Abstract
Melatonin is the chief secretory product of the pineal gland and is synthesized enzymatically from serotonin. These indoleamine derivatives play an important role in the prevention of oxidative damage. Lactoperoxidase (LPO; EC 1.11.1.7) was purified from bovine milk with three purification steps: Amberlite CG-50 resin, CM-Sephadex C-50 ion-exchange, and Sephadex G-100 gel filtration chromatography, respectively. LPO was purified with a yield of 21.6%, a specific activity of 34.0 EU/mg protein, and 14.7-fold purification. To determine the enzyme purity, SDS-PAGE was performed and a single band was observed. The Rz (A412/A280) value for LPO was 0.9. The effect of melatonin and serotonin on lactoperoxidase was determined using ABTS as chromogenic substrate. The half-maximal inhibitory concentration (IC50) values for melatonin and serotonin were found to be 1.46 and 1.29 μM, respectively. Also, the inhibition constants (Ki) for melatonin and serotonin were 0.82 ± 0.28 and 0.26 ± 0.04 μM, respectively. Both melatonin and serotonin were found to be competitive inhibitors.
Introduction
The pineal hormone melatonin (N-acetyl-5-methoxytryptamine), an indoleamine, is the chief secretory product of the pineal gland. It is synthesized enzymatically from serotonin (5-hydroxytryptamine) by the sequential action of serotonin N-acetyltransferase and hydroxyindole-O-methyltransferaseCitation1. It is also produced in other organs and found in all body fluids after its release from the pineal. It is known that melatonin influences a variety of biological processes including circadian rhythm and neuroendocrine, cardiovascular, and immune functions, as well as thermoregulationCitation2–4. Additionally, this molecule functions in protecting cell components such as nuclear DNA, membrane lipids, and cytosolic proteins from free radical damageCitation5,Citation6.
Serotonin (5-hydroxytryptamine) has been implicated in the control of many physiological (cardiovascular, respiratory, and thermoregulatory) and behavioral (feeding and sexual behavior, circadian rhythm, sleep–wake cycle, aggression, learning, and pain sensitivity) functions that could be disturbed by depressionCitation7,Citation8. It is a biogenic amine belonging to the most common neurotransmitters in nature. Serotonin is a well-established neurotransmitter produced and activated in nervous tissues and the digestive tractCitation9. In humans, serotonin modifies body temperature, blood pressure, sexual behavior, and mood. It is also involved in several interactions of the immune systemCitation10. It has been reported that serotonin is implicated in many brain functions, including those related to pleasure and the intake of food, depression, suicide, anxiety, and Parkinson’s and Alzheimer’s diseasesCitation8,Citation11.
Milk contains a variety of compounds that protect the neonate as well as the milk itself from a host of deleterious microorganisms. One of those compounds is the enzyme lactoperoxidaseCitation12. Lactoperoxidase (LPO; EC 1.11.1.7) is a heme-containing glycoprotein with a single chain of 612 residues that has a molecular mass of approximately 78 kDa and a carbohydrate content of about 10%Citation13. Lactoperoxidase catalyzes the oxidation of halides and pseudohalides at the expense of hydrogen peroxide, and generates products with a wide antimicrobial activity. Hence, it catalyzes the inactivation of a wide range of microorganismsCitation14–16. The other members of the mammalian peroxidase family include eosinophil peroxidase (EPO), thyroid peroxidase (TPO), and myeloperoxidase (MPO). LPO, EPO, and MPO contribute to the nonimmune host defense system by oxidizing halide and pseudohalide ions to produce potent antimicrobial agentsCitation17. LPO is present and active in many secretory fluids in various parts of the body including milk, tears, and salivaCitation15. It was reported that LPO carries out this function in the above exocrine secretions, while EPO and MPO play similar roles in the phagosomes of eosinophils and neutrophils, respectively, during engulfment of microorganisms. On the other hand, TPO is an intracellular membrane-bound protein, which is involved in catalysis of the iodination and coupling of thyroglobulin moieties in the biosynthesis of thyroid hormones thyroxine and triiodothyronineCitation17. Bovine milk LPO has a high activity, more than other types of LPO; therefore, most research has been carried out on bovine LPO. Also, the quantity of LPO in human milk is less than in other sources such as bovine milk LPO. The objective of this study was to evaluate the in vitro effects of melatonin and serotonin on lactoperoxidase purified from bovine milk.
Materials and methods
Chemicals
Melatonin, serotonin, Sephadex G-100, CM-Sephadex C-50, 2,2′-azino-bis(3-ethylbenzthiazoline-6 sulfonic acid) (ABTS), Coomassie Brilliant Blue R-250, and standard proteins (egg albumin, bovine albumin, and β-galactosidase) were obtained from Sigma Aldrich Chemie GmbH. Amberlite CG-50 resin was purchased from Fluka Chemie GmbH.
Determination of lactoperoxidase activity
Lactoperoxidase activity was determined by the procedure of Schindler with a slight modificationCitation18. This method is based on the oxidation of ABTS as a chromogenic substrate with hydrogen peroxide. The formed colored compound gives an absorbance at 412 nm. As a typical procedure, 2.8 mL of 1 mM ABTS in phosphate buffer (0.1 M, pH 6.0) was mixed with 0.1 mL of enzyme in phosphate buffer (1 mM, pH 6.0) and 0.1 mL of H2O2 solution (3.2 mM) and the absorbance was taken at 412 nm as a function of time every 15 s during 3 minCitation19.
Lactoperoxidase activity unit
One unit of activity is defined as the amount of enzyme catalyzing the oxidation of 1 μmol of ABTS min−1 at 298K (molar absorption coefficient: 32,400 M−1 cm−1)Citation19,Citation20.
Protein determination
Protein concentration was determined according to the method of Lowry et al.Citation21. Bovine serum albumin (BSA) was used as standard proteinCitation22,Citation23.
Purification of lactoperoxidase
Bovine milk was centrifuged at 5000 × g using the centrifuge maximum at 48°C for 15 min to remove fat. First, Amberlite CG-50 resin was equilibrated with sodium phosphate buffer (5 mM, pH 6.8), then added in the proportion of 22 g/L to the fresh, raw, skimmed bovine milkCitation21,Citation24. The supernatant was decanted and the resin was washed with distilled water, then equilibrated with 20 mM sodium phosphate buffer (pH 6.8). The bound protein was eluted with 0.5 M sodium phosphate buffer (pH 6.8). To the green-colored mixture was gradually added solid ammonium sulfate (precipitation I, saturation 90%) over a period of 30 min while it was being stirred magnetically, and the enzyme solution was dialyzed overnight against 5 mM sodium phosphate buffer (pH 6.8).
The clear greenish supernatant obtained above was loaded onto a column of CM-Sephadex C-50 (3 × 10 cm) previously equilibrated with 10 mM sodium phosphate buffer (pH 6.8). The column-bound enzyme was washed with 100 mL of 10 mM phosphate buffer (pH 6.8) containing 100 mM NaCl. The enzyme was eluted with a linear gradient of 100–200 mM NaCl in 10 mM phosphate buffer (pH 6.8) and subjected to ammonium sulfate precipitation (precipitation II, saturation 90%). Thereafter the enzyme solution was dialyzed overnight against sodium phosphate buffer (5 mM, pH 6.8)Citation19,Citation20.
Lactoperoxidase enzyme obtained from the CM-Sephadex C-50 column was applied to a column of Sephadex G-100 (2.5 × 100 cm). The column-bound enzyme was eluted with 0.1 M phosphate buffer (pH 6.8), and salted out with ammonium sulfate precipitation (precipitation III, 90% saturation). The enzyme solution was dialyzed overnight against phosphate buffer (0.5 M, pH 6.0). Fractions were lyophilized and checked for purity by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)Citation25–27.
Ammonium sulfate precipitation
Lactoperoxidase enzyme obtained from the CM-Sephadex C-50 column was subjected to ammonium sulfate fractionation and the precipitate in the 0–90% saturation range was collected by centrifugation for 60 min at 15,000 × g. The precipitate was suspended in ∼2 mL phosphate buffer (0.5 M, pH 6.0)Citation28.
SDS-PAGE
SDS-PAGE was performed under denaturing conditions after LPO purification, according to Laemmli’s procedureCitation29. The stacking and running gels comprised 3% (w/v) and 10% (w/v) acrylamide, respectively, and 0.1% (w/v) SDS. The electrode buffer was 0.025 M Tris/0.2 M glycine (pH 8.3). The sample buffer was prepared by mixing 0.65 mL of Tris-HCl (1 M, pH 6.8), 3 mL of 10% (w/v) SDS, 1 mL of neat glycerol, 1 mL of 0.1% (w/v) bromphenol blue, 0.5 mL of β-mercaptoethanol, and 3.85 mL of water. A 20 μg aliquot of enzyme (50 μL) was added into 50 μL of sample buffer and the mixture was heated in a boiling water bath for 3 min and then cooledCitation30.
LPO samples were loaded into each space of the stacking gel. LPO was analyzed separately by PAGE. Initially, an electric potential of 80 V (Hoefer Scientific Instruments, SE 600) was applied until the bromphenol dye reached the running gel. Then it was increased to 200 V for 3–4 h. Gels were stained for 1.5 h in 0.1% (w/v) Coomassie Brilliant Blue R-250 in 50% (v/v) methanol and 10% (v/v) acetic acid, and destained with methanol/acetic acidCitation31,Citation32.
Results and discussion
Lactoperoxidase enzyme (LPO) is a member of the mammalian peroxidase family. It catalyzes the oxidation of thiocyanate and halides. As reported in the literature, LPO, EPO, and TPO are monomeric proteins, while MPO is a covalently linked dimer of two identical halves, each consisting of two polypeptide chains of 108 and 466 amino acid residues as a result of a posttranslational deletion of six amino acid residuesCitation17. LPO has been intensively studied over the years, and is present and active in many secretory fluids in various parts of the body. The effect of increasing concentrations of melatonin (0.5–2.5 μM) and serotonin (0.5–2.15 μM) on LPO enzyme activity was determined.
First, LPO was purified from bovine milk using CM-Sephadex C-50 ion exchange chromatography. Then the enzyme obtained from CM-Sephadex C-50 ion exchange was applied to Sephadex G-100 gel filtration chromatography (). Specific activity was determined for each purification step. Kinetics parameters Km and Vmax were calculated by a Lineweaver–Burk plot for the ABTS substrate.
Table 1. Purification scheme of lactoperoxidase obtained after the application of different purification steps.
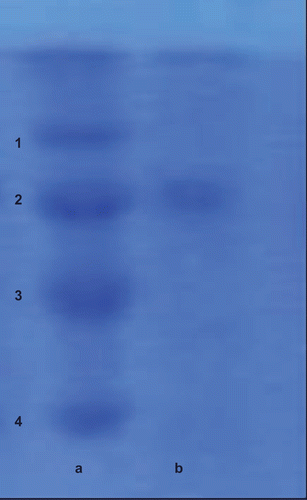
The Km value was found to be 0.358 mM; on the other hand, the Vmax value was calculated to be 18.86 μmol/mL/min. The purification of LPO was controlled by SDS-PAGE. Maltose-binding protein (MBP)–β-galactosidase (fusion of MBP and β-galactosidase, 175 kDa), MBP–paramyosin (fusion of MBP and paramyosin, 80 kDa), MBP–chitin binding domain (CBD) (fusion of MBP and CBD, 58 kDa), and CBD–Mxe Intein–2CBD (fusion of CBD and Mxe Intein followed by two CBDs, 46 kDa) were used as standard proteins. LPO purified from bovine milk, when subjected to SDS-PAGE electrophoresis, exhibited only one band of LPO, as shown in , column b.
Figure 1. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) bands of lactoperoxidase (LPO) purified from bovine milk. Column a: standard proteins, 1: maltose-binding protein (MBP)–β-galactosidase (fusion of MBP and β-galactosidase, 175 kDa); 2: MBP–paramyosin (fusion of MBP and paramyosin, 80 kDa); 3: MBP–chitin binding domain (CBD) (fusion of MBP and CBD, 58 kDa); 4: CBD–Mxe Intein–2CBD (fusion of CBD and Mxe Intein followed by two CBDs, 46 kDa). Column b: purified LPO from bovine milk.
There is no detailed study regarding the effect of melatonin and serotonin on LPO activity. In the present study, melatonin and serotonin were investigated for their inhibitory effects on LPO, and kinetics constants Ki and IC50 were evaluated. The results obtained from the present study clearly showed that melatonin and serotonin had strong inhibitory effects on LPO activity.
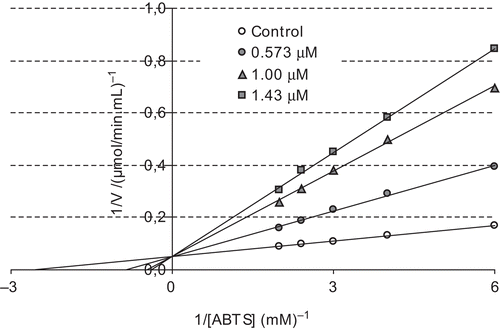
The concentration required for 50% inhibition (IC50) and inhibition constant (Ki) values are often reported in the literature, but direct comparison of these values is not possible. The concentration required to inhibit LPO activity of the purified proteins by 50% (IC50) and inhibition constants Ki were determined for each compound. The IC50 and Ki values were used to compare the inhibitory potentials of melatonin and serotonin. To determine the Ki value as well as the inhibition type, at least three different melatonin or serotonin concentrations were selected. At each melatonin or serotonin concentration, enzyme activity was measured in the presence of various substrate concentrations. The relationship of Ki and IC50 for a given compound varies, depending on the assay conditions and the compound’s mechanism of inhibition. In this study, Ki and IC50 parameters for melatonin and serotonin as inhibitors of bovine LPO were determined. The inhibitor concentrations causing up to 50% inhibition were determined from activity (%) vs. [melatonin/serotonin] plots. As can be seen in and , IC50 values for melatonin and serotonin were determined as 1.46 and 1.29 μM, respectively. In addition, Ki values were calculated from Lineweaver–Burk plots ( and ). Ki constants for melatonin and serotonin were 0.82 ± 0.28 and 0.26 ± 0.04 μM, respectively. Both melatonin and serotonin exhibited competitive inhibition. These results showed that lactoperoxidase had affinity to serotonin more than to melatonin. In comparison, in a previous study, in vitro effects of ketamine and bupivacaine as analgesic agents were determined on LPO activityCitation18. Ki constants for both anesthetic drugs were found to be 19 and 15 μM, and IC50 values were 290 and 155 μM, respectively.
Figure 2. The effect of different concentrations of melatonin (0.5–2.5 mM) on lactoperoxidase obtained from bovine milk.
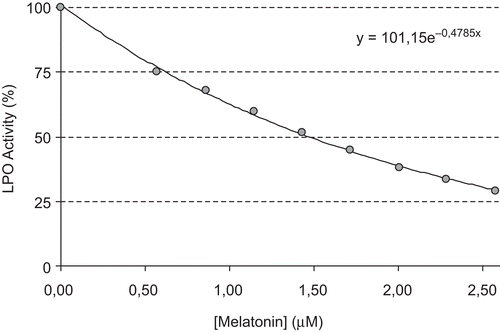
Figure 3. Lineweaver–Burk plot for different ABTS concentrations and three different melatonin concentrations for determination of Ki constant.
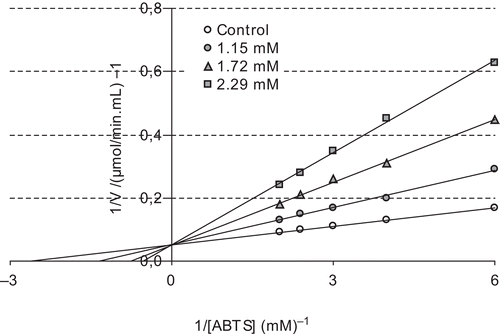
Figure 4. The effect of serotonin at different concentrations (0.5–2.15 mM) on bovine milk lactoperoxidase.
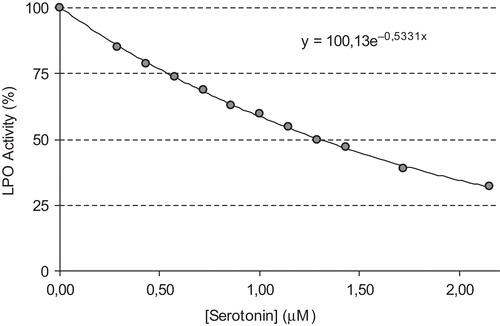
Figure 5. Lineweaver–Burk plot for different ABTS concentrations and three different serotonin concentrations for determination of Ki constant.
The binding and structural studies of bovine lactoperoxidase with three aromatic ligands, acetylsalicylic acid (ASA), salicylhydroxamic acid (SHA), and benzylhydroxamic acid (BHA), were studied by Singh and co-workersCitation33. This study showed that all three compounds bind to lactoperoxidase at the substrate binding site on the distal heme side. The binding of ASA occurs without perturbing the position of the conserved heme water molecule W-1, whereas both SHA and BHA displace it by the hydroxyl group on hydroxamic acid moieties. The acetyl group carbonyl oxygen atom of ASA forms a hydrogen bond with W-1, which in turn makes three other hydrogen bonds, one each with heme iron, His-109 N2, and Gln-105 N2. In contrast, in complexes of SHA and BHA, the OH group of the hydroxamic acid moiety in both complexes interacts with the heme iron directly. The OH is also hydrogen-bonded to His-109 N2 and Gln-105 N2. The plane of the benzene ring of ASA is inclined at 70.7° from the plane of the heme moiety, whereas the aromatic planes of SHA and BHA are nearly parallel to the heme plane. The mode of ASA binding provides information about the mechanism of action of aromatic substrates, whereas the binding characteristics of SHA and BHA indicate the mode of inhibitor bindingCitation33. In the same manner, melatonin has a carbonyl group and serotonin has also a phenolic hydroxyl group. Both molecules can bind the heme group of lactoperoxidase.
In conclusion, the results showed that melatonin or serotonin had greater inhibition on LPO than other anesthetic drugs such as ketamine and bupivacaineCitation20,Citation21. Melatonin and serotonin showed in vitro inhibition of LPO activity. According to the results obtained from the present study, both compounds were found to be marked LPO inhibitors, and could cause some side effects in lactation periods.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Saarela S, Reiter RJ. Function of melatonin in thermoregulatory processes. Life Sci 1993;54:295–311.
- Gülçin İ,Büyükokuroğlu, ME, Oktay M, Küfrevioglu Öİ. On the in vitro antioxidant properties of melatonin. J Pineal Res 2002;33:167–71.
- Gülçin İ, Büyükokuroğlu ME, Küfrevioğlu Öİ. Metal chelating and hydrogen peroxide scavenging effects of melatonin. J Pineal Res 2003;34:278–81.
- Montilla P, Cruz A, Padillo FJ, Túnez I, Gascon F, Muñoz MC, et al. Melatonin versus vitamin E as protective treatment against oxidative stress after extrahepatic bile duct ligation in rats. J Pineal Res 2001;31:138–44.
- Kim SJ, Reiter RJ, Qi W, Tan DX, Cabrera J. Melatonin prevents oxidative damage to protein and lipid induced by ascorbate-Fe3+-EDTA: comparison with glutathione and α-tocopherol. Neuroendocrinol Lett 2000;21:269–76.
- Stahl SM. Mechanism of action of serotonin selective re-uptake inhibitors: serotonin receptors and pathways mediate therapeutic effects and side effects. J Affect Disord 1998;51:215–35.
- Gülçin İ. Measurement of antioxidant ability of melatonin and serotonin by the DMPD and CUPRAC methods as trolox equivalent. J Enzyme Inhib Med Chem 2008;23:871–6.
- Bubenik GA, Ball RO, Pang SF. The effect of food deprivation on brain and gastrointestinal tissue levels of tryptophan, serotonin, 5-hydroxyindoleacetic acid and melatonin. J Pineal Res 1992;12:7–16.
- Hellstrand K, Hermodsson S. Serotonergic 5-HT1A receptors regulate a cell contact-media interaction between natural killer cells and monocytes. Scand J Immunol 1993;37:7–18.
- Hasegawa S, Watanabe A, Nishi K, Nguyen KQ, Diksic M. Selective 5-HT1B receptor agonist reduces serotonin synthesis following acute, and not chronic, drug administration: results of an autoradiographic study. Neurochem Int 2005;46:261–72.
- Bootsa JW, Floris R. Lactoperoxidase: from catalytic mechanism to practical applications. Int Dairy J 2006;16:1272–6.
- Wolf SM, Ferrari RP, Traversa S, Biemann K. Determination of the carbohydrate composition and the disulfide bond linkages of bovine lactoperoxidase by mass spectrometry. J Mass Spectrom 2000;35:210–17.
- Soukka T, Lumikari M, Tenovuo J. Combined inhibitory effect of lactoferrin and lactoperoxidase system on the viability of Streptococcus mutans, serotype c. Scand J Dent Res 1991;99:390–6.
- Dionysius DA, Grieve PA, Vos AC. Studies on the lactoperoxidase system: reaction kinetics and antibacterial activity using two methods for hydrogen peroxide generation. J Appl Bacteriol 1992;72:146–53.
- Benoy MJ, Essy AK, Sreekumar B, Haridas M. Thiocyanate mediated antifungal and antibacterial property of goat milk lactoperoxidase. Life Sci 2000;66:2433–9.
- Shindler JS, Bardsley WG. Steady-state kinetics of lactoperoxidase with ABST as chromogens. Biochem Biophys Res Comun 1975;67:1307–12.
- Singh AK, Singh N, Sharma S, Singh SB, Kaur P, Bhushan A, et al. Crystal structure of lactoperoxidase at 2.4 å resolution. J Mol Biol 2008;376:1060–75.
- Şişecioğlu M, Çankaya M, Gülçin İ, Ozdemir H. The inhibitory effect of propofol on lactoperoxidase. Protein Peptide Lett 2009;16:46–9.
- Özdemir H, Uguz MT. In vitro effects of some anaesthetic drugs on lactoperoxidase enzyme activity. J Enzyme Inhib Med Chem 2005;20:491–5.
- Uguz MT, Özdemir H. Purification of bovine milk lactoperoxidase and investigation of antibacterial properties at different thiocyanate mediated. Appl Biochem Microbiol 2005;41:349–53.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin-phenol reagents. J Biol Chem 1951;193:265–75.
- Çoban TA, Beydemir Ş, Gülçin, İ, Ekinci D. Morphine inhibits erythrocyte carbonic anhydrase in vitro and in vivo. Biol Pharm Bull 2007;30:2257–61.
- Şentürk M, Gülçin İ, Daştan A, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of human erythrocyte isozymes I and II with a series of antioxidant phenols. Bioorg Med Chem 2009;17:3207–11.
- Elagamy EI. Effect of heat treatment on camel milk proteins with respect to antimicrobial factors: a comparison with cows’ and buffalo milk proteins. Food Chem 2000;68:227–32.
- Beydemir Ş, Gülçin İ, Küfrevioğlu Öİ, Çiftçi M. Glucose 6-phosphate dehydrogenase: in vitro and in vivo effects of dantrolene sodium. Pol J Pharmacol 2003;55:787–92.
- Şentürk M, Gülçin İ, Çiftci M, Küfrevioğlu Öİ. Dantrolene inhibits human erythrocyte glutathione reductase. Biol Pharm Bull 2008;31:2036–9.
- Çoban TA, Beydemir Ş, Gülçin İ, Ekinci D. The inhibitory effect of ethanol on carbonic anhydrase isoenzymes: in vivo and in vitro studies. J Enzyme Inhib Med Chem 2008;23:266–70.
- Köksal E, Gülçin İ. Purification and characterization of peroxidase from cauliflower (Brassica oleracea L.)buds. Protein Peptide Lett 2008;15:320–6.
- Laemmli DK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–3.
- Hisar O, Beydemir Ş, Gülçin İ, Küfrevioğlu Öİ. Effect of low molecular weight plasma inhibitors of rainbow trout (Oncorhynchus mykiss) on human erythrocytes carbonic anhydrase-II isozyme activity in vitro and rat erythrocytes in vivo. J Enzyme Inhib Med Chem 2005;20:35–9.
- Gülçin İ, Beydemir Ş, Büyükokuroğlu ME. In vitro and in vivo effects of dantrolene on carbonic anhydrase enzyme activities. Biol Pharm Bull 2004;27:613–16.
- Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc 1934;56:658–66.
- Singh AK, Singh N, Sinha M, Bhushan A, Kaur P, Srinivasan A, et al. Binding modes of aromatic ligands to mammalian heme peroxidases with associated functional implications: crystal structures of lactoperoxidase complexes with acetylsalicylic acid, salicylhydroxamic acid, and benzylhydroxamic acid. J Biol Chem 2009;284:20311–18.