Abstract
A series of benzoic acid derivatives 1–10 have been synthesised by two different methods. Compounds 1–6 were synthesised by a facile procedure for esterification using N,N’-dicyclohexylcarbodiimide (DCC) as a coupling agent, methylene chloride as a solvent system and dimethylaminopyridine (DMAP). While 7–10 were synthesised by converting benzoic acid into benzoyl chloride by treating with thionyl chloride in the presence of benzene and performing a further reaction with amine in dried benzene. The structures of all the synthesised derivatives of benzoic acid (1–10) were assigned on the basis of extensive NMR studies. All of them showed inhibitory potential against tyrosinase. Among them, compound 7 was found to be the most potent (1.09 μM) when compared with the standard tyrosinase inhibitors of kojic acid (16.67 μM) and L-mimosine (3.68 μM). Finally in this paper, we have discussed the structure–activity relationships of the synthesised molecules.
Introduction
Drug discovery is one of the areas at the forefront of modern medicinal chemistry. The search for and development of new biologically active compounds have recently become of great interest. The enzyme tyrosinase (EC 1.14.18.1, syn. polyphenol oxidase, PPO; monophenol; dihydroxy-Lphenylalanine; oxidoreductase) is known to be a multifunctional copper-containing enzyme from the oxidase superfamily, which is the key protein involved in the biosynthesis of the large biological pigment melanin. This enzyme catalyses two distinct reactions of melanin biosynthesis, the hydroxylation of a monophenol and the conversion of an o-diphenol to the corresponding o-quinone. In both of these oxidation reactions, oxygen is used as an oxidant and o-quinone as the product can inactivate the tyrosinase. Quinones are usually formed rapidly and undergo non-enzymic conversion to form more stable intermediates. These intermediates subsequently undergo slow oligomerisation reactions that ultimately yield high molecular weight, insoluble polyphenols [Citation1]. Inhibitors of this protein have a huge impact on industry and the economy [Citation2]. A large number of mild to potent inhibitors of tyrosinase from several classes, such as phenolics, terpenes, steroids, chalcones, flavonoids, alkaloids, long-chain fatty acids, coumarins, sildenafil analogues, bipiperidines, biscoumarins, oxadiazole, and tetraketones have been reported in recent years [Citation2].
Derivatives of benzoic acid offer promise as compounds that have multifunctional physiological activity such as hypocholesterolaemic, antitumour, antithrombic without causing hypervitaminosis and other side effects [Citation3–14]. Benzoic acid derivatives have exhibited antitumour, antioxidant, antibacterial, antiinflammatory activities and also displayed an immunopotentiatory effect [Citation15–18]. Several derivatives have been identified as novel retinoid X receptor (RXR) antagonists [Citation19]. Benzoic acid derivatives are also able to inhibit human breast carcinoma cell proliferation [Citation20]. An investigation to determine the structure verses activity characteristics would make a definite contribution to the development of the goal-directed synthesis of biologically active compounds. Therefore it seemed to us that ester and amide derivatives of benzoic acid might also show interesting biological properties. In view of this, we have synthesised some new derivatives of benzoic acid using two methods, with the aim that they may be potential biomarkers.
The present paper describes the tyrosinase inhibitory activities and the structure–activity relationships (SAR) of these benzoic acid derivatives, including their synthetic procedures and characterisation utilising conventional spectroscopic techniques.
Materials and methods
Column chromatography was carried out for purification of the crude materials using silica gel of 230–400 mesh (Seoul, Merck) as the stationary phase. Alumina sheets precoated with silica gel 60 F254 (20 × 20 cm, 0.2-mm thick; Merck) were used for TLC to check the purity and were visualised under UV light (254 and 365 nm) using ceric sulphate reagent. All the chemicals were purchased from the Aldrich (Seoul) and all the solvents were HPLC grade. The Fourier transform infrared spectrometer (FT-IR) spectra were recorded in KBr dispersion on an Excalibur Series FT-IR (Seoul, DIGLAB) instrument. Mass spectra (EI and HREIMS) were measured on on Finnigan (Seoul) (Varian MAT) 112 and Finnigan (Varion MAT) 312 spectrometers and the ions are given in m/z (%). The 1H and 13C NMR spectra were recorded on a Bruker AMX-400 spectrometer (Seoul) in CDCl3. The chemical shifts (δ) were reported in ppm and the coupling constants (J) in hertz (Hz). The chemical shift standard was internal tetramethylsilane (TMS) for both 1H and 13C NMR. The following abbreviations were used: s, d, dd and m for singlet, doublet, doublet of doublet and multiplet, respectively.
Chemistry
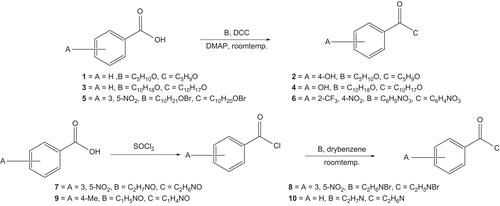
General procedure for the synthesis of esters 1–4
Compounds 1–4 were synthesised by esterification of benzoic acids with alcohol by a synthetic procedure in which alcohols (5 mL/mmol) were added to the benzoic acids (1.3 equiv) dissolved in anhydrous CH2Cl2 (10 mL). Dimethylaminopyridine (DMAP) (0.15 equiv) and N,N’-dicyclohexylcarbodiimide (DCC) (1.25 equiv) were added and stirred for up to 12 h. The N,N-Dicyclohexylurea was filtered and the filtrate was concentrated. The crude compounds 1–4 (1.32–140 g) were subjected to flash column chromatography over silica gel, successively eluting with n-hexane–ethyl acetate (4:6 to 3:7) afforded 1–4 (15–20 mg).
General procedure for the synthesis of esters 5–10
All the target compounds (5–10) were prepared according to . The starting material and the corresponding benzoic acid (1.54 g, 0.01 mol) were suspended in 30 mL of dried benzene (with care as it is carcinogenic), then thionyl chloride (2.4 g, 0.02 mol) was added. The mixture was refluxed for 6 h, after the reaction was completed the solvent was removed by evaporation, and the residue was washed with ether to afford benzoyl chloride. To a solution of 1 g (0.006 mol) of benzoyl chloride in 60 mL of dried benzene was added compound B (0.012 mol) and the individual mixture was reacted at room temperature for 2 h, after the reaction was completed the solvents were removed by evaporation. The residues were purified by chromatography on silica gel with elution with CH2Cl2 and re-crystallisation from benzene yielded compounds 5–10.
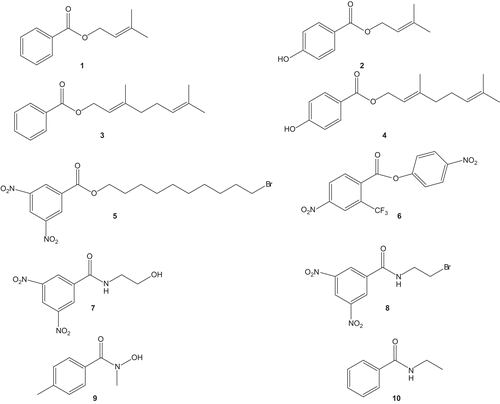
3-Methyl-2-butenylbenzoate (1)
Colourless oil, IR (KBr), υmax cm−1: 3100–3000 (=C-H), 2950–2860 (C-H), 1740 (C=O), 1662 (C=C), 1610–1600 (aromatic C=C) and 1200–1100 (C-O), HREIMS: m/z M+ 190.0997 (calcd 190.0994 for C12H14O2), 1H NMR: δ 8 (2H, d, J = 7 Hz), 7.54 (1H, m), 7.43 (2H, m), 5.47 (1H, t, J = 1.2 Hz), 4.8 (2H, m), 1.78 (3H, s) and 1.6 (3H, s) and 13C NMR: δ 167.1, 133.6, 132.2, 130, 129.2 × 2, 128 × 2, 122.2, 62.1, 24 and 18.7.
3-Methyl-2-butenyl-4-hydroxybenzoate (2)
Colourless oil, IR (KBr), υmax cm−1: 3300–3200 (O-H), 3100–3000 (=C-H), 2950–2860 (C-H), 1740 (C=O), 1669 (trans C=C), 1658 (cis C=C), 1610–1600 (aromatic C=C) and 1200–1100 (C-O), HREIMS: m/z M+ 206.0947 (calcd 206.0943 for C12H14O3), 1H NMR: δ 7.8 (2H, d, J = 7 Hz), 6.9 (2H, d, J = 7 Hz), 5.47 (1H, t, J = 1.2 Hz), 4.8 (2H, m), 1.78 (3H, s) and 1.6 (3H, s) and 13C NMR: δ 167, 161, 130.9 × 2, 122.8, 115 × 2, 132.2, 62.1, 24.0 and 18.7.
(2E)-3,7-Dimethyl-2,6-octadienylbenzoate (3)
Colourless oil, IR (KBr), υmax cm−1: 3100–3000 (=C-H), 2950–2860 (C-H), 1740 (C=O), 1662 (C=C), 1610–1600 (aromatic C=C) and 1200–1100 (C-O), HREIMS: m/z M+ 258.1625 (calcd 258.162 for C17H22O2), 1H NMR: δ 8. (2H, d, J = 7 Hz), 7.54 (1H, m), 7.43 (2H, m), 5.47 (1H, m), 5.25 (1H, m), 4.8 (2H, m), 2.2 (2H, m), 2.18 (2H, m), 1.78 (3H, s), 1.65 (3H, s) and 1.6 (3H, s) and 13C NMR: δ 167, 138.2, 133.4, 132.3, 130, 129.2 × 2, 128 × 2, 121.5, 62, 40.1, 23.8, 24.5, 18.7 and 17.
(2E)-3,7-Dimethyl-2,6-octadienyl-4-hydroxybenzoate (4)
Colourless oil, IR (KBr), υmax cm−1: 3300–3200 (O-H), 3100–3000 (=C-H), 2950–2860 (C-H), 1740 (C=O), 1669 (trans C=C), 1658 (cis C=C), 1610-1600 (aromatic C=C) and 1200–1100 (C-O), HREIMS: m/z M+ 274.1574 (calcd 274.1569 for C17H22O3), 1H NMR: δ 7.8 (2H, d, J = 7 Hz), 6.9 (2H, d, J = 7 Hz), 5.47 (1H, m), 5.25 (1H, m), 4.8 (2H, m), 2.2 (2H, m), 2.18 (2H, m), 1.78 (3H, s), 1.65 (3H, s) and 1.6 (3H, s) and 13C NMR: δ 167, 161, 138.2, 133.4, 130.9 × 2, 122.8, 122, 121.5, 115 × 2, 62, 40.1, 23.8, 24.5, 18.7 and 17.
10-Bromo-decyl-(3,5-dinitro)-benzoate (5)
White crystal, mp 210–213°C, IR (KBr), υmax cm−1: 3100–3000 (=C-H), 2950–2860 (C-H), 1735 (C=O), 1610–1600 (aromatic C=C), 1600–1500 (N=O) and 1300–1100 (C-O, N-O), HREIMS: m/z M+ 430.0743 (calcd 430.0739 for C17H23BrN2O6), 1H NMR: δ 9.23 (1H, s), 9.17 (2H, d, J = 1.9 Hz), 4.2 (2H, m), 3.27 (2H, m), 1.74 (2H, m), 1.71 (2H, m) and 1.29 (12H, brs) and 13C NMR: δ 167.2, 148.7 × 2, 132, 130.5 × 2, 122.8, 65.9, 33.9, 32.5, 30 × 2, 29.8, 29.7, 29, 28.9 and 25.9.
4-Nitro-phenyl-(4-nitro-2-trifluoromethyl)-benzoate (6)
Colourless oil, IR (KBr), υmax cm−1: 3100–3000 (=C-H), 1735 (C=O), 1610–1600 (C=C) and 1600–1500 (N=O) and 1300–1100 (C-O, N-O, C-F), HREIMS: m/z M+ 356.0259 (calcd 356.0256 for C14H7F3N2O6), 1H NMR: δ 8.49 (1H, s), 8.2 (1H, d, J = 7.9 Hz), 8.29 (1H, d, J = 7.9 Hz), 8.23 (2H, d, J = 8.1 Hz) and 7.38 (2H, d, J = 8.1 Hz) and 13C NMR: δ 163.8, 158.9, 153.5, 144.8, 133.5, 133.4, 130.7, 126.5, 123.7 × 2, 121.9 × 2, 120 and 110.8.
N-(2-Hydroxy-ethyl)-3,5-dinitro-benzamide (7)
Colourless powder, mp: 125–-127°C, IR (KBr), υmax cm−1: 3300–3200 (O-H), 3100–3000 (=C-H), 2950–2860 (C-H), 1685 (C=O), 1610–1600 (aromatic C=C), 1600–1500 (N=O) and 1300–1100 (C-O, N-O), HREIMS: m/z M+ 255.0495 (calcd 255.0491 for C9H9N3O6), 1H NMR: δ 9.23 (1H, s), 9.17 (2H, d, J = 1.9 Hz), 4 (2H, m) and 3.7 (2H, m) and 13C NMR: δ 167.6, 148.5 × 2, 134.9, 128 × 2, 128.4 × 2, 121.6, 46.5 and 63.9.
N-(2-Bromo-ethyl)-3,5-dinitro-benzamide (8)
Colourless powder, mp: 103–105°C, IR (KBr), υmax cm−1: 3100–3000 (=C-H), 2950–2860 (C-H), 1685 (C=O), 1610–1600 (aromatic C=C), 1600–1500 (N=O) and 1300–1100 (C-O, N-O), HREIMS: m/z M+ 316.9651 (calcd 316.9647 for C9H8BrN3O5), 1H NMR: δ 9.23 (1H, s), 9.17 (2H, d, J = 1.9 Hz), 3.75 (2H, m) and 3.6 (2H, m) and 13C NMR: δ 167.6, 148.5 × 2, 134.9, 128 × 2, 128.4 × 2, 121.6, 46.7 and 33.9.
N-Hydroxy-4,N-dimethyl-benzamide (9)
Colourless powder, mp: 121–123°C, IR (KBr), υmax cm−1: 3300–3200 (O-H), 3100–3000 (=C-H), 2950-–2860 (C-H), 1685 (C=O), 1610–1600 (aromatic C=C), and 1300–1100 (C-O, C-N), HREIMS: m/z M+ 165.0794 (calcd 165.0794 for C9H11NO2), 1H NMR: δ 7.41 (2H, d, J = 8 Hz), 7.2 (2H, d, J = 8 Hz), 3.3 (3H, s) and 2.3 (3H, s) and 13C NMR: δ 162.1, 140.8, 130.1, 129.2 × 2, 127 × 2, 36.5 and 21.1.
N-Ethyl-benzamide (10)
Colourless powder, mp: 68–70°C, IR (KBr), υmax cm−1: 3100–3000 (=C-H), 2950–2860 (C-H), 1685 (C=O), 1610–1600 (aromatic C=C), and 1300–1100 (C-O, C-N), HREIMS: m/z M+ 149.0846 (calcd 149.0846 for C9H11NO), 1H NMR: δ 7.97 (2H, m), 7.92 (2H, m), 7.49 (1H, m), 7.42 (2H, m), 3.23 (2H, m) and 1.2 (3H, t, J = 7.01 Hz) and 13C NMR: δ 167.8, 132.9, 131.7, 128.5 × 2, 127 × 2, 37 and 15.7.
In vitro tyrosinase inhibition assay
Tyrosinase inhibition assays were performed in a 96-well microplate format using the SpectraMax 340 microplate reader (Molecular Devices, CA) according to the method developed by Hearing [Citation1]. First the samples were screened for the o-diphenolase inhibitory activity of mushroom tyrosinase (Lyophilised, ≥2000 units/mg, Sigma, Montana, USA) using L-3,4-dihydroxyphenylalanine (L-DOPA) (Sigma) as substrate. All the active samples from the preliminary screening were subjected to half maximal inhibitory concentration (IC50) studies. The samples were dissolved in methanol/DMSO to a final concentration of 0.5%. At higher concentrations like 3.3–6.7% DMSO shows a dose dependent inhibitory activity against the enzyme tyrosinase [Citation22]. So the final concentration.forDMSO at 0.5% should be comparatively safe.
Thirty units of mushroom tyrosinase (28 nM) were preincubated with the test compounds in 50 nM Na-phosphate buffer (pH 6.8) for 10 min at 25°C. Then the L-DOPA (0.5 mM) was added to the reaction mixture and the reaction was monitored by measuring the change in absorbance at 475 nm (at 37°C) due to the formation of the DOPA chrome for 10 min. The percentage inhibition of the enzyme was calculated as follows, by using an MS Excel™ 2000-based program (Microsoft, USA) developed for this purpose (Equation 1):
Where B and S are the absorbances for the untreated control and samples, respectively. After the preliminary screening of the compounds the IC50 values of the active compounds were calculated. All the studies were carried out at least in triplicate and the results represents the mean ± SEM. Compounds like Kojic acid and L-mimosine were used as standard inhibitors for the tyrosinase. All the chemicals and reagents were purchased from Sigma.
Results and discussion
Characterisation of compounds (1–10)
Given the widespread utility and broad spectrum of biological activities of these compounds, the synthesis of more potent efficacious, new ester derivatives is of interest. All these derivatives were synthesised by following the published procedures [Citation23,Citation24]. The benzoic acid derivatives were coupled with various alcohols using steglich conditions (DCC/DMAP) with methylene chloride as solvent (Scheme 1), by converting benzoic acid into benzoyl chloride then treating with thionyl chloride in the presence of benzene and a further reaction with amine in dried benzene providing the desired products. These were then confirmed by mass, 1H NMR, 13C NMR, homonuclear multiple-quantum coherence (HMQC) and heteronuclear multiple-bond connectivity (HMBC) experiments.
Compound 1 showed a molecular ion peak in the high-resolution mass spectrum at m/z 190.0997, corresponding to the molecular formula C12H14O2 (calcd 190.0994 for C12H14O2). The 1H NMR spectrum (experimental part) showed a signal at 8 (2H, d, J = 7 Hz), 7.54 (1H, m) and 7.43 (2H, m) which could be assigned to the aromatic protons. The spectrum also showed signals for the side chain protons at δ 5.47 (1H, m), 4.8 (2H, m) and two singlets at δ 1.78 (3H) and 1.6 (3H) supporting the presence of a double bond, methylene protons and a pair of methyls in the side chain. The 13C NMR spectrum (BB and DEPT) (experimental part) showed 12 carbon signals, two methyl, one methylene, six methine and three quaternary carbons. The low-field region of the 13C NMR spectrum showed signals at δ 167.1, 133.6, 132.2, 130, 129.2 × 2, 128 × 2 and 122.2 which could be assigned to the carbonyl carbon of the ester, aromatic ring carbons and double bond carbons of the side chain, respectively. One oxygenated methylene carbon resonated at δ 62.1 while methyl carbons appeared at δ 18.7 and 24. The positions of the substituent were confirmed by the homonuclear multiple-quantum coherence (HMQC) and heteronuclear multiple-bond connectivity (HMBC) experiments. Thus compound 1 was confirmed as 3-methyl-2-butenylbenzoate. Structures of all the other compounds were confirmed in the same way using spectral data and were found as 3-methyl-2-butenyl-4-hydroxybenzoate (2), (2E)-3,7-dimethyl-2,6-octadienylbenzoate (3), (2E)-3,7-dimethyl-2,6-octadienyl-4-hydroxybenzoate (4), 10-bromo-decyl-(3,5-dinitro)-benzoate (5), 4-nitro-phenyl-(4-nitro-2-trifluoromethyl)-benzoate (6), N-(2-Hydroxy-ethyl)-3,5-dinitro-benzamide (7), N-(2-Bromo-ethyl)-3,5-dinitro-benzamide (8), N-hydroxy-4,N-dimethyl-benzamide (9) and N-Ethyl-benzamide (10).
Structure-activity relationship (SAR)
Addition of the OH functional group at the 4-position makes compound 2 (IC50 = 14.83 µM) about six times more active than compound 1 (IC50 = 83.39 µM); whereas compound 4 is inactive although it has the same OH group at this position (shown in and ). This is probably due to the incremental effect of chain length. Compound 3 is also inactive probably due to a similar reason. Addition of two NO2 functional groups at the 3 and 5 positions in compound 7 creates a strong inhibitor of the enzyme tyrosinase having an IC50 value of 1.09 µM and in the case of compound 8, replacement of the OH group with Br gives it a two-fold decrease in potency (IC50 = 3.08 µM) compared to compound 7. A structurally similar compound like 5 is not active, probably due to the change of class from ester to amide as well as the chain length.
Compound 6 showed relatively less potency (IC50 = 29.72 µM) against tyrosinase; this is probably due to the presence of a 4-nitro-phenyl in the benzoate. Compound 9 exhibited stronger potency (IC50 = 2.09 µM) against tyrosinase, better than both the standard inhibitors Kojic acid and L-mimosine. But the structurally similar compound 10 did not show strong potency (IC50 = 11.83 µM), probably due to the lack of an N–OH group in the side chain as well as a methyl group at the 4 position.
Table 1. Tyrosinase inhibitory activities of the compounds 1–10.
Conclusion
Benzoic acid derivatives 1–10 have been synthesised successfully. Compounds 1, 2 and 6–10 showed activity against tyrosinase and compound 7 had an IC50 value of 1.09 µM which could be used as a “Lead” molecule for further development of molecules against tyrosinase as well as against melanoma related to over-expression of the protein.
Declaration of interest
This work was supported by Korea Association of Industry, Academy and Research Institute grant funded by the Korea government (SMBA) (00037459) and National Research Foundation of Korea grant funded by the Korean Government (MEST); contract grant number; NRF-2009-C1AAA001-0092926.
References
- Khan MTH. Molecular design of tyrosinase inhibitors: A critical review of promising novel inhibitors from synthetic origins. Pure Appl Chem 2007;79:2277–2295.
- Shora HME, Metwally M. Use of tyrosinase enzyme from Bacillus thuringiensis for the decontamination of water polluted with phenols. Biotech 2008;7:305–310.
- Jetten AM, Anderson K, Deas MA, Kagechika H, Lotan R, Rearick JI, Shudo K. New benzoic acid derivatives with retinoid activity: lack of direct correlation between biological activity and binding to cellular retinoic acid binding protein. Cancer Res 1987;47:3523–3527.
- Correa-Basurto J, Espinosa-Raya J, Vazquez-Alcantara I, Flores-Sandoval CA, Trujillo-Ferrara J. Ex vivo anticholinesterase activity of benzoic acid derivatives. Chem Biol Interact 2005;157–158:353–434.
- Sato M, Shudo K, Hiragun A, Functional studies of newly synthesized benzoic acid derivatives: Identification of highly potent retinoid-like activity. J Cell Physiol 2005;135:179–188.
- Shin H, Gennadios HA, Whittington DA, Christiansonb DW. Amphipathic benzoic acid derivatives: Synthesis and binding in the hydrophobic tunnel of the zinc deacetylase LpxC. Bioorgan Med Chem 2007;15:2617–2623.
- Kelly PN, Pretre A, Devoy S, O’Rielly I, Devery R, Goel A, Gallagher JF, Lough AJ, Kenny PTM. Synthesis, structural characterisation and biological activity of novel N-(ferrocenylmethyl)benzene-carboxamide derivatives. J Organomet Chem 2007;692:1327–1331.
- Jackman JE, Raetz CRH, Fierke CA. UDP-3-O-(R-3-Hydroxymyristoyl)-N-acetylglucosamine deacetylase of Escherichia coli is a zinc metalloenzyme. Biochemistry 1999;38:1902–1911.
- Anderson MS, Bulawa CE, Raetz CRH. The biosynthesis of gram-negative endotoxin. Formation of lipid A precursors from UDP-GlcNAc in extracts of Escherichia coli. J Biol Chem 1985;260:15536–15541.
- Anderson MS, Robertson AD, Macher I, Raetz CRH. Biosynthesis of lipid A in Escherichia coli: identification of UDP-3-O-[(R)-3-hydroxymyristoyl]-.alpha.-D-glucosamine as a precursor of UDP-N2,O3-bis[(R)-3-hydroxymyristoyl]-.alpha.-D-glucosamine. Biochemistry 1988;27:1908–1917.
- Anderson MS, Bull HG, Galloway SM, Kelly TM, Mohan S, Radika K, Raetz CRH. UDP-N-acetylglucosamine acyltransferase of Escherichia coli. The first step of endotoxin biosynthesis is thermodynamically unfavorable. J Biol Chem 1993;268:19858–19865.
- Young K, Silver LL, Bramhill D, Cameron P, Eveland SS, Raetz CRH, Hyland SA, Anderson MS. The envA permeability/cell division gene of Escherichia coli encodes the second enzyme of lipid A biosynthesis: UDP-3-O-(R-3-hydroxymyristoyl)-N-acetylglucosamine deacetylase. J Biol Chem 1995;270: 30384–30391.
- Chand P, Babu YS, Bantia S, Chu N, Cole LB, Kotian PL, Laver WG, Montgomery JA, Pathak VP, Petty SL, Shrout DP, Walsh DA, Walsh GM. Design and synthesis of benzoic acid derivatives as influenza neuraminidase inhibitors using structure-based drug design. J Med Chem 1997;40:4030–4052.
- Lin FH, Lin JY, Gupta RD, Tournas JA, Burch JA, Selim MA, Riviere MNA, Grichnik JM, Zielinski J, Pinnell SR. Ferulic acid stabilizes a solution of vitamins C and E and doubles its photoprotection of skin. J Invest Dermatol 2005;125:826–832.
- Barakat ESS, Ghorab MM, Saker HM, Abd Rabo MM. Novel quinazolinone derivatives as possible antitumor agents. Phosphorus, Sulfur and Silicon and the Related Elements 2007;182:65–77.
- Szwajgier D, Pielecki J, Targonski Z. Antioxidant activities of cinnamic and benzoic acid derivatives. Acta Sci Pol Technol Aliment 2005;4:129–142.
- Udupi RH, Pasha TY, Bhat AR. Synthesis and biological activities of 2 - substituted- 4-nitro-(amino)-benzoic acid derivatives. Journal of the Institution of Chemists (India) 1998;70:139–141.
- Kinchington D, Ng T, Mathews N, Tisdale M, Devine D, Ayuko WO. T cell costimulation by derivatives of benzoic acid. Antiviral Chem Chemother 1997;8:121–130.
- Ebisawa M, Umemiya H, Ohta K, Fukasawa H, Kawachi E, Christoffel G, Gronemeyer H, Tsuji M, Hashimoto Y, Shudo K, Kagechika H. Retinoid X receptor-antagonistic diazepinylbenzoic acids. Chem Pharm Bull 1999;47:1778–1786.
- Fontana JA, Hobbs PD, Dawson IM. Inhibition of mammary carcinoma growth by retinoidal benzoic acid derivatives. Exp Cell Biol 1988;56:254–263.
- Hearing VJ. Methods in Enzymology. New York: Academic Press, 1987 142:154–165.
- Yu L. Inhibitory effects of (S)- and (R)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acids on tyrosinase activity. J Agric Food Chem 2003;51:2344–2347.
- Eicher T, Ott M, Speicher A. Bryophyte constituents; 7: new synthesis of (+)-rosmarinic acid and related compounds. Synthesis 1996;6:755– 762.
- Chan HC, Kuo SC, Liu SC, Liu CH, Hsu SL. 4-Fluoro-N-butylphenylacetamide: a synthetic phenylacetate derivative that upregulates Bcl-XS, activates caspase cascade and induces apoptosis in human squamous lung cancer CH27 cells. Cancer Lett 2002;186:211–221.
- Karbassi F, Saboury AA, Khan MT, Choudhary MI, Saifi ZS. Mushroom tyrosinase inhibition by two potent uncompetitive inhibitors. J Enzyme Inhib Med Chem 2004;19:349–353.
- Gheibi N, Saboury AA, Mansuri-Torshizi H, Haghbeen K, Moosavi-Movahedi AA. The inhibition effect of some n-alkyl dithiocarbamates on mushroom tyrosinase. J Enzyme Inhib Med Chem 2005;20:393–399.