Abstract
Tabun belongs to the most toxic nerve agents. Its mechanism of action is based on acetylcholinesterase (AChE) inhibition at the peripheral and central nervous systems. Therapeutic countermeasures comprise administration of atropine with cholinesterase reactivators able to reactivate the inhibited enzyme. Reactivation of AChE is determined mostly biochemically without specification of different brain structures. Histochemical determination allows a fine search for different structures but is performed mostly without quantitative evaluation. In rats intoxicated with tabun and treated with a combination of atropine and HI-6, obidoxime, or new oxime K048, AChE activities in different brain structures were determined using biochemical and quantitative histochemical methods. Inhibition of AChE following untreated tabun intoxication was different in the various brain structures, having the highest degree in the frontal cortex and reticular formation and lowest in the basal ganglia and substantia nigra. Treatment resulted in an increase of AChE activity detected by both methods. The highest increase was observed in the frontal cortex. This reactivation was increased in the order HI-6 < K048 < obidoxime; however, this order was not uniform for all brain parts studied. A correlation between AChE activity detected by histochemical and biochemical methods was demonstrated. The results suggest that for the mechanism of action of the nerve agent tabun, reactivation in various parts of the brain is not of the same physiological importance. AChE activity in the pontomedullar area and frontal cortex seems to be the most important for the therapeutic effect of the reactivators. HI-6 was not a good reactivator for the treatment of tabun intoxication.
Introduction
The basic mechanism of action of organophosphates (OPs)/nerve agents is considered to be acetylcholinesterase (AChE, EC 3.1.1.7) inhibition and subsequent accumulation of neuromediator acetylcholine at the cholinergic synapses, either peripheral or central, leading to cholinergic hyperstimulation and development of the symptoms of poisoning, followed by metabolic dysbalance and, without effective treatment, leading to death. Tabun (O-ethyl N,N-dimethyl phosphoramidocyanidate; GA) is a nerve agent acting at both peripheral and central levels but with prevailing central effectsCitation1–3.
The treatment of nerve agent poisoning consists of the administration of parasympatholytics, cholinesterase reactivators (oximes), and anticonvulsants. Atropine is considered as the most common parasympatholytic drug. Among anticonvulsants, diazepam is frequently used. The choice of oximes is broad (e.g. pralidoxime, obidoxime, HI-6, and others) but not so simple, and depends very much on the type of OPCitation1,Citation4.
There is a general opinion that obidoxime is a good reactivator for OP insecticide poisoningCitation1,Citation5. For nerve agents, HI-6 seems to be a relatively good reactivator; however, in the case of tabun intoxication it is not effectiveCitation1,Citation6,Citation7. Therefore, some attempts to synthesize new reactivators with the aim of making them universal or more effective, especially against tabun-inhibited AChE, have been made. After their synthesis, the reactivation efficacy was tested in vitro followed by in vivo studiesCitation4,Citation6. In some cases, however, the in vivo results did not correspond to the in vitro observations. This discrepancy could be caused by different inhibition/reactivation of AChE originating from various sources (human brain, rat erythrocytes, brain, etc.) or by different penetration of the agent to the target organs. It is generally accepted that reactivation on the periphery is without doubt, but with one exception: after nerve agent intoxication followed by monoisonitrosoacetone (MINA) treatment, reactivation of peripheral AChE was not observed, but AChE in the brain (central compartment) was reactivatedCitation8.
K048 (1-(4-hydroxyiminomethylpyridinium)-3-(4-carbamoylpyridinium)butane dibromide) is one of the newly synthesized reactivators having some reactivation efficacy against tabun-inhibited AChE in vitro and comparable therapeutic efficacy with obidoxime in vivoCitation9–14. On the other hand, HI-6 is less effective than obidoxime. We compared the reactivation effects of these three reactivators () in vivo on AChE activity in different parts of the brain in tabun-intoxicated rats.
Materials and methods
Chemicals
Tabun was obtained from the Military Technical Institute of Protection (Brno, Czech Republic). It was of minimal 95% purity and was stored in glass ampoules (1 mL). Solutions of the agents for experiments were prepared before use. All oximes (obidoxime, HI-6, K048) were synthesized at the Department of Toxicology of the Faculty of Military Health Sciences (Hradec Kralove, Czech Republic) Their purities were analyzed using the high performance liquid chromatography (HPLC) technique. All other chemicals of analytical purity were obtained commercially and used without further purification. All substances were administered intramuscularly (i.m.) at a volume of 1 mL/kg body weight.
Animals
Female Wistar rats (Velaz, Prague), weighing 200–220 g, were used in this study. The animals were divided into groups of 10 animals each (six for biochemical and four for histochemical examination). Housing of the rats was realized in the Central Vivarium of the Faculty of Military Health Sciences under veterinary control (light cycle 12h/12h, standard laboratory diet and water ad libitum). All the experiments were performed with the permission and under the supervision of the Ethics Committee of the Faculty of Military Health Sciences, Hradec Kralove (permission No 153/06) according to §17 of Czech law No 207/2004, permission of responsible person No 0001/94 – M 699.
Intoxication and treatment
Control group
The animals were injected with saline i.m. and 1 min later they were injected once again with saline i.m. (1.0 mL/kg). Decapitation and brain removal (sampling) was realized 30 min after the last saline injection.
Tabun group
The animals were injected with tabun (i.m.) at a dose of 1 × LD50, i.e. 200 μg/kg, and 1 min later they were injected with saline. Then, 31 min after the intoxication, the animals were decapitated and the brains were removed.
Treated groups
HI-6 group The animals were injected with tabun (i.m.) at a dose of 1 × LD50, i.e. 200 μg/kg; 1 min later, the animals were injected with one injection (i.m.) of atropine (21 mg/kg) and HI-6 dichloride. Then, 31 min after the intoxication, the animals were decapitated and the brains without perfusion were removed and used for histochemical or biochemical examination.
Obidoxime group The animals were injected with tabun (i.m.) at a dose of 1 × LD50, i.e. 200 μg/kg; 1 min later, the animals were injected with one injection (i.m.) of atropine (21 mg/kg) and diobidoxime chloride. Then, 31 min after the intoxication, the animals were decapitated and the brains without perfusion were removed and used for histochemical or biochemical examination.
K048 group The animals were injected with tabun (i.m.) at a dose of 1 × LD50, i.e. 200 μg/kg; 1 min later, the animals were injected with one injection (i.m.) of atropine (21 mg/kg) and K048 chloride. Then, 31 min after the intoxication, the animals were decapitated and the brains without perfusion were removed and used for histochemical or biochemical examination.
The doses of all oximes (obidoxime, HI-6, and K048) were equal to 5% of their LD50, i.e. corresponding to human therapeutic doses.
Six animals per group were used for biochemical examination. For histochemical examination, four animals per group were used.
Histochemical determination of AChE
The removed brains were rapidly frozen and cut into series of 20 μm sections using a cryostat. For neuroanatomical mapping according to a rat brain atlasCitation15, AChE detectionCitation16 was used. The Karnovski–Roots method is based on the hydrolysis of artificial substrate acetylthiocholine (the same as in biochemical examination) and detection of the released reaction product (thiocholine). For digital microphotography, an Olympus BX51 light microscope equipped with CCD (charge coupled device) was used.
Quantitative evaluation was done using 3D Doctor softwareCitation17,Citation18. The image was transposed to a gray scale with density distribution (expressed in pixels) ranging from 0 to 255. A lower pixel number indicates high activity, a higher number shows inhibition. As the density is on a linear scale, the difference (255 – determined density) gives information about the AChE activity. This pixel density was compared in control and intoxicated animals for each structure examined, in absolute and relative (%) values.
We selected the following sections and groups of nuclei to compare quantitative histochemistry with the biochemical determination of AChE: nucleus ruber (NR) – section at level 5.8 mm dorsally from bregma; frontal cortex (FC) – level of 2.2 mm rostrally to bregma, containing areas F1, F2, F3; dorsal septum (DS) – level of bregma, including lateral septal nucleus, dorsal and intermediate parts; hypothalamus (HTh) – 2.8 mm dorsally to bregma, including ventromedial and dorsomedial hypothalamic nuclei, perifornical, arcuate, periventricular, and supraoptic nuclei, tuber cinereum, dorsal and lateral hypothalamic areas; hippocampus (Hipp) – 3.5 mm dorsally to bregma; medial thalamus (Th) – 2.8 mm dorsally from bregma including mediodorsal nuclei, intermediodorsal, paraventricular, paracentral, central medial, and centrolateral nuclei; pontomedullar area (PM) – section at level of 13 mm dorsally to bregma, we evaluated only nucleus gigantocellularis as representative of AChE activity, its size, and function. The distances from the bregma and terminology of nuclei are quoted from reference 15.
Biochemical determination of AChE
The brains were frozen and the parts (NR, FC, DS, HTh, Hipp, Th, PM) were prepared. After thawing, the tissue was homogenized (1:10, distilled water, Ultra-Turrax homogenizer) and homogenates were used for enzymatic analysis. The concentration of wet-weight tissue was 2 mg per cuvette (2 mL). AChE activity was determined using the method of Ellman et al.Citation19 as described elsewhereCitation20. Acetylthiocholine iodide (0.5 mM) was used as substrate (Tris-HCl buffer, pH 7.6) and 5,5′-dithiobis-2-nitrobenzoic acid (0.5 mM) as chromogen. A Uvikon 752 spectrophotometer was used for determination of the absorbance at 412 nm. The activity is expressed as µmol of substrate hydrolyzed/60 min.kg wet-weight tissue or as % of control value. In the control group, distinguishing between AChE and butyrylcholinesterase activity was performed, and the butyrylcholinesterase activity was found to be negligible (less than 5%).
Statistical evaluation
Enzyme activities determined by the biochemical method are expressed as mean ± SD or % of control values and statistical differences were tested by t-test. Histochemical results are expressed as mean only. Transformation of curves and their equations and correlation coefficients were evaluated by the least-squares method using relevant PC programs.
Results
Mortality
Following tabun poisoning, all animals survived for 30 min after tabun injection. However, three animals were clearly ante finem. Salivation, tremor, and convulsions were observed in all animals. After tabun intoxication and treatment with atropine and reactivators, fine tremor was observed 10–30 min after the administration only, and all animals survived.
Biochemical
Data dealing with normal AChE activity in the brain areas are presented in . AChE activity varied from high (PM, Th, Hipp) to low (NR, FC) values. The administration of tabun caused different inhibitions of AChE in all areas studied. Percent inhibition (lower activity) was highest in FC and Hipp, containing about 30–40% of the control AChE activity. Lower inhibition of AChE was observed in the remaining brain structures and in NR. The highest AChE activity increase was observed following obidoxime therapy. This increase, detected by the biochemical method, was statistically significant in all brain structures studied except Th (obidoxime). AChE activity in three structures (FC, Hipp, PM) was significantly increased following treatment with K048. The increase of AChE activity following treatment with HI-6 was statistically significant only in Hipp and DS (). Percent expression of AChE activity is shown in .
Table 1. Control values of AChE activity in different brain areas detected by biochemical and histochemical methods.
Table 2. AChE activities in the brain parts of intoxicated and treated animals.
Table 3. Percent values of AChE activity in brain parts following intoxication with tabun and its treatment.
Histochemical
The histochemical results of AChE distribution in the brain structures showed a picture similar to that of biochemical examination (). The most sensitive areas to tabun were FC and Hipp (about 35–40% of control values); other high AChE activity following untreated tabun intoxication was observed in NR. Histochemical images of three areas, the relatively sensitive FC, PM, and NR are shown in –. The quantitative evaluation following intoxication with tabun and treatment with atropine and reactivator is also shown in –, where it can be seen that AChE inhibition in all parts had a similar tendency, as observed by biochemical examination. Quantitative evaluation of AChE activity is shown in and percent changes following treatment using different oximes are given in . Quantitative histochemical evaluation showed that the activities were shifted to lower densities, and pixel frequencies were different (–).
Figure 2. Top: Microphotography of 20 μm sections of rat brain (reticular formation, ncl. gigantocellularis, position marked by arrows) following different treatments: 1, control (not intoxicated); 2, tabun (intoxicated); intoxicated with tabun and treated: 3, HI-6; 4, obidoxime; 5, K048. Magnification: ×40. Staining: AChE. Bottom: Quantitative evaluation of histochemical data: density curves of microphotographs.
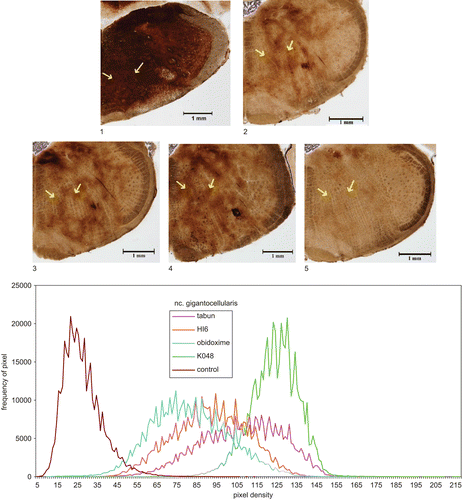
Figure 3. Top: Microphotography of 20 μm sections of rat brain (frontal cortex) following different treatments: 1, control (not intoxicated); 2, tabun (intoxicated); intoxicated with tabun and treated: 3, HI-6; 4, obidoxime; 5, K048. Magnification: ×40. Staining: AChE. Bottom: Quantitative evaluation of histochemical data: density curves of microphotographs.
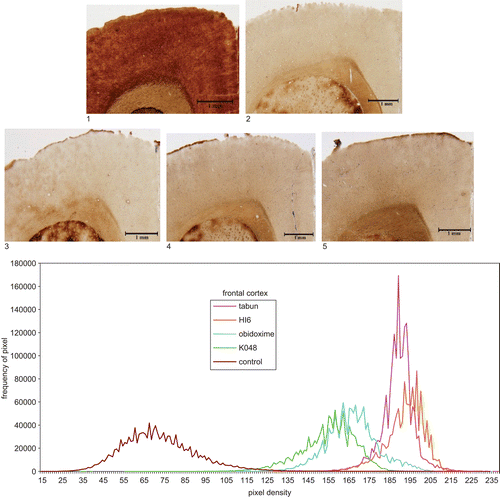
Figure 4. Top: Microphotography of 20 μm sections of rat brain (nucleus ruber) following different treatments: 1, control (not intoxicated); 2, tabun (intoxicated); intoxicated with tabun and treated: 3, HI-6; 4, obidoxime; 5, K048. Magnification: ×40. Staining: AChE. Bottom: Quantitative evaluation of histochemical data: density curves of microphotographs.
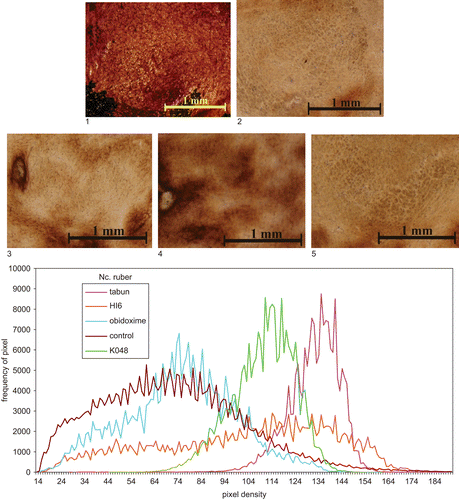
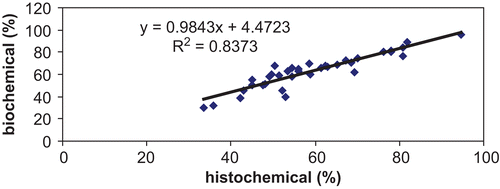
Correlation between results obtained using histochemical and biochemical methods
The comparison of percentage residual AChE activities after the administration of tabun, atropine, and reactivator in seven brain regions () shows a good correlation between results obtained by histochemical and biochemical methods of AChE detection.
Discussion
The main cholinergic pathways in the rat brain are represented by different structures, i.e. the septal nuclei, thalamus, cortex, and hippocampusCitation21. AChE activity in these structures is very different, varying from high to low activityCitation20,Citation22. It appears from our results that AChE activity in these structures is influenced differently following tabun intoxication and its treatment. Non-uniform AChE inhibition in different brain structures following untreated intoxication with nerve agents in rats and guinea-pigsCitation3,Citation20,Citation21,Citation23,24 has been demonstrated.
K048 was developed with the aim of obtaining a universal reactivator, but later on, it was tested as a prospective reactivator for tabun and OP pesticide poisoningCitation11–13; it showed better antidotal efficacy than that of HI-6, and AChE reactivation in the brain in vivo (whole brain homogenate) was higher in comparison with HI-6Citation13. Simultaneously, the antidotal efficiency of K048 against tabun poisoning was comparable with trimedoximeCitation9. Prospective antidotal efficacy of K048 against paraoxonCitation25 and methyl-paraoxonCitation26 was also shown. However, its potency to reactivate AChE in the brain inhibited by tabun in vivo as demonstrated in our results was not so unambiguous. In their excellent review, Lorke et al.Citation27 demonstrated that the entry of oximes into the brain is a currently hotly debated question, and that only part of the oximes present in the bloodstream are able to penetrate the blood–brain barrier.
Our results showed that different AChE reactivations could be observed in various parts of the brain following treated tabun poisoning; K048 was more effective than HI-6. However, when comparing reactivation effects in the brain parts, obidoxime was the most effective. When the reactivation efficacy in vivo is tested using whole brain homogenate as an enzyme source, the results obtained may be a mix of activities of the structures involved.
AChE reactivation in some structures can be functionally more important than that in other areas. These areas can be identified as the frontal cortex, pontomedullar area, and some parts of the limbic system. The importance of AChE inhibition/reactivation in these structures seems to shed more light on the pathogenesis of OP/nerve agent intoxication as well as contributing to the understanding of neurotoxicology in general.
Acknowledgements
The authors are indebted to Mrs J. Ellingerova and J. Uhlirova for skillful technical assistance. The financial support of the Ministry of Defense (grant No FVZ 0000501) and Grant Agency of Charles University (grant No GA UK 82/2005) is gratefully acknowledged.
Declaration of interest: The authors report no conflict of interest.
References
- Bajgar J. Organophosphates/nerve agent poisoning: mechanism of action, diagnosis, prophylaxis and treatment. Adv Clin Chem 2004;38:151–216.
- Cabal J, Bajgar J. Tabun – reappearance 50 years later. Chem Listy 1999;93:27–31. [in Czech]
- Fusek J, Bajgar J, Kassa J, Hajek P, Slizova D, Krs O. Differences in the action of G- and V- agents. In: Bajgar J, ed. Central and Peripheral Nervous System: Effects of Highly Toxic Organophosphates and their Antidotes. Kerala, India: Research Signpost, 2009, in press.
- Kuca K, Musilek K, Jun D, Bajgar J, Kassa J. Novel oximes. In: Gupta RC, ed. Handbook of Toxicology of Chemical Warfare Agents. Oxford: Elsevier, 2009, 997–1021.
- Eyer P. The role of oximes in the management of organophosphorus pesticides poisoning. Toxicol Rev 2003;22:165–90.
- Kassa J, Kuca K, Karasova J, Musilek K, Jun D, Bajgar J, et al. Development of new reactivators of tabun inhibited acetylcholinesterase and the evaluation of their efficacy by in vitro and in vivo methods. Presented at: HFM-149 Symposium on “Defense Against the Effects of Chemical Toxic Hazards: Toxicology, Diagnosis and Medical Countermeasures,” Edinburgh, Scotland, 8–10 October 2007. Session 4, OP Medical Countermeasures, No 17.
- Marrs TC, Rice P, Vale JA. The role of oximes in the treatment of nerve agent poisoning in civilian casualties. Toxicol Rev 2006;25:297–323.
- Shih T-M, Skovira JW, O’Donnell JC, McDonough JH. Central cholinesterase reactivation by oximes improves survival and terminates seizures following nerve agent intoxication. Presented at: HFM-149 Symposium on “Defense Against the Effects of Chemical Toxic Hazards: Toxicology, Diagnosis and Medical Countermeasures,” Edinburgh, Scotland, 8–10 October 2007. Session 4, OP Medical Countermeasures, No 18.
- Berend S, Lucic-Vrdoljak A, Radic B, Kuca K. New bispyridinium oximes: in vitro and in vivo evaluation of their biological efficiency in soman and tabun poisoning. Chem Biol Interact 2008;175:413–16.
- Pohanka M, Jun D, Kuca K. In vitro reactivation of trichlorfon-inhibited butyrylcholinesterase using obidoxime, pralidoxime and K048. J Enzyme Inhib Med Chem 2009;24:680–3.
- Kuca K, Cabal J, Jun D, Bajgar J, Hrabinova M. Potency of new structurally different oximes to reactivate cyclosarin-inhibited human brain acetylcholinesterase. J Enzyme Inhib Med Chem 2006;21:663–6.
- Musilek K, Holas O, Kuca K, Jun D, Dohnal V, Opletalova V, et al. Synthesis of monooxime-monocarbamoyl bispyridinium compounds bearing (E)-but-2-ene linker and evaluation of their reactivation activity against tabun- and paraoxon-inhibited acetylcholinesterase. J Enzyme Inhib Med Chem 2008;23:70–6.
- Kassa J, Kuca K, Cabal J, Paar M. A comparison of the efficacy of new asymmetric bispyridinium oximes (K027, K048) with currently available oximes against tabun by in vivo methods. J Toxicol Environ Health A 2006;69:1875–82.
- Kuca K, Kassa J. A comparison of the ability of a new bispyridinium oxime – 1-(4-hydroxyiminomethylpyridinium)-4-(carbamoylpyridinium)butane dibromide and currently used oximes to reactivate nerve agent-inhibited rat brain acetylcholinesterase by in vitro methods. J Enzyme Inhib Med Chem. 2003;18:529–35.
- Paxinos G, Watson C. The Rat Brain in Stereotactic Coordinates. New York: Academic Press, 1987.
- Lojda Z, Gossrau RR, Schiebler TH. Enzyme Histochemistry – A Laboratory Manual. New York: Springer Verlag, 1979.
- Hammond PI, Jelacic T, Padilla S, Brimijoin S. Quantitative, video-based histochemistry to measure regional effects of anticholinesterase pesticides in rat brain. Anal Biochem 1996;241:82–92.
- Benali A, Leefken I, Eysel U, Weile E. A computerized image analysis system for quantitative analysis of cells in histological brain sections. J Neurosci Methods 2003;125:33–43.
- Ellman GL, Courtney DK, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961;7:88–95.
- Bajgar J, Hajek P, Slizova D, Krs O, Fusek J, Kuca K, et al. Changes of acetylcholinesterase activity in different brain areas following intoxication with nerve agents: biochemical and histochemical study. Chem Biol Interact 2007;165:14–21.
- Giacobini G. Cholinesterases in human brain: the effect of cholinesterase inhibitors on Alzheimer’s disease and related disorders. In: Giacobini E,Pepeu G, eds. The Brain Cholinergic System in Health and Disease. Abingdon: Informa Healthcare, 2006:235–64.
- Gupta RC. Brain regional heterogeneity and toxicological mechanisms of organophosphates and carbamates. Toxicol Mech Methods 2004;14:103–43.
- Gupta RC, Patterson GT, Dettbarn W-D. Biochemical and histochemical alterations following acute soman intoxication in the rat. Toxicol Appl Pharmacol 1987;87:393–402.
- Shih T-M, Kan RK, McDonough JH. In vivo cholinesterase inhibitory specificity of organophosphorus nerve agents. Chem Biol Interact 2005;157–8:293–303.
- Petroianu GA, Nurulain SM, Nagelkerke N, Al-Sultan MAH, Kuca K, Kassa J. Five oximes (K-27, K-33, K-48, BI-6 and methoxime) in comparison with pralidoxime: survival in rats exposed to the organophosphate paraoxon. J Appl Toxicol 2006;26:262–8.
- Petroianu GA, Nurulain SM, Nagelkerke N, Shafiullah M, Kassa J, Kuca K. Five oximes (K-27, K-48, obidoxime, HI-6 and trimedoxime) in comparison with pralidoxime: survival in rats exposed to methyl-paraoxon. J Appl Toxicol 2007;27:453–7.
- Lorke DE, Kalasz H, Petroianu GA, Tekes K. Entry of oximes into the brain: a review. Curr Med Chem 2008;15:743–53.