Abstract
Inhibitory effects of some drugs on glucose 6-phosphate dehydrogenase from the erythrocytes of human have been investigated. For this purpose, at the beginning, erythrocyte glucose 6-phosphate dehydrogenase was purified 2256 times in a yield of 44.22% by using ammonium sulphate precipitation and 2’, 5’-ADP Sepharose 4B affinity gel. Temperature of +4°C was maintained during the purification process. Enzyme activity was determined with the Beutler method by using a spectrophotometer at 340 nm. This method was utilized for all kinetic studies. Ketotifen, dacarbazine, thiocolchicoside, meloxicam, methotrexate, furosemide, olanzapine, methylprednizolone acetate, paricalcitol, ritodrine hydrochloride, and gadobenate-dimeglumine were used as drugs. All the drugs indicated the inhibitory effects on the enzyme. Ki constants for glucose 6-phosphate dehydrogenase were found by means of Lineweaver-Burk graphs. While methylprednizolone acetate showed competitive inhibition, the others displayed non-competitive inhibition.
In addition, IC50 values of the drugs were determined by plotting Activity% vs [I].
Introduction
Glucose 6-phosphate dehydrogenase (D-glucose-6-phosphate: NADP+ oxidoreductase EC 1.1.1.49; G6PD) is the key enzyme of the pentose phosphate metabolic pathway and it is widespread in all tissues and blood cells. The pentose phosphate shunt occurs widely in living cells where one of its main functions is to provide the NADPH necessary for the synthesis of fatty acids and other specific reductions. The enzymes, glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase, produce the necessary NADPH. G6PD reaction is an important site of metabolic control. This view is supported by several investigators who have shown variations in enzyme activity as a function of hormone and nutritional levels, enzyme quaternary structure and various metabolites including ATP, ADP, spermidine and palmitoyl-CoA [Citation1,Citation2]. Deficiency of the enzyme in red blood cells causes haemolytic anaemia. Deficiency of glucose 6-phosphate dehydrogenase is one of the most common genetic abnormalities, affecting more than 150 million males. It has a polymorphic frequency that is second after to the haemoglobinopathies, 400 variants having been described [Citation3]. Many antibiotics are being used in therapies. There is few literature reports related with changing of enzyme activities. It has been reported that some increase and decline were found on human liver enzyme activity levels such as aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase [Citation4-Citation7].
Since the effects of some drugs have not been analyzed on glucose 6-phosphate dehydrogenase, in the present study, the in vitro effect of ketotifen, dacarbazine, thiocolchicoside, meloxicam, methotrexate, furosemide, olanzapine, methylprednizolone acetate, paricalcitol, ritodrine hydrochloride, and gadobenate-dimeglumine on G6PD purified from human erythrocytes was investigated. By using the obtained Ki and IC50 values, undesirable side-effects can be diminished on G6PD activity and body metabolism in therapy.
Materials and methods
Materials
2’, 5’ ADP-Sepharose 4B was obtained from Pharmacia. NADP+, glucose-6-phosphate, protein assay reagents, chemicals for electrophoresis and all other chemicals used were obtained from either Sigma or Sigma-Aldrich Co. (Germany) and the drugs were purchased from Hoechst Marian Roussel (Turkey).
Preparation of the hemolysate
Fresh human blood (age: 34, male) collected in EDTA was centrifuged (15 min, 2500xg). The red cells were isolated and washed three times with 0.16 M KCl, and haemolysed with five volumes of ice-cold water, and then centrifuged at 4°C, 10,000xg for 20 min to remove the ghosts and intact cells [Citation8-Citation10].
Ammonium sulphate fractionation and dialysis
Hemolysate was brought among 35-65% (NH4)2SO4 saturation with solid (NH4)2SO4. The precipitate was separated by centrifugation at 5000xg for 15 min and dissolved in a small amount of 50 mM phosphate buffer_pH 7.0., and then dialysed at 4°C in 50 mM K-acetate / 50 mM K-phosphate buffer (pH 7.0) for 2 h with two changes of buffer [Citation8].
Purification of G6PD by affinity chromatography
Dry 2’, 5’ ADP-Sepharose 4B was resuspended in 0.1 M K-acetate + 0.1 M K-phosphate buffer (pH 6.0), then used to pack a small column (1x10 cm) which was equilibrated in the same buffer. The dialysed enzyme solution obtained above was loaded on the column. The gel was then sequentially washed with 25 ml of 0.1 M K-acetate + 0.1 M K-phosphate (pH 6.0), with 25 ml of 0.1 M K-acetate + 0.1 M K-phosphate buffer (pH 7.85), and finally with 25 ml of 0.1 M KCl + 0.1 M K-phosphate buffer (pH 7.85). Elution was carried out with 80 mM K-phosphate 80 mM KCl + 0.5 mM NADP+ + 10 mM EDTA (pH 7.85) solution. The flow rates of the washings and eluting steps were 50 ml h−1 and 20 ml h−1, respectively. In elutes of 2-ml volume, activities of G6PD were determined in all fractions. It was not performed protein determination at 280 nm in elutes, since the NADP+ absorbance masked the actual protein absorbance. Active fractions were collected. All procedures were performed at 4°C [Citation8, Citation11].
Measurement of G6PD activity
G6PD was measured spectrophotometrically at 25°C as described by Beutler. Briefly, the enzyme sample was added to the 1 ml of (final volume) incubation mixture containing 0.1 M Tris-HCl + 0.5mM EDTA (pH 8.0), 10 mM MgCl2, 0.2 mM NADP+ and 0.6 mM glucose-6 phosphate. The activity measurement was done by monitoring the increase in absorption at 340 nm due to the reduction of NADP+ at 25°C. One enzyme unit represents the reduction of 1 µmol of NADP+ min−1 at 25°C, pH 8.0 [Citation8, Citation12].
Protein determination
Quantitative protein determination was done by absorbance measurement at 595 nm according to Bradford, with bovine serum albumin as a standard [Citation13].
SDS polyacrylamide gel electrophoresis
SDS polyacrylamide gel electrophoresis was done after the purification of the enzyme. It was carried out in 10% and 4% acrylamide concentrations for the running and the stacking gel, respectively, containing 0.1% SDS according to Laemmli [Citation14]. To the sample and standard 20 mg bovine serum albumin was applied to the electrophoresis medium. Gels were stained overnight in 0.1% Coomassie Brillant Blue R-250 in 50% methanol and 10% acetic acid, then destained with many changes of the same solvent without dye.
In vitro inhibitor studies
Ketotifen, dacarbazine, thiocolchicoside, meloxicam, methotrexate, furosemide, olanzapine, methylprednizolone acetate, paricalcitol, ritodrine hydrochloride, and gadobenate-dimeglumine were used as inhibitors. In the media with inhibitor or without inhibitor, the substrate concentrations were 0.012 mM, 0.030 mM, 0.060 mM, 0.120 mM, and 0.450 mM. Inhibitor solutions were added to the reaction medium, resulting in three different fixed concentrations of inhibitors in 1 ml total reaction volume. To draw Lineweaver-Burk graphs by using 1/V vs 1/[S] values, regression analysis was carried out and equations obtained from regression analysis were used to draw graphs for each fixed inhibitor concentration. Ki values were calculated from these Lineweaver-Burk graphs. In order to determinate IC50 values, inhibition percent values were obtained from five different inhibitors concentrations with 0.6 mM constant substrate concentration.
Statistical analysis
Data were presented as means ± SD. Three parallel measurements were analyzed by Student’s t-test. Means were compared by Kruskal-Wallis one way analysis of variance. Drug concentrations which produce 50% inhibition (IC50) were calculated from activity (%)-drug concentration curves.
Results
Human G6PD was purified by 2’, 5’ ADP-Sepharose 4B gel affinity column. As shown in , specific activity was calculated for the hemolysate and purified enzyme solution as 14.44 EU/mg protein, a yield of 44.22% and a purification coefficient of 2256-fold. Purification steps were controlled by SDS-PAGE. As shown , and , the obtained IC50 values for ketotifen, dacarbazine, thiocolchicoside, meloxicam, methotrexate, furosemide, olanzapine, methylprednizolone acetate, paricalcitol, ritodrine hydrochloride, and gadobenate-dimeglumine were 0.008, 0.021, 0.056, 0.067, 0.114, 0.315, 0.53, 2.23, 2.28, 8.11, and 115 mM, respectively. Ki values (Tables II) were calculated from Lineweaver-Burk graphs. Ki constants for the drugs were 0.0052 ± 0.0012, 0.0154 ± 0.0011, 0.0397 ± 0.0063, 0.0579 ± 0.0061, 0.0910 ± 0.0166, 0.1874 ± 0.0136, 0.4972 ± 0.0710, 0.5405 ± 0.1528, 4.2867 ± 1.0670, 8.2081 ± 1.3522, and 48.4380 ± 2.176 mM respectively. Inhibition types were found as non competitive for the drugs, except methylprednizolone acetate (competitive). Representative graphs are shown for ketotifen ( and ).
Figure 1. Activity % vs [Ketotifen] regression analysis graphs for human erythrocytes G6PD in the presence of 5 different ketotifen concentrations.
![Figure 1. Activity % vs [Ketotifen] regression analysis graphs for human erythrocytes G6PD in the presence of 5 different ketotifen concentrations.](/cms/asset/65a4ba00-2e16-417a-8aa9-f375864dd4cb/ienz_a_449374_f0001_b.gif)
Figure 2. Lineweaver-Burk graph for 5 different substrate (G6P) concentrations and 3 different ketotifen concentrations for determination of Ki.
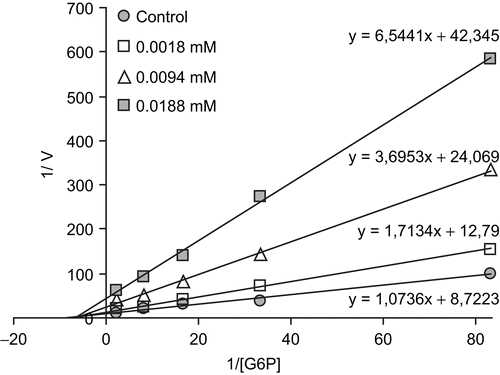
Table I. Purification scheme of G6PD dehydrogenase from human erythrocyte.
Table II. IC50 values and Ki constants obtained from inhibition percent values vs five different inhibitors concentrations and Lineweaver–Burk graphs respectively in the presence of three fixed inhibitors and five substrate concentrations for different drugs.
Discussion
Currently, there are many patients with G6PD deficiency disorder in some regions of Turkey and world-wide G6PD deficiency is frequently seen in African, Mediterranean, Middle Eastern and Far Eastern nations and their lineages with a frequency ranging from 5%–40% [Citation11,Citation15]. Pamaquine used in malaria therapy resulted in some severe side-effects in some patients [Citation16] i.e. dark colored urine, jaundice and anemia. Later, G6PD deficiency was found in these individuals. Use of this and some other drugs cause hemolysis and are connected with complication. The importance of G6PD in metabolism has been well known for many years. GSH is used as an antioxidant defense mechanism and its production requires NADPH to be synthesized in the pentose phosphate metabolic pathway in which G6PD and 6PGD participate [Citation17]. For this reason, G6PD and 6PGD have been considered as antioxidant enzymes [Citation18]. Inhibitory effects of many drugs on G6PD enzyme activity in different animal species and human beings have been reported in many investigations [Citation19]. For example, it has been reported that thiamphenicol, amikacin, gentamicin, netilmicin, chloramine-T and CuSO4 inhibit rainbow trout erythrocyte G6PD [Citation20]. Effects of many drugs such as antibiotics, analgesic and anesthetic have been investigated on human G6PD [Citation21, Citation22], sheep erythrocyte G6PD [Citation23] and sheep liver G6PD [Citation24]. Several study reports concerning strong inhibitory effects of antibiotic and anesthetics on erythrocytes G6PD enzyme have been published [Citation21,Citation22]. Some anesthetics have been reported to inhibit Ca+2-ATP as and Na+/Ca+2 exchanger of the synaptosomal plasma membrane [Citation25]. The an other study, anesthetics inhibited Na+,K+-ATPase, Mg+2-ATPase and acetylcholinesterase activity from rat cerebral cortex [Citation25].
However, to the best of our knowledge, the inhibitory effects of the drugs examined here on G6PD in human erythrocyte G6PD have not been studied. In order to show inhibitory effects, while the most suitable parameter is the Ki constant, some researchers use the IC50 value [Citation26,Citation27]. Therefore, in this study, both the Ki and IC50 parameters of these drugs for G6PD were determined.
In the present study, an investigation of the effects of some selected drugs on human erythrocyte G6PD was proposed. For this purpose, G6PD was purified 2256-fold from human erythrocytes in 44.22 yields by ammonium sulfate precipitation and then 2’, 5’-ADP Sepharose 4B affinity chromatography. IC50 values of ketotifen, dacarbazine, thiocolchicoside, meloxicam, methotrexate, furosemide, olanzapine, methylprednizolone acetate, paricalcitol, ritodrine hydrochloride, and gadobenate-dimeglumine were 0.008, 0.021, 0.056, 0.067, 0.114, 0.315, 0.53, 2.23, 2.28, 8.11, and 115 mM, respectively. Ki constants of ketotifen, dacarbazine, thiocolchicoside, meloxicam, methotrexate, furosemide, olanzapine, methylprednizolone acetate, paricalcitol, ritodrine hydrochloride, and gadobenate-dimeglumine were 0.0052 ± 0.0012 (non-competitive), 0.0154 ± 0.0011(non-competitive), 0.0397 ± 0.0063 (non-competitive), 0.0579 ± 0.0061(non-competitive), 0.0910 ± 0.0166(non-competitive), 0.1874 ± 0.0136 (non-competitive), 0.4972 ± 0.0710 (non-competitive), 0.5405 ± 0.1528 (competitive), 4.2867 ± 1.0670 (non-competitive), 8.2081 ± 1.3522 (non-competitive), and 48.4380 ± 2.176 mM (non-competitive), respectively (Table II). In this study, the drugs inhibited G6PD activity compared with the control group. The drugs can cause non-competitive inhibition by binding to other sites affecting the three dimensional structure of the enzyme except methylprednizolone acetate. Methylprednizolone acetate causes competitive inhibition by binding at the active site of G6PD [Citation1].
Ki values show that ketotifen had the highest inhibitory effect, followed by dacarbazine, thiocolchicoside, meloxicam, methotrexate, furosemide, olanzapine, methylprednizolone acetate, paricalcitol, ritodrine hydrochloride, and gadobenate-dimeglumine, respectively. IC50 values showed the same trend.
Table II shows that the enzyme is mostly inhibited by ketotifen, dacarbazine, thiocolchicoside, meloxicam, methotrexate, furosemide and olanzapine drugs. The chemical structures of all these drugs contain an active group of carbonyl, sulfur and nitrogen, only dacarbazin does not contain a sulfur group. It is quite remarkable that those drugs like methylprednizolone acetate, paricalcitol, ritodrine hydrochloride and gadobenate-dimeglumine which have a less inhibitory effect on the enzyme’s activity, generally contain methyl and hydroxyl groups, but do not contain sulfur and carbonyl groups.
In this investigation, by using the obtained Ki and IC50 values, undesirable side effects of these drugs on G6PD activity and body metabolism and fatty acid synthesis can be reduced. The dosage of the examined dacarbazine (iv) used clinically give blood drug concentrations as 0.2 mM [Citation28]. By taking into account this concentration, the inhibition data calculated from plot was found to be 98%. According to this data, if it is required to give dacarbazine to patients, its dosage should be very well controlled to decrease hemolytic and other side effects due to possible inhibition of G6PD.
Declaration of interest
This research was financed by grants from of the Scientific and Technological Research Council of Turkey (TÜBİTAK) (Project No: 108T213).
References
- Lehninger AL, Nelson DL, Cox MM. Principles of biochemistry. 2nd edn. 2000. p 558–560.
- Kanji MI, Toews ML, Carpar WR. A kinetic study of glucose-6-phosphate dehydrogenase. J Biol Chem 1976; 25: 2258.
- Yuregir GT, Aksoy K, Arpaci A, Unlukurt I, Tuli A. Studies on red cell glucose-6-phosphate dehydrogenase, evaluation of reference values. Ann Clin Biochem 1994;31:50–5.
- Honjo T, Watanabe A. Clinical experience on sulbactamrcefoperazone in the pediatric field. Jpn J Antibiot 1984;37: 32–36.
- Pickering LK, O’Connor DM, Anderson D, Bairan AC, Feigin RD, Cherry JD. Clinical and pharmacologic evaluation of cefazolin in children. J Infect Dis 1973;128: 407–414.
- Turck M, Clark RA, Beaty HN, Holmes KK, Karney WW, Reller LB. Cefazolin in the treatment of bacterial pneumonia. J Infect Dis 1973;128:382–385.
- Singhvi SM, Heald AF, Gadebusch HH, Resnick ME, Difazio LT, Leitz MA. Human serum protein binding of cephalosporin antibiotic in vitro. J Lab Clin Med 1977;89:414–420.
- Ninfali P, Orsenigo T, Barociani L, Rapa S. Rapid purification of glucose-6-phosphate dehydrogenase from mammal’s erythrocytes. Prep Biochem 1990;20:297–309.
- Delgado C, Tejedor C, Luquue J. Partial purification of glucose-6-phosphate dehydrogenase and phosphofructokinase from rat erythrocyte haemolysate by partitioning in aqueous two-phase systems. J Chromatogr 1990;498:159–168.
- Shreve DS, Levy HR. On the molecular weight of human glucose-6-phosphate dehydrogenase. Biochem Biophy Res Com 1977;78:1369–1375.
- Morelli A, Benatti U, Gaetani GF, De Flora A. Biochemical mechanisms of glucose-6 phosphate dehydrogenase deficiency. Proc Natl Acad Sci 1978; 75: 1979–1983.
- Beutler E. Red cell metabolism manual of biochemical methods. London: Academic Press, 1971:68-70.
- Bradford MM. A rapid and sensitive method for the quantition of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–251.
- Laemmli DK. Clevage of structual proteins during in assembly of the head of bacteriophage T4. Nature 1970;227:680–683.
- Laurence DR, Bennett PN, Brown MJ. Clinical Pharmacology. 8th ed. Singapore: Churchill Livingstone;1997.
- Keha EE, Kufrevioglu. Biyokimya, (Turkish) 2nd ed. Aktif Yayinevi: Istanbul; 2000. p 338–349.
- Gaetani GF, Galiano S, Canepa L, Ferraris AM, Kirkman HN. Catalase and glutathione peroxidase are equally active indetoxification of hydrogen peroxide in human erythrocytes. Blood 1989;73(1):334–339.
- Deutsch J. Glucose-6-phosphate dehydrogenase. In: Bergmeyer J, Bergmeyer HU, editors. Methods of enzymatic analysis., 3 VCH: Verlagsgerellschaff; 1983. p 190–196.
- Ciftci M, Kufrevioglu OI, Gundogdu M, Ozmen I. Effects of some antibiotics on enzyme activity of glucose-6-phosphate dehydrogenase from human erythrocytes. Pharmacol Res 2000;41:109–113.
- Erdogan O, Ciftci M, Ciltas A, Hisar O. Inhibition Effects of some antibiotics on the activity of glucose 6-phosphate dehydrogenase enzyme from rainbow trout (oncorhynchus mykiss walbaum, 1792) Erythrocytes. Turk J Vet Anim Sci 2004;28:675–681.
- Altikat S, Ciftci M, Buyukokuroglu ME. In vitro effects of some anesthetic drugs on enzymatic activity of human red blood cell glucose 6-phosphate dehydrogenase. Polish J Pharmacol 2002;54:67–71.
- Ozdemir H, Ciftci M. In vitro effects of some drugs on human red blood cell glucose-6-phosphate dehydrogenase enzyme activity. J Enz Inhib Med Chem 2006;21(1):75–80.
- Beydemir S, Ciftci M, Kufrevioglu OI. Purification and characterization of glucose 6- phosphate dehydrogenase from sheep erythrocytes, and inhibitory effects of some antibiotics on enzyme activity. J Enz Inhib Med Chem 2002;17(4):271–277.
- Turkoglu V, Aldemir S, Ciftci M. Purification and characterization of glucose-6 phosphate dehydrogenase from sheep liver. Turk J Chem 2003;27:395–402.
- Sidek HM, Nayquist-Battie C, Vanderkooi G. Biochim Biophys Acta 1984;801:26–33.
- Ciftci M, Beydemir S, Yılmaz H, Bakan E. Effects of some drugs on rat erythrocyte 6- phosphogluconate dehydrogenase: An in vitro and in vivo study. J Pharmacol 2002;54:275–280.
- Akyuz M, Erat M, Ciftci M, Gumustekin K, Bakan N. Effects of some antibiotics on human erythrocyte 6-phosphogluconate dehydrogenase: An in vitro and in vivo study. J Enzyme Inhibit Med Chem 2004;(19):361–365.
- Kayaalp SO: Rasyonal tedavi yonunden tibbi farmakoloji. Ankara, Hacettepe-Tas 2002. Yayincilik (Turkish).