Abstract
In this study the potent scavenging activity of “Lycopi Herba” (LH) extract was studied using the following: evaluation of the total phenolics, measuring the antioxidant activity by Trolox equivalent antioxidant concentration, measuring the scavenging effects on reactive oxygen species, on reactive nitrogen species, and measuring the inhibitory effect on Cu2+ induced human low-density lipoprotein oxidation in vitro. The ethyl acetate fraction from the LH extracts were found to have a potent scavenging activity against all of the reactive species tested, as well as an inhibitory effect on LDL oxidation. Therefore, we isolated and identified luteolin-7-O-β-D-glucuronide methyl ester as the major compound from the ethyl acetate fraction of LH and their antioxidant activities were evaluated.
Introduction
Oxidative stress occurs when intracellular antioxidant mechanisms are overwhelmed by reactive oxygen species (ROS) or reactive nitrogen species (RNS). It is considered to be an important pathogenic factor in degenerative diseases such as cardiovascular dysfunctions, atherosclerosis, inflammation, carcinogenesis, drug toxicity, reperfusion injury and neurodegenerative diseases [Citation1]. Ongoing research indicates that the abundance of ROS or RNS in vasculature results in an increased oxidation of proteins that induces oxidized low-density lipoprotein (Ox-LDL). This initiates an inflammatory process which causes damage to arterial walls [Citation2] and Ox-LDL is a primary constituent of atherosclerotic lesions. If natural antioxidant nutrients in herbs have the ability to inhibit the formation of Ox-LDL, they could be used to prevent and treat atherosclerosis or other cardiovascular diseases (CVD).
The antioxidant activity of plants is significantly influenced by their qualitative and quantitative composition, which can be reversible depending on the method of evaluation and whether the results show positive or negative correlation. In recent years, different methods have been proposed for evaluation of the antioxidant capacity of plants. The methods used are techniques based on using either biological oxidants (superoxide anion, hydroxyl radical, nitric oxide radical, and peroxynitrite) or on non-biological oxidants (scavenging of 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulphonate). Radical cations (Trolox equivalent antioxidant concentration (TEAC) assay), scavenging of 2,2-diphenyl-1-picrylhydrazyl radical (DPPH assay) and electrochemical total reducing capacity) are also used. Each method has advantages and shortcomings. [Citation3].
Lycopi Herba (LH) is a traditional herbal medicine that has been used to reduce fever, exclusion of a thrombus, menstrual irregularity, as a detoxicant and a diuretic. It has been reported that extracts of LH have pharmacological activities such as being anti-allergic [Citation4], anti-inflammatory [Citation5] and has inhibitory effects on platelet aggregation and thrombus formation [Citation6]. To date no studies concerning the antioxidant properties of LH have been reported. Therefore, this study was conducted to assess the in vitro scavenging activity and inhibitory effect of LDL oxidation of pro-oxidant reactive species in response to treatment with LH and luteolin-7-O-β-D-glucuronide methyl ester (LGME) using various screening methods including biological and non-biological oxidants.
Materials and methods
Chemicals and instruments
Ascorbic acid (AA), butylated hydroxytoluene (BHT) were purchased from Sigma Chemical, St. Louis, MO. Thin layer chromatography (TLC) was performed on pre-coated silica gel G and GP Uniplates from Analtech (IL, USA) and visualized with 254–nm UV light. Vacuum liquid chromatography was carried out on silica gel 60 (Scientific Adsorbents, MO, USA). All other chemicals used were of analytical grade. 1H NMR and 13C NMR spectra were recorded on a Bruker DPX 400 at 400 MHz and 100 MHz (Germany). The chemical shifts are reported in parts per million (ppm) downfield from tetramethylsilane, and J–values are in Hz. Mass spectra were recorded with a Waters Micromass ZQ LC–Mass system was measured with a Bruker BioApex FTMS system by direct injection using an electrospray interface (ESI) (Munich, Germany).
Preparation of 70% ethanol extract, solvent fractionation and LGME isolation
The heartwood of LH (500 g, purchased from Dongguk University Gyeongju Oriental Hospital, Gyeongju, Gyeongbuk) was ground (max particle size 0.4 mm) and refluxed three times (12 h, 6 h, 3 h) with 70% ethanol (ethanol: water, 70:30, E) solution (20-fold) and then filtered through a glass filter funnel (G4). The extract was gathered and the ethanol was evaporated under reduced pressure at 45°C in a rotary vacuum evaporator (Buchi®II, Buchi, Switzerland), followed by lyophilisation. The dried extract was then suspended in 50 mL distilled water and the aqueous suspension was partitioned sequentially with hexane (H), dichloromethane (DCM), ethyl acetate (EA), n-butanol (B) and aqueous (A) in a 1:1 ratio (V/V) at room temperature. The resulting extracts were evaporated under a rotary vacuum evaporator to give H, DCM, EA, B and A fractions. They were then quantitatively re-dissolved in 30% ethanol solution. The stock solutions were kept at 4°C in the dark until further analysis. Prior to analysis, the solution was filtered through a 1 μm syringe filter. Vacuum liquid chromatography (150 g, 6 × 30 cm) of the EA fractions (5 g), using n-hexane:CH2Cl2 (1:0–0:1) and CH2Cl2:MeOH (1:0–0:1) step gradients, produced 11 fractions with the yellow colour fraction. These were pooled by TLC profile into four fractions (LH-EA-1~LH-EA-4), in which fraction LH-EA-3 (1.4 g) eluted with petroleum ether-acetone (1:0 (500 mL), 2:1 (500 mL), 1:1 (500 mL), 1:2 (500 mL), 1:5 (500 mL), and 0:1 (500 mL)). The major compound was purified by preparative high-performance liquid chromatography (Econosil C-18, 10 × 250 mm; 1 mL/min; MO. USA) with MeCN:MeOH (3:1) to afford compound 1 (532 mg). The compound was then quantitatively re-dissolved in 1% DMSO as stock solution. luteolin-7-O-β-D-glucuronide methyl ester (LGME): A yellow amorphous powder, FAB-MS: m/z 477 [M+H]+; 1H-NMR (DMSO-d6, 500 MHz) δ:13.0 (1H, s, OH-5), 7.51 (1H, dd, J = 8.5, 2 Hz, H-6’), 7.48 (1H, d, J = 2 Hz, H-2’), 6.96 (1H, d, J = 8.5 Hz, H-5”), 6.88 (1H, d, J = 2 Hz,H-8), 6.81 (1H, s, H-3), 5.39 (1H, d, J = 7.5 Hz, H-l”), 4.27 (1H, d, J = 9.5 Hz, H-5”), 3.73 (s, OCH3), 3.3-3.6 (3H, m, Glu H-2”, 3”, 4”); 13C-NMR (DMSO-d6, 125 MHz) δ:181.86 (C-4), 169.19 (Glu-6”), 164.54 (C-2), 162.4 (C-7), 161.19 (C-5), 156.95 (C-9), 150.14 (C-4’), 145.83 (C-3’), 121.19 (C-1’), 119.19 (C-6’), 115.97 (C-5’), 113.5 (C-2’), 105.47 (C-10), 103.12 (C-3), 99.31 (C-6), 99.05 (Glu-l”), 94.5 (C-8), 75.37 (Glu-3”), 75.14 (Glu-5”),72.72 (Glu-2”), 71.3 (Glu-4”), 51.98 (OCH3).
Determination of total phenolics
The content of the total phenolic compounds was determined by the Folin-Ciocalteu’s reaction [Citation7], using gallic acid as a standard. A 40 μL aliquot of LH extract (1 mg/mL) was mixed with 200 μL of Folin-Ciocalteu’s reagent and 1160 μL of distilled water. The mixture was allowed to stand for 3 min at room temperature, after which 600 μL of 20% sodium carbonate was added. After shaking for 2 h at room temperature, the absorbance was measured at 765 nm using a microplate reader (VERSAmax, Molecular Device, MO, USA). The concentration of total phenolic compounds was expressed as gallic acid equivalents (μg of GA eq/mg).
Antioxidant activity as determined by the ABTS·+ assay
The total antioxidant activity of LH extracts was measured by the ABTS+ radical cation (ABTS·+) decolourization assay [Citation8]. ABTS was dissolved in water to a 7 mM concentration to form a stock solution. ABTS·+ was then produced by reacting the ABTS stock solution with 2.45 mM potassium persulphate (final concentration) and allowing the mixture to stand in the dark, at room temperature for 12 h prior to use. Oxidation of the ABTS commenced immediately, however, the absorbance was not maximal or stable until more than 6 h had elapsed. The radical cation was stable in this form for more than 2 days of storage in the dark at room temperature. Prior to assay, the solution was diluted in ethanol to give an absorbance of 0.7 ± 0.02 at 734 nm using a UV/visible spectrophotometer (Ultraspec 6300 pro, Amersham)in a 1 cm cuvette after being equilibrated to 37°C, which was the temperature at which all assays were performed. The stock solution of the LH extracts, AA and BHT in ethanol were diluted such that, after introduction of a 10 μL aliquot of each dilution into the assay produced between 20% and 80% inhibition of the absorbance of the blank. After the addition of 1 mL of diluted ABTS·+ solution to 10 μL of antioxidant compounds or Trolox standards (final concentration 0–15 μM) prepared in ethanol, the sample was then incubated at 37°C for 30 min. Appropriate solvent blanks were also run in each assay. Triplicate determinations were made at each dilution of the standard, and the percentage of inhibition was calculated using the absorbance of the blank at 734 nm and plotted as a function of the Trolox concentration. The activity of the LH extracts, AA and BHT were estimated using a minimum of three different concentrations within the range of the dose–response curve, and the mean value was then derived as the Trolox equivalent antioxidant capacity value (TEAC). The unit of total antioxidant activity was defined as the concentration of Trolox having an equivalent antioxidant activity expressed as mM. AA and BHT were used as positive controls.
Free radical scavenging activity as determined by DPPH assay
DPPH radical scavenging activity of LH extract was determined by the method according to Gyamfi et al. [Citation9] with a slight modification. Briefly, different concentrations of CSL extract (50 μL) were mixed with 1 mL of 0.1 mM DPPH ethanol solution and 450 μL of 50 mM tris-HCl buffer (pH 7.4). The mixture was shaken and incubated for 30 min at room temperature. The absorbance was measured at 517 nm using a microplate reader (VERSAmax, Molecular Device) and DPPH radical scavenging activity was calculated as follows:
AA and BHT were used as positive controls.
Superoxide anion and hydroxyl radical scavenging activity
In this assay, when O2 is generated, NBT is reduced, which produces a blue formazan colour associated with an increase in the absorbance at 560 nm. When a scavenger compound is added, it competes with the NBT for oxidation of the generated superoxide anions, which leads to a decrease in the rate of the NBT reduction and therefore a reduction in absorbance. The more effective a compounds is at scavenging radicals, the lower the concentration would be required to inhibit the NBT reduction by 50% (IC50). The conditions of the NBT assay were adapted from Gotoh and Niki [Citation10]. The hydroxyl radical (·OH) scavenging activity of LH extracts and LGME were assessed using the method described by Halliwell and Gutteridge [Citation11]. AA and BHT were used as positive controls.
Nitric oxide radical and peroxynitrite scavenging activity
A 4,5-diaminofluorescein diacetate (DAF-2) assay [Citation12] was used to measure the nitric oxide radical (NO) scavenging ability. The peroxynitrite (ONOO−) scavenging activity of the LH extracts and LGME were determined using the method described by Kooy et al. [Citation13] with a slight modification. Briefly, 10 μL of LH extracts of different concentrations were mixed with 175.8 μL of rhodamine buffer (50 mM sodium phosphate dibasic, 50 mM sodium phosphate monobasic, 90 mM sodium chloride and 5 mM potassium chloride) containing 4 μL of 5 mM diethylene triamine pentacetic acid (DTPA) and 0.2 μL of 5 mM dihydrorhodamine (DHR) 123. The reaction was then initiated by adding 10 μL of 10 μM peroxynitrite. After ten min at room temperature, the fluorescent intensity of the mixture was monitored at excitation and emission wavelengths of 480 and 530 nm, respectively, using a fluorescence microplate reader (Molecular Device, Sunnyvale, CA). The scavenging effect of the extract was expressed as the percentage inhibition of DHR 123 oxidation. AA and BHT were used as positive controls.
Relative electrophoretic mobility (REM) assay
The REM of human LDL was determined by agarose gel electrophoresis according to the method described by Yoon et al. [Citation14]. AA was used as a positive control.
Inhibitory effects of CuSO4-induced human LDL oxidation
The inhibitory effects of LH extracts on CuSO4-induced human LDL oxidation were determined spectrophotometrically by measuring the amount of TBARS generated [Citation15]. AA and BHT were used as positive controls.
Statistical analysis
All experiments were performed at least three times by conducting each assay in triplicate. Data was analyzed by SPSS (version 14.0) and is expressed as the mean ± SE of triplicate measurements. Statistical analyses were conducted using analysis of variance (ANOVA–Tukey test) and a p level of 0.05 or less was considered significant.
Results and discussion
The extract yields ranged from 0.55 g/100 g LH (DCM extract) to 21.32g/500 g LH (extract E), and increased in the following order: E> A> H> B> EA> DCM (). The total phenol content of the extracts, as estimated by the Folin-Ciocalteu reagent method, ranged from 96.67 μg GA eq/mg (DCM fraction) to 576.83 μg GA eq/mg (EA fraction), and increased in the following order: EA> B> E> A> H> DCM fraction ().
Table 1. Extraction yields and contents of total phenolics in the extracts of Lycopi Herba.
shows the antioxidant capacities of LH extracts as determined by the TEAC assay. The extracts show high antioxidant capacities that range from 0.046 to 0.483 mmol Trolox equivalents. In addition, the difference in the antioxidant capacities of the various extracts was also very large, up to 10.5-fold. The EA fraction of the LH possessed the highest antioxidant capacity (0.483 mmol Trolox equivalent). So, TLC and preparative HPLC methods of the EA-soluble fraction of the EtOH extract of LH led to the isolation of one major compound (luteolin-7-O-β-D-glucuronide methyl ester (LGME)) ().
Table 2. Antioxidant activities of the extracts and 1 from Lycopi Herba as determined by the ABTS·+ and DPPH assays.
The spectral data for compound 1 was in good agreement with those previously reported in the literature [Citation16]. The antioxidant activities of LGME showed significant differences (p < 0.05) and their TEAC value was 1.990 mmol Trolox equivalent units. In addition, the antioxidant activities of AA and BHT, which were used as positive controls were 2.349 and 1.869 mmol Trolox equivalent units, respectively. AA is natural antioxidant and BHT is well known as a synthetic antioxidant. AA is relatively expensive antioxidant, while BHT is toxic to humans and therefore inappropriate for chronic human consumption. Therefore, screening for inexpensive, non-toxic antioxidants from a natural source is needed. Consequently, we evaluated objectively the antioxidant activities of LH by comparing with the available natural and synthetic antioxidants.
The free radical scavenging effects of LH extract on DPPH are shown in . Among the extracts examined, the EA fraction exhibited the strongest efficiency and showed over 50% scavenging effect of DPPH at a concentration of 129.78 ± 3.5 μg/mL. These values were similar to those obtained from the compounds that were tested, which was 123.58 ± 1.90 (AA). These values were superior to the positive control LGME which showed effective scavenging activities, with an IC50 value of 60.92 μg/mL. These results suggest that LH might contain reductones, which could react with free radicals to stabilize and terminate the radical chain reactions. The IC50 values of the superoxide anion scavenging activity of all of the test samples from LH are shown in . LH had a significant scavenging activity on the superoxide anion and this effect occurred in a dose-dependent manner. In addition, the superoxide anion-scavenging activity of LH extract was significantly different from that of AA (p > 0.05). The EA fraction exerted the strongest scavenging activity showing 1.7-fold, 2.4-fold, 2.9-fold, 3.6-fold and five-fold greater activity when compared with B, E, A, H, and DCM, respectively. In addition, AA and BHT showed no effect for scavenging on superoxide anion. These values were better than that of the positive control of LGME, which showed effective scavenging activities with an IC50 value of 937.61 ± 14.63 μg/mL and these data imply that LH has a high hydrogen-donating capacity. Since phenolic compounds present in LH are good electron donors, they may accelerate the conversion of H2O2–H2O. According to our results, LGME bearing the unsubstituted -OH group proved to be able to scavenge free stable DPPH radicals, though they showed less activity than the antioxidant standards used (AA and BHT). The scavenging activities of LH on hydroxyl radicals are shown in . LH showed a high enough scavenging activity to be considered a potent hydroxyl radical-scavenger. The IC50 values of the EA, H and DCM fraction of LH were 16.76 ± 0.47, 315.08 ± 3.48 and 368.51 ± 2.96 μg/mL, respectively. The hydroxyl radical activity of LGME, which was isolated from EA of LH, had an IC50 value of 99.40 μg/mL. The ability of the above mentioned extracts to quench hydroxyl radicals seems to be directly related to prevention of propagation of the process of lipid peroxidation, the extract also appears to be a good scavenger of active oxygen species. The LH extract inhibited the NO induced oxidation of DAF-2 to triazole fluorescein (), which was indicated by IC50 values of 3.78 ± 0.15, 6.01 ± 0.58 and 28.62 ± 3.3 μg/mL for EA, B and E fractions of LH, respectively. In addition, the scavenging activities of LGME on nitric oxide radical exhibited excellent scavenging effects with an IC50 value of 7.84 ± 0.16 μg/mL.
Taken together, these data imply that the LH extract and its compounds may be effective scavengers of RNS. The peroxynitrite scavenging activity of LH was investigated and the results were compared with those of reference antioxidants (). The need for a higher extract concentration to scavenge radicals indicates a lower antioxidant activity. The LH extract inhibited the peroxynitrite induced oxidation of the DHR reaction mixture, with the peroxynitrite scavenging activity being the highest in the EA fraction. The order of the peroxynitrite scavenging activity of the LH extracts was as follows: EA > B > H > E > DCM > A. The inhibitory effects of LGME on peroxynitrite scavenging activity were powerful, and their IC50 values were 7.04 ± 0.22 μg/mL. Together, this data implies that LH extract is an effective scavenger of RNS.
Table 3. ROS (superoxide anion and hydroxyl radical) and RNS (nitric oxide radical and peroxynitrite) scavenging activities of the extracts and 1 from Lycopi Herba.
shows the protective effect of the LH extract on LDL oxidation induced by Cu2+. The peroxidation of LDL was significantly inhibited in the presence of LH, and the protective action of LH on LDL oxidation occurred in a concentration-dependent manner. The IC50 values for the inhibition of LDL oxidation were 52.44 ± 2.47, 55.14 ± 4.51, 110.15 ± 11.25, 125.4 ± 13.04, 128.04 ± 10.35 and 129.67 ± 8.76 for the B, DCM, E, A, EA and H fractions, respectively, indicating that these extracts prevented oxidation of LDL. The inhibitory effects of LGME on LDL oxidation induced by Cu2+ were powerful, and the IC50 value was 71.13 ± 0.40 μg/mL. Lipid peroxidation resulting in Ox-LDL production is a common occurrence in patients with systemic autoimmune diseases and in chronic inflammatory disorders. Moreover, Ox-LDL can stimulate endothelial cells and monocytes to produce tissue factors, which may contribute to thrombus formation in retyped plaques as well as enhance spontaneous fibrin deposition. These phenomena result in the gradual thickening of arteries, causing decreased elasticity, narrowing, reduced blood supply, and ultimately leading to atherosclerosis [Citation17]. Based on the data shown in and , LH has the potential to prevent atherosclerosis via suppression of LDL oxidation. Collectively, these remarkable properties indicate that LH has significant antioxidant activity.
Table 4. Inhibitory effect on Cu2+-induced LDL oxidation of the extracts and 1 from Lycopi Herba.
Figure 2. The relative electrophoretic mobility (REM) of human LDL incubated with Cu2+, with or without extracts and one from Lycopi Herba. * LDL (120 μg/mL) was oxidized with 10 μM CuSO4 at 37°C in the presence of LH extracts for 12 h. (A): Lane 1: native LDL; Lane 2: LDL and Cu2+; Lane 3,4: LDL and Cu2+ and 5, 10 μg of E; Lanes 5,6: LDL and Cu2+ and 5, 10 μg of H; Lanes 7,8: LDL and Cu2+ and 5, 10 μg of DCM; Lanes 9,10: LDL and Cu2+ and 5, 10 μg of EA; Lanes 11,12: LDL and Cu2+ and 5, 10 μg of B; Lanes13,14: LDL and Cu2+ and 5, 10 μg of A; Lanes 15,16: LDL and Cu2+ and 5, 10 μg of luteolin-7-O-β-D-glucuronide methyl ester (#1); Lanes 17,18: LDL and Cu2+ and 5, 10 μg of AA. (B): Protection rate (%), (Each value represents the mean ± SE of triplicate measurements.).
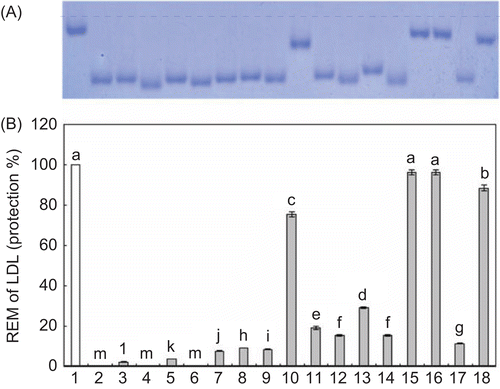
shows the effect of LH on the REM of LDL peroxidation induced by Cu2+. If the REM of native LDL is assumed to be one, the addition of Cu2+ caused the REM to increase to 2.83 in response. In addition, the data showed that LDL peroxidation can be suppressed by the addition LH extracts, as indicated by the REM value being reduced to 1.50 and 1.83 in response to treatment with a concentration of 1 μg/mL of the EA and B fraction, respectively. Moreover, the REM value was decreased to 1.33 in response to treatment with a concentration of 5 μg/mL of the DCM fraction. In this study, the ability of LH to scavenge free radicals was further confirmed by the inhibition of LDL peroxidation. These results revealed that LH extracts could convert free radicals to more stable products and terminate the radical chain reaction, thereby supplying antioxidant action.
Conclusion
These data imply that at least part of the observed antioxidant activity may be a result of the phenolic compounds of LH. Furthermore, the studied extract may be helpful for preventing lipid peroxidation and protecting excipient bases and medicines from oxidative damage. Nevertheless, it is still necessary to evaluate its potential toxicity prior to application on a practical scale.
Declaration of interest
This work was supported by the Dongguk University Research Fund and the MRC program of MOST/KOSEF (grant #: R13-2005-01001-0).
References
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature 2000;408:239–247.
- Schmitz G, Grandl M. Role of redox regulation and lipid rafts in macrophages during Ox-LDL-mediated foam cell formation. Antioxid Redox Signal 2007;9:1499–1518.
- Magalhães LM, Segundo MA, Reis S, Lima JLFC. Methodological aspects about in vitro evaluation of antioxidant properties. Analytica Chimica Acta 2008;613:1–19.
- Shin TY, Kim SH, Suk K, Ha JH, Kim I, Lee MG, Jun CD, Kim SY, Lim JP, Eun JS, Shin HY, Kim HM. Anti-allergic effects of Lycopus lucidus on mast cell-mediated allergy model. Toxicol Appl Pharmacol 2005;209:255–262.
- Lee YJ, Kang DG, Kim JS, Lee HS. Lycopus lucidus inhibits high glucose-induced vascular inflammation in human umbilical vein endothelial cells. Vascul Pharmacol 2008;48:38–46.
- Shi HZ, Gao NN, Li YZ, Yu JG, Fan QC, Bai GE. Effects of active fractions from Lycopus lucidus L. F04 on platelet aggregation and thrombus formation. Space Med Eng (Beijing) 2004;17:313–317.
- Kujala TS, Loponen JM, Klika KD, Pihlaja K. Phenolic and betacyanins in red beetroot (Beta vulgaris) root: distribution and effects of cold storage on the content of total phenolics and three individual compounds. J Agri Food Chem 2000;48:5338–5342.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999;26:1231–1237.
- Gyamfi MA, Yonamine M, Aniya Y. Free-radical scavenging action of medicinal herbs from Ghana: Thonningia sanguinea on experimentally induced liver injuries. Gen. Pharmacol 1999;32:661–667.
- Gotoh N, Niki E. Rates of interactions of superoxide with vitamin E, vitamin C and related compounds as measured by chemiluminescence. Biochem Biophys Acta 1992;1115:201–207.
- Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview. Method Enzymol 1990;186:1–85.
- Nagata N, Momose K, Ishida Y. Inhibitory effects of catecholamines and anti-oxidants on the fluorescence reaction of 4,5-diaminofluorescein, DAF-2, a novel indicator of nitric oxide. J Biochem (Tokyo) 1999;125:658–661.
- Kooy NW, Royall JA, Ischiropoulos H, Beckman JS. Peroxynitrite-mediated oxidation of dihydrorhodamine 123. Free Radic Biol Med 1994;16:149–156.
- Yoon MA, Jeong TS, Park DS, Xu MZ, Oh HW, Song KB, Lee WS, Park HY. Antioxidant effect of quinoline alkaloid and 2,4-di-tert-butylphenol isolated from Scolopendra subspinipes. Biol Pharm Bull 2006;29:735–739.
- Xu MZ, Lee WS, Han JM, Oh HW, Park DS, Tian GR, Jeong TS, Park HY. Antioxidant and anti-inflammatory activities of N-acetyldopamine dimers from Periostracum cicadae. Bioorg Med Chem 2006;14:7826–7834.
- Du Q, Xu Y, Li L, Zhao Y, Jerz G, Winterhalter P. Antioxidant constituents in the fruits of Luffa cylindrica (L.) Roem. J Agric Food Chem 2006;54(12):4186–4190.
- Stoll G, Bendszus M. Inflammation and atherosclerosis: Novel insights into plaque formation and destabilization. Stroke 2006;37:1923–1932.