation23. Generally, the findings reveal that the inhibitory activity of the essential oil results from a complex interaction between the constituents, which produce both synergistic and antagonistic responses between the component terpenes.
Various previous studies have demonstrated the biological activity of essential oils and their components after oral administrationCitation40–42. Moreover, Perry et al.Citation43 reviewed the pharmacological activity of S. lavandulaefolia essential oil for the treatment of Alzheimer’s disease in a study involving oral administration. After 6 weeks of treatment a significant reduction in neuropsychiatric symptoms and an improvement in attention were observed. Recently, focus has also been on the nasal mucosa as an alternative administration route. A number of drugs including lipophilic compounds are administered intranasally, and can be rapidly absorbed into the brainCitation44,Citation45. Therefore, following oral or nasal administration, Pinus essential oils and/or identified compounds could be tested in vivo to evaluate their potential beneficial therapeutic effects in AD treatment.
Conclusions
In summary, the essential oils from three Pinus species, namely P. nigra subsp. nigra, P. nigra var. calabrica, and P. heldreichii subsp. leucodermis, were evaluated for their chemical composition and acetylcholinesterase and butyrylcholinesterase inhibitory activity. Our results demonstrated that Pinus essential oils and some constituents have properties for the treatment of Alzheimer’s disease and provide further data supporting the value of carrying out clinical studies using these species. In fact, further studies of the essential oils and/or the identified compounds on the pharmacokinetics, particularly on administrative routes or mode of action, are warranted.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Hodges JR. Alzheimer’s centennial legacy: origins, landmarks and the current status of knowledge concerning cognitive aspects. Brain 2006;129:2811–22.
- Hasselmo ME, Giocomo LM. Cholinergic modulation of cortical function. J Mol Neurosci 2006;30:133–5.
- Loizzo MR, Tundis R, Menichini F, Menichini F. Occurring natural products and their derivatives as cholinesterase inhibitors in the treatment of neurodegenerative disorders: an update. Curr Med Chem 2008;15:1209–28.
- Darvesh S, Hopkins DA, Geula C. Neurobiology of butyrylcholinesterase. Nat Rev Neurosci 2003;4:131–8.
- Greig NH, Sambamurti K, Yu QS, Perry TA, Holloway HW, Haberman F, et al. In: Giacobini E, ed. Butyrylcholinesterase: Its Function and Inhibition. London: Martin Dunitz, 2003:69–90.
- Ballard CG, Greig NH, Guillozet-Bongaarts AL, Enz A, Darvesh S. Cholinesterases: roles in the brain during health and disease. Curr Alzheimer Res 2005;2:307–18.
- Francotte P, Graindorge E, Boverie S, de Tullio P, Pirotte B. New trends in the design of drugs against Alzheimer’s disease. Curr Med Chem 2004;11:1757–78.
- Houghton PJ, Ren Y, Howes MJ. Acetylcholinesterase inhibitors from plants and fungi. Nat Prod Rep 2006;23:181–99.
- Mata AT, Proença C, Ferreira AR, Serralheiro MLM, Nogueira JMF, Araújo MEM. Antioxidant and antiacetylcholinesterase activities of five plants used as Portuguese food spices. Food Chem 2007;103:778–86.
- Moreno L, Bello R, Primo-Yufera E, Esplugues J. Pharmacological properties of the methanol extract from Mentha suaveolens Ehrh. Phytother Res 2002;16:10–13.
- Ferreira A, Proença C, Serralheiro MLM, Araújo MEM. The in vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from Portugal. J Ethnopharmacol 2006;108:31–7.
- Orhan I, Kartal M, Kan Y, Sener B. Activity of essential oils and individual components against acetyl- and butyrylcholinesterase. Z Naturforsch C 2008;63:547–53.
- Dohi S, Terasaki M, Makino M. Acetylcholinesterase inhibitory activity and chemical composition of commercial essential oils. J Agric Food Chem 2009 Apr 9. [Epub ahead of print]
- Mills C, Cleary BJ, Gilmer JF, Walsh JJ. Inhibition of acetylcholinesterase by tea tree oil. J Pharm Pharmacol 2004;56:375–9.
- Miyazawa M, Yamafuji C. Inhibition of acetylcholinesterase activity by tea tree oil and constituent terpenoids. Flavour Fragr J 2006;21:198–201.
- Perry NS, Houghton PJ, Theobald A, Jenner P, Perry EK. In vitro inhibition of human erythrocyte acetylcholinesterase by Salvia lavandulaefolia essential oil and constituent terpenes. J Pharm Pharmacol 2000;52:895–902.
- Perry NS, Houghton PJ, Jenner P, Keith A, Perry EK. Salvia lavandulaefolia essential oil inhibits cholinesterase in vivo. Phytomedicine 2002;9:48–51.
- Loizzo MR, Menichini F, Tundis R, Bonesi M, Conforti F, Nadjafi F, et al. In vitro biological activity of Salvia leriifolia Benth essential oil relevant to the treatment of Alzheimer’s disease. J Oleo Sci 2009;58:443–6.
- Loizzo MR, Menichini F, Conforti F, Tundis R, Bonesi M, Saab AM, et al. Chemical analysis, antioxidant, antiinflammatory and anticholinesterase activities of Origanum ehrenbergii Boiss and Origanum syriacum L. essential oils. Food Chem 2009;117:174–80.
- Menichini F, Tundis R, Loizzo MR, Bonesi M, Marrelli M, Statti GA, et al. Acetylcholinesterase and butyrylcholinesterase inhibition of ethanolic extract and monoterpenes from Pimpinella anisoides V Brig. (Apiaceae). Fitoterapia 2009;80:297–300.
- Miyazawa M, Watanabe H, Kameoka H. Inhibition of acetylcholinesterase activity by monoterpenoids with a p-menthane skeleton. J Agric Food Chem 1997;45:677–9.
- Miyazawa M, Yamafuji C. Inhibition of acetylcholinesterase activity by bicyclic monoterpenoids. J Agric Food Chem 2005;53:1765–8.
- Savelev S, Okello E, Perry NSL, Wilkins RM, Perry EK. Synergistic and antagonistic interactions of anticholinesterase terpenoids in Salvia lavandulaefolia essential oil. Pharmacol Biochem Behav 2003;75:661–8.
- Savelev SU, Okello E, Perry EK. Butyryl- and acetyl-cholinesterase inhibitory activities in essential oils of Salvia species and their constituents. Phytother Res 2004;18:315–24.
- Tundis R, Menichini F, Conforti F, Loizzo MR, Bonesi M, Statti G, et al. A potential role of alkaloid extracts from Salsola species (Chenopodiaceae) in the treatment of Alzheimer’s disease. J Enzyme Inhib Med Chem 2009;24:818–24.
- Clevenger JF. Apparatus for the determination of volatile oils. J Am Pharm Assoc 1928;17:341–6.
- Jennings W, Shibamoto T. Qualitative Analysis of Flavor and Fragrance Volatiles by Glass Capillary Gas Chromatography. New York: Academic Press, 1980.
- Adams RP. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy. Carol Stream, IL: Allured Publishing, 2001.
- Ellman GL, Courtney KD, Andres V Jr, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961;7:88–95.
- Macchioni F, Cioni P, Flamini G, Maccioni S, Ansaldi M. Chemical composition of essential oils from needles, branches and cones of Pinus pinea, P. halepensisi, P. pinaster and P. nigra from central Italy. Flavour Fragr J 2003;18:139–43.
- Rafii Z, Dodd R, Zavarin E. Monoterpene variability of Pinus monticola wood. Biochem Syst Ecol 1996;24:325–39.
- Rezzi S, Bighelli A, Mouillot D, Casanova J. Composition and chemical variability of the needle essential oil of Pinus nigra subsp. laricio from Corsica. Flavour Fragr J 2001;16:379–83.
- Roussis V, Petrakis PV, Ortiz A, Mazomenos B. Volatile constituents of five Pinus species grown in Greece. Phytochemistry 1995;39:357–61.
- Nikolić B, Ristić M, Bojović S, Marin PD. Variability of the needle essential oils of Pinus heldreichii from different populations in Montenegro and Serbia. Chem Biodivers 2007;4:905–16.
- Murray AP, Gurovic MS, Rodriguez SA, Murray MG, Ferrero AA. Acetylcholinesterase inhibition and antioxidant activity of essential oils from Schinus areira L. and Schinus longifolia (Lindl.) Speg. Nat Prod Commun 2009;4:873–6.
- Giacobini E. Drugs that target cholinesterases. In: Buccafusco JJ (ed). Cognitive enhancing drugs. Birkhäuser, Basle-Boston-Berlin, 2004:11–36.
- Mukherjee PK, Kumar V, Mal M, Houghton PJ. In vitro acetylcholinesterase inhibitory activity of the essential oil from Acorus calamus and its main constituents. Planta Med 2007;73:283–5.
- Souza A, Silva MC, Cardoso-Lopes EM, Cordeiro I, Sobral ME, Young MC, et al. Differential acetyl cholinesterase inhibition by volatile oils from two specimens of Marlierea racemosa (Myrtaceae) collected from different areas of the Atlantic Rain Forest. Nat Prod Commun 2009;4:1143–6.
- Miyazawa M, Watanabe H, Umemoto K, Kameoka H. Inhibition of acetylcholinesterase activity by essential oils of Mentha species. J Agric Food Chem 1998;46:3431–4.
- Santos FA, Rao VSN, Silveira ER. Investigations on the antinociceptive effect of Psidium guajavai leaf essential oil and its major constituents. Phytother Res 1998;12:24–7.
- Santos FA, Rao VSN. Antiinflammatory and antinociceptive effects of 1,8-cineole, a terpenoid oxide present in many plant essential oils. Phytother Res 2000;14:240–4.
- Yoshida N, Koizumi M, Adachi I, Kawakami J. Inhibition of P-glycoprotein-mediated transport by terpenoids contained in herbal medicines and natural products. Food Chem Toxicol 2006;44:2033–9.
- Perry NS, Bollen C, Perry EK, Ballard C. Salvia for dementia therapy: review of pharmacological activity and pilot tolerability clinical trial. Pharmacol Biochem Behav 2003;75:651–9.
- Christensen K, Rogers E, Green GA, Hamilton DA, Mermelstein F, Liao E, et al. Safety and efficacy of intranasal ketamine for acute postoperative pain. Acute Pain 2007;9:183–92.
- Illum L. Nasal drug delivery: possibilities, problems and solutions. J Control Release 2003;87:187–98.
