Abstract
Using Triton WR-1339-induced hyperlipidemic rats as an experimental model, we investigated whether compound 4 [N-(9,10-dihydro-9,10-dioxoanthracen-2-yl)bezofuran-2-carboxamide] and compound 5 [N-(4-benzoylphenyl)benzofuran-2-carboxamide], two novel anti-hyperlipidemic agents, have any effect on plasma triglyceride (TG), total cholesterol (TC), and high-density lipoprotein cholesterol levels (HDL-C) levels. The tested animals were divided into control (CG), hyperlipidemic (HG), and compounds 4, 5, and bezafibrate (BF) treated groups. At a dose of 15 mg/kg body weight, compounds 4, 5, and BF significantly reduced elevated plasma TG levels after 7 and 24 h. Furthermore, HDL-C levels were remarkably increased in all treated groups after 7 and 24 h compared to the hyperlipidemic control group. However, only compounds 4 and 5 treated groups clearly showed a significant reduction in plasma total cholesterol levels after 7 and 24 h. It is therefore reasonable to assume that compounds 4 and 5 may have promising potential in the treatment of hyperlipidemia and atherosclerosis.
Introduction
Cardiovascular diseases are the most common cause of death in industrialized countriesCitation1. Hyperlipidemia is one of the important risk factors involved in the development of cardiovascular diseaseCitation2. Many clinical trials have demonstrated that increases in plasma total cholesterol (TC) and triglyceride (TG) levels are implicated in the development of atherosclerosisCitation3,Citation4.
Triton WR-1339 (a nonionic detergent that results in a milky serum lasting up to 48 h) has been widely used to produce acute hyperlipidemia in animal models in order to screen natural and chemical drugsCitation5. The accumulation of plasma lipids by Triton WR-1339 appears to be due to the inhibition of lipoprotein lipase activityCitation6.
Fibrate derivatives are among the most widely used anti-hyperlipidemic drugs in the world, and have been shown to be effective in preventing coronary heart diseases in hyperlipidemic patientsCitation7, and in patients with a low level of high-density lipoprotein cholesterol (HDL-C)Citation8. The major pharmacological mechanism of fibrates, including bezafibrate, is supposed to be increased hydrolysis of TG by the induction of lipoprotein lipase and reduction of apolipoprotein C-III synthesisCitation9.
During the past decade, much attention has been given to studies focused on the synthesis of benzofuran-containing agents and their pharmacological activitiesCitation10,Citation11. From these studies it was found that compounds containing the benzofuran ring have a promising potential effect as lipid-lowering agentsCitation12,Citation13.
Given the importance of correcting hyperlipidemia to improve the risk of developing cardiovascular disease, the present study focused on the synthesis and pharmacological evaluation of novel derivatives of benzofuran-2-carboxamide, compound 4 [N-(9,10-dihydro-9,10-dioxoanthracen-2-yl)bezofuran-2-carboxamide] and compound 5 [N-(4-benzoylphenyl)benzofuran-2-carboxamide], as lipid-lowering agents ().
Materials and methods
Chemical studies
Melting points (m.p.) were determined using a Stuart Scientific electrothermal melting point apparatus and are uncorrected. Infrared (IR) spectra were recorded on an Avatar Thermo Nicolet Impact 400 FT-IR spectrophotometer using Smart Omni-Transmission software; all samples were prepared as potassium bromide (Acros, Belgium) disks. 1H- and 13C-nuclear magnetic resonance (NMR) spectra were measured on a Bruker UltraShield 300 MHz instrument operating at 300 MHz (1H) and 75 MHz (13C), respectively. Elemental analysis of C, H, and N was performed on a Euro elemental analyzer (model EA3000 A; Italy). The analytical results for the elements were within ±0.4% of the theoretical values.
All starting materials were purchased from Sigma-Aldrich (St. Louis, MO, USA) and used without further purification. Experiments were performed in purified solvents.
Synthesis of benzofuran-2-carbonyl chloride (2)
A mixture of (2 g, 12.3 mmol) benzofuran-2-carboxylic acid and (6 mL, 83 mmol) SOCl2 in 40 mL of dry dichloromethane (DCM) was stirred under reflux for 6 h. After cooling to room temperature, DCM and the excess SOCl2 were evaporated under reduced pressure. The residue was stirred for 10 min in DCM and flashed through a short chromatography column (SiO2, DCM). The solvent was removed under reduced pressure and the product dried in vacuo to afford 1.8 g (81%) as a white precipitate; m.p.: 55–57°C; IR (KBr, cm−1): 3108 (aromatic CH), 1702 (CO). 1H-NMR: (300 MHz, CDCl3) δ (ppm): 7.31–7.80 (4H, aromatic), 8.21 (1H’, aromatic).
Synthesis of N-(9,10-dihydro-9,10-dioxoanthracen-2-yl)benzofuran-2-carboxamide (4)
Benzofuran-2-carbonyl chloride (0.8 g, 4.4 mmol) was added to a solution (0.64 g, 4.4 mmol) of 2-aminoanthraquinone and (1.3 mL, 8.8 mmol) Et3N in dry N,N-dimethylformamide (DMF). The reaction mixture was stirred for 24 h under reflux, and then cooled to room temperature. DMF and the excess Et3N were evaporated under reduced pressure, and the residue was stirred for 10 min in CHCl3 and flashed through a short chromatography column (CHCl3:MeOH, 993:7). The solvent was removed under reduced pressure and the product dried in vacuo to afford compound 4 as a yellow solid (yield: 51%); m.p.: 259°C; IR (KBr, cm−1): 1671, 1721, 1734 (CO), 3321 (NH); 1H-NMR: (300 MHz, CDCl3) δ (ppm): 6.97 (m, 3H, aromatic), 7.47 (s, 1H, H-3), 7.68 (d, 1H, J = 8.8 Hz aromatic), 7.70–7.75 (m, 4H, aromatic), 7.78 (s, 1H, aromatic), 8.18 (d, 1H, J = 8.7 Hz aromatic), 8.25 (d, 1H, J = 8.9 Hz aromatic), 9.15 (s, 1H, NH); MS (CI/ESI negative mode): m/z (%) = 367 (12), 321 (61), 245 (100), 237 (29). Anal. calcd. for C23H13NO4: C, 75.20; H, 3.57; N, 3.81. Found: C, 74.81; H, 4.01; N, 3.43%.
Synthesis of N-(4-benzoylphenyl)benzofuran-2-carboxamide (5)
Benzofuran-2-carbonyl chloride (0.8 g, 4.4 mmol) was added to a solution (0.87 g, 4.4 mmol) of 4-aminobenzophenone and (1.3 mL, 8.8 mmol) Et3N in dry N,N-dimethylformamide (DMF). The reaction mixture was stirred for 24 h under reflux, and then cooled to room temperature. DMF and the excess Et3N were evaporated under reduced pressure, and the residue was stirred for 10 min in CHCl3 and flashed through a short chromatography column (CHCl3:MeOH, 993:7). The solvent was removed under reduced pressure and the product dried in vacuo to afford compound 5 as a yellow solid (yield: 47%); m.p.: 217°C; IR (KBr, cm−1): 1681, 1729 (CO), 3395 (NH); 1H-NMR: (300 MHz, CDCl3) δ (ppm): 7.30–7.58 (m, 8H, aromatic), 7.62 (s, 1H, H-3), 7.70 (d, 2H, J = 2.6 Hz aromatic), 7.79 (d, 1H, J = 8.6 Hz aromatic), 7.87 (m, 2H, aromatic) 8.63 (s, 1H, NH); MS (CI/ESI negative mode): m/z (%) = 341 (18), 339 (100), 237 (34); Anal. calcd. for C22H15NO3: C, 77.41; H, 4.43; N, 4.10. Found: C, 77.01; H, 4.80; N, 3.72%.
Animals and treatments
Fifty-four adult male Wistar rats, weighing around 180 g, bred in the animal care center of the Faculty of Pharmacy, Al-Zaytoonah University, Amman, Jordan, were provided ad libitum access only to tap water throughout the experimental duration. Rats were maintained in a 12 h light–dark cycle under constant humidity and a temperature of 22 ± 2°C. All experiments were performed in accordance with the Guidelines for Animal Welfare Committee of Al-Zaytoonah University.
Triton model of hyperlipidemia
Triton WR-1339 was dissolved in dimethylsulfoxide (DMSO) and administered intraperitoneally to the rats (300 mg/kg body weight) in order to induce hyperlipidemia.
Pharmacological experimental design
Overnight-fasted rats were randomly divided into five groups of six animals each. The first group, serving as the control group (CG), received intraperitoneal administration of normal saline; the second, hyperlipidemic group (HG) received an intraperitoneal injection of Triton and were gavaged with 4% DMSO (in distilled water). In the third group, compound 4 was intraperitoneally injected with Triton, followed by an intragastric administration of compound 4 (15 mg/kg body weight) dissolved in 4% DMSO; in the rats of the fourth group, compound 5 was also intraperitoneally injected with Triton, followed by an intragastric administration of compound 5 (15 mg/kg body weight) dissolved in 4% DMSO. The last group (BF) was also intraperitoneally injected with Triton, and intragastrically treated with bezafibrate (100 mg/kg body weight) dissolved in 4% DMSO.
After 7 and 24 h from treatment, animals were anesthetized with diethyl ether and blood was collected. The blood samples were immediately centrifuged (3000 rpm for 10 min) and the plasma was used for lipid analysis by an enzymatic method with an automatic analyzer (Erba XL-300; Mannheim, Germany).
Statistical analysis
Results are expressed as mean values and standard deviations. Data obtained were analyzed using Student’s t-test, and differences with p < 0.05 were considered statistically significant.
Results
Synthesis
Benzofuran-2-acyl chloride (2) was prepared in good yield by direct reaction of benzofuran-2-carboxylic acid (1) with excess thionyl chloride (SOCl2) under reflux. The purification of benzofuran-2-acyl chloride (2) from its corresponding acid (1) was carried out by column chromatography to afford a white precipitate ().
Scheme 1. Synthesis route for the preparation of compounds 4 and 5. Reagents and conditions: (a) dichloromethane, reflux 6 h; (b) triethylamine; (c), (d) N,N-dimethylformamide, 24 h.
In spite of the weakness of 4-aminobenzophenone and 2-aminoanthraquinone as nucleophiles, their reaction with benzofuran-2-acyl chloride (2) in the presence of triethylamine (Et3N) produced compounds 4 and 5, respectively (). This problem was overcome by applying Et3N to convert the reactive acyl chloride (2) into a more reactive species, acyl ammonium chloride (3) (Scheme 1), in addition to its role as soluble base.
Pharmacological activity
Induction of hyperlipidemia by Triton WR-1339
The levels of plasma total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) in the HG and CG after 7 and 24 h treatment are shown in . In comparison with the normal control group (CG), Triton WR-1339 caused a significant increase in cholesterol and triglyceride plasma concentrations measured either 7 or 24 h after Triton injection. After 7 h, the plasma total cholesterol was increased by 44% (p < 0.0001) and triglycerides more than nine-fold (). After 24 h, the total cholesterol increase was 20% (p < 0.05) and triglyceride levels were markedly increased more than seven-fold ().
Figure 2. Effect of Triton WR-1339 on lipid profile after (a) 7 h and (b) 24 h. Values are mean ± SEM from six animals in each group. CG, control group; HG, hyperlipidemic control group; TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol. HG is compared to CG: *p < 0.05, **p < 0.0001.
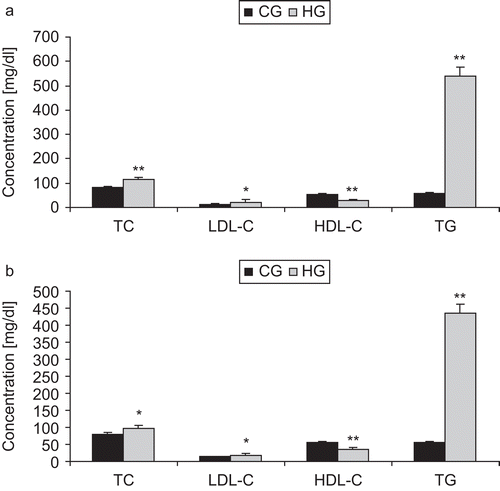
Triton WR-1339 caused a significant decrease in HDL cholesterol levels (p < 0.0001) in the hyperlipidemic control group (HG), at both 7 and 24 h after Triton administration, in comparison with the CG. In fact, the decrease of plasma HDL-C concentration in the HG was 49% and 37% after 7 h and 24 h, respectively, compared to the CG.
When the HG was compared with the CG, we observed that after 7 h from Triton injection (), LDL cholesterol increased by 50% (p < 0.05). This effect was maintained until 24 h from Triton injection ().
Effect of compounds 4, 5, and bezafibrate on rat plasma lipid profile
The plasma total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL-C), and low-density lipoprotein (LDL-C) levels of BF and compounds 4 and 5 treated rats after 7 and 24 h are shown in . Importantly, the elevated plasma TG levels produced by Triton WR-1339 administration were significantly (p < 0.0001) suppressed in BF (66%, 57%), compound 5 (93%, 68%), and compound 4 (80%, 68%) groups after 7 h and 24 h, respectively, with respect to the hyperlipidemic control group HG.
Table 1. Effect of compounds 4, 5, and bezafibrate on plasma lipid levels in Triton WR-1339-induced hyperlipidemic rats after 7 h and 24 h.
HDL-cholesterol levels were significantly increased 7 h after Triton administration (+80% and +111%, p < 0.0001) in BF and compound 4, respectively, and also (+48%, p < 0.001) in compound 5, compared to HG (). On the other hand, the increases in HDL-cholesterol levels after 24 h were not considered highly significant (+36%, +27%, and +22%, p < 0.05) in BF and compounds 4 and 5 groups, respectively, compared to HG ().
Seven hours after treatment, LDL-cholesterol levels were lowered (34% and 32%, p < 0.05) in BF and compound 5 goups, respectively, and especially (58%, p < 0.0001) in the group administered compound 4 (). However, after 24 h, only the compound 5 treated group had significantly lower LDL-cholesterol (36%, p < 0.05) compared to the hyperlipidemic control group HG ().
Treated groups clearly showed a significant reduction in plasma total cholesterol levels after 7 and 24 h, except for the BF treated group (). In fact, it was found that total cholesterol levels were reduced (by 50% and 18%) after 7 h and also (by 14% and 9%) after 24 h in compound 5 and compound 4 groups, respectively.
Discussion
Triton WR-1339 has been widely used as a model to produce acute hyperlipidemia in animals by blocking the clearance of triglyceride-rich lipoproteinsCitation5,Citation14. This model is commonly used in rats for screening agents with lipid-lowering activity, as the rat is convenient in terms of length of treatment period and handling. With this aim, many novel anti-hyperlipidemic agents have been assessed for their hypolipidemic activity in the Triton WR-1339-induced hyperlipidemic modelCitation15,Citation16.
In fact it was demonstrated that parenteral administration of Triton WR-1339 to adult rats induced hyperlipidemia. The maximum plasma total cholesterol and triglyceride levels were reached at 20 h, followed by a decline to normal valuesCitation17,Citation18. In our hands, the same model gave a similar pattern of lipid profile changes at either 7 h or 24 h after Triton WR-1339 administration ().
It is clear from our results that both compounds 4 and 5 at a dose of 15 mg/kg body weight decreased both plasma total cholesterol and triglyceride levels in a marked manner, at either 7 h or 24 h () after Triton treatment.
The large decrease in plasma HDL-C levels due to Triton WR-1339 injection results mostly from a progressive displacement of the apo A-1 protein from the HDL surface without loss of lipidCitation19. Meanwhile, the large increase in plasma TG and TC levels due to Triton administration results mostly from an increase of very low-density lipoprotein (VLDL) secretion by the liver, accompanied by a strong reduction of VLDL and LDL catabolismCitation20.
Thus, since the proportion of triglycerides in VLDL is many times higher than cholesterol, it is not surprising that the hypolipidemic activity of compounds 4 and 5 was significantly higher for triglycerides than for cholesterol. This result suggests that our compounds are able to restore, at least partially, the catabolism of B-lipoproteins, as hypothesized by many works with other lipid-lowering agentsCitation21,Citation22.
The reduction of plasma total cholesterol by compounds 4 and 5 was associated with a decrease of its LDL fraction, which is a major risk factor for cardiovascular disease. This result suggests that the cholesterol-lowering activity of these novel compounds can result from the enhancement of LDL catabolism through the hepatic receptorCitation23.
In addition, both compounds 4 and 5 increased HDL levels, which are reported to have a preventive function against atherogenesis. HDL facilitates the mobilization of triglycerides and cholesterol from plasma to the liver, where it is catabolized and eliminated in the form of bile acidsCitation24,Citation25.
The reduction in plasma triglyceride levels induced by compounds 4 and 5 at a dose of 15 mg/kg body weight 7 and 24 h after Triton injection is more significant than the reduction induced by bezafibrate at a dose of 100 mg/kg body weight, which in this study has been used as the standard reference hypolipidemic drug. Furthermore, total cholesterol levels were not significantly changed, which agrees with the mechanism of action of fibrates in that their total cholesterol-lowering activity is not strongly marked, but the triglyceride-decreasing effect of them is very impressive, especially by stimulation of the gene expression of lipoprotein lipaseCitation26.
Conclusion
Benzofuran-2-carboxamide derivatives, compounds 4 and 5, improved lipid abnormalities such as hypertriglyceridemia and hypercholesterolemia, and then elevated HDL levels in Triton-induced hyperlipidemic rats, suggesting that these compounds may be useful in the treatment of patients with lipid abnormalities. The results found are encouraging for further assessment, to elucidate the exact mechanism of action of these novel compounds as lipid-lowering agents.
Acknowledgements
The authors wish to express their sincere appreciation to Al-Zaytoonah Private University of Jordan for financial support, and to Sameer Al-Kouz for technical support.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper
References
- Epstein FM. The hepatorenal syndrome–newer perspectives. N Engl J Med 1992;327:1774–8.
- Libby P, Schoenbeck U, Mach F, Selwyn AP, Ganz P. Current concepts in cardiovascular pathology: the role of LDL cholesterol in plaque rupture and stabilization. Am J Med 1998;104:18S–27S.
- Martin MJ, Hulley SB, Browner WS, Kuller LH, Wentworth D. Serum cholesterol, blood pressure, and mortality: implications from a cohort of 361,662 men. Lancet 1986;2:933–6.
- West KM, Ahuja MS, Bennet PH. The role of circulating glucose and triglyceride concentrations and their interactions with other “risk factors” as determinants of arterial disease in nine diabetic population samples from the WHO multinational study. Diabetes Care 1983;6:361–9.
- Schurr PE, Schultz JR, Parkinson TM. Triton-induced hyperlipidemia in rats as an animal model for screening hypolipidemic drugs. Lipids 1972;7:69–74.
- Hayashi H, Niinobe S, Matsumoto Y, Suga T. Effects of Triton WR-1339 on lipoprotein lipolytic activity and lipid content of rat liver lysosomes. J Biochem 1981;89:573–9.
- Frick MH, Elo O, Haapa K, Heinonen OP, Heinsalmi P, Helo P, et al. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med 1987;317:1237–45.
- Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med 1999;341:410–18.
- Schoonjans K, Staels B, Auwerx J. Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J Lipid Res 1996;37:907–25.
- Ross BC, Middlemiss D, Scopes D, Jack T, Cardwell K, Dowle M, et al. Benzofuran derivatives. US Patent 5498722, May 15, 1992.
- Holzemann G, Schiemann K, Bottcher H, Heinrich T, Seyfried C, Leibrock J, et al. Benzofuran oxyethylamines as antidepressants and anxiolytics. US Patent 7425574, September 16, 2008.
- Parker RA. Alkoxy benzofuran carboxylic acids and salts and esters thereof as hypolipidemic agents. US Patent 4229467, October 21, 1980.
- Sohda T, Odaka H, Momose Y. Benzofuran compounds and their use. US Patent 5723479, March 3, 1998.
- Kellner A, Correll JW, Ladd AT. The influence of intravenously administered surface-active agents on the development of experimental atherosclerosis in rabbits. J Exp Med 1951;93:385–98.
- Ohmori K, Yamada H, Yasuda A, Yamamoto A, Matsuura N, Kiniwa M. Effects of a novel anti-hyperlipidemic agent, S-2E, on blood lipid levels in rats with fructose-induced hypertriglyceridemia. Pharmacology 2004;72:240–6.
- Harnafi H, Bouanani N, Aziz M, Serghini H, Ghalim N, Amrani S. The hypolipidaemic activity of aqueous Erica multiflora flowers extract in Triton WR-1339 induced hyperlipidaemic rats: a comparison with fenofibrate. J Ethnopharmacol 2007;109:156–60.
- Lauk L, Galati EM, Forestieri AM, Kirjavainen S, Trovato A. Mucuma pruriens infusion lowers cholesterol and total lipid plasma levels in the rat. Phytother Res 1989;3:263–4.
- Khanna AK, Chauder R, Chandan S, Srivastava AK, Kapoor NK. Hypolipidemic activity of Achyranthus aspera Linn in normal and Triton induced hyperlipemic rats. Indian J Exp Biol 1992;30:128–30.
- Yamamoto K, Byrne R, Edelstein C, Shen B, Scanu AM. In vitro effect of Triton WR-1339 on canine plasma high density lipoproteins. J Lipid Res 1984;25:770–9.
- Otway S, Robinson DS. The use of a non-ionic detergent (Triton WR 1339) to determine rates of triglyceride entry into the circulation of the rat under different physiological conditions. J Physiol 1967;190:309–19.
- Campillo JE, Torres MD, Dominguez E, Romero A, Perez C. Ficus carica leaf administration reduces hypertrygliceridaemia in streptozotocin diabetic rats. Diabetologia 1994;37:A213.
- Perez C, Canal JR, Campello JE, Adelaida R, Torres MD. Hypotriglyceridaemic activity of Ficus carica leaves in experimental hypertriglyceridaemic rats. Phytother Res 1999;13:188–91.
- Khanna AK, Rizvi F, Chander R. Lipid lowering activity of Phyllanthus niruri in hyperlipemic rats. J Ethnopharmacol 2002;82:19–22.
- Malloy MJ, Kan JP. Medical management of hyperlipidemic states. Adv Intern Med 1994;39:603–31.
- Anila L, Vijayalakshmi NR. Flavonoids from Emblica officinalis and Mangifera indicia - effectiveness for dyslipidemia. J Ethnopharmacol 2002;79:81–7.
- Staels B, Dallongville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart JC. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation 1998;98:2088–93.
![Figure 1. Chemical structures of compound 4 [N-(9,10-dihydro-9,10-dioxoanthracen-2-yl)benzofuran-2-carboxamide] and compound 5 [N-(4-benzoylphenyl)benzofuran-2-carboxamide].](/cms/asset/fb278730-97c9-4ac9-8d00-0227d1e3e268/ienz_a_439166_f0001_b.gif)