Abstract
In this study, a series of novel 3-(substituted phenyl)-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]isoxazole analogues were synthesized and evaluated for antimycobacterial activity against Mycobacterium tuberculosis (MTB) H37Rv and isoniazid resistant M. tuberculosis (INHR-MTB). All the newly synthesized compounds were showing moderate to high inhibitory activities. The compound 6,7-dimethoxy-3-(4-chloro phenyl)-4H-indeno[1,2-c]isoxazole (4b) was found to be the most promising compound, active against MTB H37Rv and INHR-MTB with minimum inhibitory concentrations of 0.22 and 0.34 μM.
Introduction
Many human illnesses are especially caused by infections with microbes such as virus, bacteria, or fungi. Among various illnesses to human beings, certain viral, tubercular, bacterial, and fungal infections are more common and dangerous. This is because of their tendency to develop new strains under any circumstances for developing resistance with the available drugs. This condition has paved the way for scientists to make the efforts to work on several molecules for coming out with novel entities to combat the illnesses.
Tuberculosis (TB) is making a worldwide resurgence. Several factors may be responsible for the increase in the infection rate such as infection with human immunodeficiency virus, change in economic and social circumstances, and decline in TB control programs.Citation1 In addition, outbreaks of multidrug resistant TB have been identified.Citation2 When the AIDS pandemic began, one-third of the world population was infected with Mycobacterium tuberculosis (MTB). Each year, eight to 10 million people are developing active disease and three million people die from TB. Currently, the available first-line anti-TB agents such as rifampcin, ethambutol, streptomycin, and pyrazinamide are highly effective and are generally well tolerated. Problems in the chemotherapy of TB arise when any patients develop resistance to any of these drugs. This is due to the fact that the second-line drugs such as p-aminosalicylic acid, amikacin, cycloserine, capreomycin, and ethionamide are less effective and more toxic.Citation3 The global mortality rate for TB is very high and the development of new kinds of TB such as Multi-drug-resistant tuberculosis (MDR-TB) and Extensively drug-resistant tuberculosis (XDR-TB) alarm for the discovery of new drugs to reduce the potential hazards caused by the fatal disease.
In the past decade, most heterocyclic systems have been used as a source to discover new compounds with varied biological potentials. Especially, nitrogen-containing heterocyclic systems such as novel pyrazolines, substituted oxadiazole, and substituted triazole moieties play a vital role in discovering novel candidates having antimicrobial potentials.Citation4–9 Recently, we reported that the azole derivatives possess anti-TB activity.Citation10–12 Also, substituted azole derivatives have been evaluated for their antiproliferative activity against human lung cancer A549.Citation13
From the above facts, it was evident that the microbial infections making worldwide resurgence and the whole scientific community have to pave their useful hands in exploring novel entities with good therapeutic response without allowing the microbes to develop their resistance. In view of these findings and in continuation of our previous work on the synthesis of isoxazole derivatives, we report the synthesis and evaluation of antimycobacterial activity against MTB and isoniazid resistant M. tuberculosis (INHR-MTB).
Materials
The entire chemicals were supplied by Merck Limited, Worli, Mumbai, Maharastra, India and S. D. Fine Chemicals, Mumbai, Maharastra, India. Melting points were determined by open-tube capillary method and are uncorrected. Purity of the compounds was checked on thin layer chromatography (TLC) plates (silica gel G) in the solvent system toluene–ethyl formate–formic acid (5:4:1) and benzene–methanol (8:2), the spots were located under iodine vapors or UV light. Infra-red (IR) spectrums were obtained on a Perkin–Elmer 1720 Fourier transform infrared spectroscopy (FT-IR) (KBr pellets). Proton nuclear magnetic resonance spectroscopy (1H-NMR) spectra were recorded on a Bruker AC 300 MHz spectrometer using Tetramethylsilane (TMS) as internal standard in Dimethyl sulfoxide (DMSO)/CDCl3. The mass spectra under Electron impact-Mass spectroscopy (EI-MS) were recorded at 70 eV ionizing voltage with a VG ProSpec instrument and are presented as m/z.
Methods
Chemistry
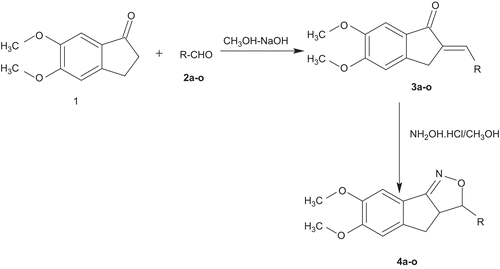
General method for the preparation of 2-[(E)-1-(substituted aryl)methylidene]-5,6-dimethoxy-1-indanone (3a–3o)
5,6-Dimethoxy-1-indanone (1.92 g, 0.01 mol) and appropriate aldehyde (1.02 g, 0.01 mol) were dissolved in ethanol and sodium hydroxide (30%, 5 ml) with 10 ml of petroleum ether. The reaction mixture was stirred at room temperature for 4 h. The resulting solution was allowed to stand overnight then poured into ice-cold water followed by neutralization with HCl. The solid separated was filtered, dried, and purified from ethanol.Citation5,Citation6
General method for the preparation of 3-(3-substituted aryl)-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]isoxazole (4a–4o)
To 2-[(E)-1-(substituted phenyl)methylidene]-5,6-dimethoxy-1-indanone (3a–3o) (0.001 mol) in 15 ml of glacial acetic acid, 0.002 mol hydroxylamine hydrochloride was added and the reaction mixture was refluxed for 15 h and cooled. Excess of solvent was removed under reduced pressure and the reaction mixture was cooled and poured on to crushed ice (20 g). The product obtained was filtered, washed with water, and purified from ethanol by recrystallization.
3-(4-Methoxyphenyl)-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]isoxazole (4a)
IR: (KBr) cm−1: 3042 (CH), 1320 (C–N); 1H-NMR (DMSO-d6) ppm: 3.0–3.2 (2H, m, CH2), 3.82 (6H, s, OCH3), 3.97–4.0 (1H, m, CH), 5.17 (d, J = 6.4 Hz, 1H, CH), 6.6–7.3 (6H, m, aromatic); 13C-NMR (75 MHz, CDCl3): 28.4, 35.1, 56.1, 56.1, 56.1, 91.3, 104.0, 110.4, 112.5, 120.3, 131.3, 131.9, 147.1, 149.9, 147.2, 147.8, 148.4, 152.2, 157.2; EI–MS (m/z): 326 (M+1); C19H19NO4; calculated: C = 70.14, H = 5.89, N = 4.30; found: C = 70.12, H = 5.87, N = 4.32%.
3-(4-Chlorophenyl)-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]isoxazole (4b)
IR: (KBr) cm−1: 3042 (CH), 1320 (C–N), 786 (C–Cl); 1H-NMR (DMSO-d6) ppm: 3.0–3.2 (2H, m, CH2), 3.82 (6H, s, OCH3), 3.97–4.0 (1H, m, CH), 5.17 (d, J = 6.4 Hz, 1H, CH), 6.6–7.4 (6H, m, aromatic); 13C-NMR (75 MHz, CDCl3): 28.4, 35.1, 56.1, 56.1, 91.3, 104.0, 112.0, 127.9 128.7, 128.7, 128.9, 128.9,130.6, 131.0, 147.2, 147.8, 152.2, 157.2; EI–MS (m/z): 330 (M+); C18H16ClNO3; calculated: C = 65.56, H = 4.89, N = 4.25; found: C = 65.58, H = 4.87, N = 4.26%.
3-(4-Dimethyl aminophenyl)-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]isoxazole (4c)
IR: (KBr) cm−1: 3042 (CH), 1320 (C–N); 1H-NMR (DMSO-d6) ppm: 2.82 (6H, s, NCH3), 3.0–3.2 (2H, m, CH2), 3.82 (6H, s, OCH3), 3.97–4.0 (1H, m, CH), 5.17 (d, J = 6.4 Hz, 1H, CH), 6.6–7.0 (6H, m, aromatic); 13C-NMR (75 MHz, CDCl3): 28.4, 35.1, 41.3, 41.3, 56.1, 56.1, 91.3, 104.0, 112.0, 113.0, 113.0, 127.2, 128.0,127.2, 131.2, 147.2, 147.8, 148.4,152.2, 157.2; EI–MS (m/z): 339 (M+1); C20H22N2O3; calculated: C = 70.99, H = 6.55, N = 8.28; found: C = 70.98, H = 6.53, N = 8.26%.
6,7-Dimethoxy-3-phenyl-3a,4-dihydro-3H-indeno[1,2-c]isoxazole (4d)
IR: (KBr) cm−1: 3042 (CH), 1320 (C–N); 1H-NMR (DMSO-d6) ppm: 3.0–3.2 (2H, m, CH2), 3.82 (6H, s, OCH3), 3.97–4.0 (1H, m, CH), 5.17 (d, J = 6.4 Hz, 1H, CH), 6.6–7.6 (7H, m, aromatic); 13C-NMR (75 MHz, CDCl3): 28.4, 35.1, 56.1, 56.1, 91.3, 104.0, 112.0, 126.0, 128.0, 128.1, 128.8, 128.8,130.5, 131.2, 147.2, 147.8, 152.2, 157.2; EI–MS (m/z): 296 (M+1); C18H17NO3; calculated: C = 73.20, H = 5.80, N = 4.74; found: C = 73.22, H = 5.78, N = 4.76%.
3-(3,4-Dimethoxyphenyl)-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]isoxazole (4e)
IR: (KBr) cm−1: 3042 (CH), 1320 (C–N); 1H-NMR (DMSO-d6) ppm: 3.0–3.2 (2H, m, CH2), 3.82, 3.85 (12H, s, OCH3), 3.97–4.0 (1H, m, CH), 5.17 (d, J = 6.4 Hz, 1H, CH), 6.6–7.0 (5H, m, aromatic); 13C-NMR (75 MHz, CDCl3): 28.4, 35.1, 56.1, 56.1, 56.1, 56.1, 91.3, 104.0, 110.4, 112.5, 120.3, 131.3, 131.9, 147.1, 149.9, 147.2, 147.8, 148.4, 152.2, 157.2; EI–MS (m/z): 356 (M+1); C20H21NO5; calculated: C = 65.59, H = 5.96, N = 3.94; found: C = 67.57, H = 5.97, N = 3.96%.
3-(3,4,5-Trimethoxyphenyl)-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]isoxazole (4f)
IR: (KBr) cm−1: 3042 (CH), 1320 (C–N); 1H-NMR (DMSO-d6) ppm: 3.0–3.2 (2H, m, CH2), 3.82, 3.86 (15H, s, OCH3), 3.97–4.0 (1H, m, CH), 5.17 (d, J = 6.4 Hz, 1H, CH), 6.6–7.0 (4H, m, aromatic); EI–MS (m/z): 386 (M+1); 13C-NMR (75 MHz, CDCl3): 28.4, 35.1, 56.1, 56.1,56.1, 56.1, 56.1, 91.3, 104.0, 110.4, 112.5, 120.3, 131.3, 131.9, 147.1, 149.9, 147.2, 147.8, 148.4, 152.2, 157.2; C21H23NO6; calculated: C = 65.46, H = 6.01, N = 3.59; found: C = 65.42, H = 6.00, N = 3.61%.
3-(4-Flurophenyl)-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]isoxazole (4g)
IR: (KBr) cm−1: 3042 (CH), 1320 (C–N), 776 (C–F); 1H-NMR (DMSO-d6) ppm: 3.0–3.2 (2H, m, CH2), 3.82 (6H, s, OCH3), 3.97–4.0 (1H, m, CH), 5.17 (d, J = 6.4 Hz, 1H, CH), 6.6–7.2 (6H, m, aromatic); 13C-NMR (75 MHz, CDCl3): 28.4, 35.1, 56.1, 56.1, 91.3, 104.0, 112.0, 115.6, 115.6, 127.9, 127.9, 131.2, 134.1, 147.2, 147.8, 152.2, 157.2, 160.2; EI–MS (m/z): 314 (M+1); C18H16FNO3; calculated: C = 69.00, H = 5.15, N = 4.47; found: C = 69.02, H = 5.13, N = 4.46%.
3-(2-Chlorophenyl)-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]isoxazole (4h)
IR: (KBr) cm−1: 3042 (CH), 1320 (C–N), 786 (C–Cl); 1H-NMR (DMSO-d6) ppm: 3.0–3.2 (2H, m, CH2), 3.82 (6H, s, OCH3), 3.97–4.0 (1H, m, CH), 5.17 (d, J = 6.4 Hz, 1H, CH), 6.6–7.4 (6H, m, aromatic); 13C-NMR (75 MHz, CDCl3): 28.4, 35.1, 56.1, 56.1, 91.3, 104.0, 112.0, 126.9,127.4 128.9, 133.4, 128.9, 135.2, 131.0, 147.2, 147.8, 152.2, 157.2; EI–MS (m/z): 330 (M+1); C18H16ClNO3; calculated: C = 65.56, H = 4.89, N = 4.25; found: C = 65.58, H = 4.87, N = 4.26%.
3-(2,6-Dichlorophenyl)-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]isoxazole (4i)
IR: (KBr) cm−1: 3042 (CH), 1320 (C–N), 786 (C–Cl); 1H-NMR (DMSO-d6) ppm: 3.0–3.2 (2H, m, CH2), 3.82 (6H, s, OCH3), 3.97–4.0 (1H, m, CH), 5.17 (d, J = 6.4 Hz, 1H, CH), 6.6–7.5 (5H, m, aromatic); 13C-NMR (75 MHz, CDCl3): 28.4, 35.1, 56.1, 56.1, 91.3, 104.0, 112.0, 127.8,128.9,130.0, 130.0,133.8, 134.8, 131.2, 147.2, 147.8, 152.2, 157.2; EI–MS (m/z): 364 (M+1); C18H15Cl2NO3; calculated: C = 59.36, H = 4.15, N = 3.85; found: C = 59.34, H = 4.13, N = 3.83%.
3-(4-Nitrophenyl)-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]isoxazole (4j)
IR:(KBr) cm−1:3042 (CH), 1320 (C–N); 1H-NMR (DMSO-d6) ppm: 3.0–3.2 (2H, m, CH2), 3.82 (6H, s, OCH3), 3.97–4.0 (1H, m, CH), 5.17 (d, J = 6.4 Hz, 1H, CH), 6.6–7.7 (6H, m, aromatic); 13C-NMR (75 MHz, CDCl3): 28.4, 35.1, 56.1, 56.1, 91.3, 104.0, 112.0, 124.0, 124.0, 129.0, 129.0, 131.2, 144.6, 145.2,147.2, 147.8, 152.2, 157.2; EI–MS (m/z): 341 (M+1); C18H16N2O5; calculated: C = 63.53, H = 4.74, N = 8.23; found: C = 63.51, H = 4.76, N = 8.26%.
3-(2-Furyl)-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]isoxazole (4k)
IR: (KBr) cm−1: 3042 (CH), 1320 (C–N); 1H-NMR (DMSO-d6)ppm: 3.0–3.2 (2H, m, CH2), 3.82 (6H, s, OCH3), 3.97–4.0 (1H, m, CH), 5.17 (d, J = 6.4 Hz, 1H, CH), 6.6–7.4 (2H, m, aromatic), 7.2–7.8 (3H, m, furyl); 13C-NMR (75 MHz, CDCl3): 28.4, 35.1, 56.1, 56.1, 91.3, 104.0, 110.2, 110.2, 112.0, 131.2, 141.5, 147.2, 147.8, 152.2, 153.5, 157.2; EI–MS (m/z):286 (M+1); C16H15NO4; calculated: C = 67.36, H = 5.30, N = 4.91; found: C = 67.35, H = 5.28, N = 4.90%.
6,7-Dimethoxy-3-(2-thienyl)-3a,4-dihydro-3H-indeno[1,2-c]isoxazole (4l)
IR: (KBr) cm−1: 3042 (CH), 1320 (C–N); 1H-NMR (DMSO-d6) ppm: 3.0–3.2 (2H, m, CH2), 3.82 (6H, s, OCH3), 3.97–4.0 (1H, m, CH), 5.17 (d, J = 6.4 Hz, 1H, CH), 6.6–7.0 (2H, m, aromatic), 7.2–7.5 (3H, m, thiophenyl); 13C-NMR (75 MHz, CDCl3): 28.4, 35.1, 56.1, 56.1, 91.3, 104.0, 112.0, 124.9, 125.1, 126.4, 131.2, 142.9, 147.2, 147.8, 152.2, 157.2; EI–MS (m/z):302 (M+1); C16H15NO3S; calculated: C = 63.77, H = 5.02, N = 4.65; found: C = 63.75, H = 5.00, N = 4.66%.
3-(4-Bromophenyl)-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]isoxazole (4m)
IR: (KBr) cm−1: 3042 (CH), 1320 (C–N); 1H-NMR (DMSO-d6) ppm: 3.0–3.2 (2H, m, CH2), 3.82 (6H, s, OCH3), 3.97–4.0 (1H, m, CH), 5.17 (d, J = 6.4 Hz, 1H, CH), 6.6–7.2 (6H, m, aromatic); 13C-NMR (75 MHz, CDCl3): 28.4, 35.1, 56.1, 56.1, 91.3, 104.0, 112.0, 120.4, 128.2, 128.2, 131.2, 131.7, 137.5, 147.2, 147.8, 152.2, 157.2, 160.2; EI–MS (m/z): 374 (M+1); C18H16BrNO3; calculated: C = 57.77, H = 4.31, N = 3.74; found: C = 57.74, H = 4.29, N = 3.76%.
4-(6,7-Dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]isoxazol-3-yl)-2-benzonitrile (4n)
IR:(KBr) cm−1: 3042 (CH), 1320 (C–N); 1H-NMR (DMSO-d6) ppm: 3.0–3.2 (2H, m, CH2), 3.82 (6H, s, OCH3), 3.97–4.0 (1H, m, CH), 5.17 (d, J = 6.4 Hz, 1H, CH), 6.6–7.2 (6H, m, aromatic); 13C-NMR (75 MHz, CDCl3): 28.4, 35.1, 56.1, 56.1, 91.3, 104.0, 109.9, 112.0, 118.6, 127.3, 127.3, 131.2, 132.3, 132.3, 142.8, 147.2, 147.8, 152.2, 157.2; EI–MS (m/z): 321 (M+1); C19H16N2O3; calculated: C = 71.24, H = 5.03, N = 8.74; found: C = 71.22, H = 5.01, N = 8.72%.
4-(6,7-Dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]isoxazol-3-yl)-2-methoxyphenol (4o)
IR: (KBr) cm−1: 3042 (CH),1320 (C–N); 1H-NMR (DMSO-d6) ppm: 3.0–3.2 (2H, m, CH2), 3.82 (9H, s, OCH3), 3.97–4.0 (1H, m, CH), 5.17 (d, J = 6.4 Hz, 1H, CH), 5.6 (1H, s, OH), 6.6–7.3 (5H, m, aromatic); 13C-NMR (75 MHz, CDCl3): 28.4, 35.1, 56.1, 56.1, 56.1, 91.3, 104.0, 109.9, 110.8, 112.0, 120.7, 132.1, 142.8, 146.0, 147.6, 147.2, 147.8, 152.2, 157.2; EI–MS (m/z):342 (M+1); C19H19NO5; calculated: C = 66.85, H = 5.61, N = 4.10; found: C = 66.83, H = 5.59, N = 4.08%.
Results and discussion
3-(Substituted aryl)-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]isoxazole analogues (4a–o) described in this study are shown in and a reaction sequence for the preparation is outlined in . In the initial step, 5,6-dimethoxy-2-[(E)-1-arylmethylidene]-1-indanone was synthesized by condensing 5,6-dimethoxy-1-indanone with appropriate aromatic aldehyde in dilute methanolic sodium hydroxide solution at room temperature. The product 5,6-dimethoxy-2-[(E)-1-phenylmethylidene]-1-indanone obtained in the first step is treated with hydroxylamine hydrochloride in the presence of glacial acetic acid to get titled compounds in 62–84% yield after recrystallization with ethanol. The purity of the compounds was checked by TLC and elemental analyses. Both analytical and spectral data (1H-NMR, IR) of all the synthesized compounds were in full agreement with the proposed structures. The elemental analysis results were within ±0.4% of the theoretical values.
Table 1. Physical constants and antimycobacterial activity of the synthesized compounds.
The synthesized compounds (4a–o) were tested for their antimycobacterial activity in vitro against MTB and INHR-MTB by agar dilution method using double-dilution technique similar to that recommended by the National Committee for Clinical Laboratory Standards.Citation14 The INHR-MTB clinical isolate was obtained from Tuberculosis Research Center, Alwar, India. The MIC was defined as the minimum concentration of compound required to inhibit 90% of bacterial growth and MICs of the compounds were reported in with standard drug INH for comparison.
Among the 15 compounds synthesized eight compounds were found to be most active compounds with minimum inhibitory concentration of <6 μΜ and were more active than INH against MTB. Compounds with electron withdrawing group substituted on the aryl ring were showing better activity. Among the 15 newly synthesized compounds, compound 6,7-dimethoxy-3-(4-chloro phenyl)-4H-indeno[1,2-c]isoxazole (4b) was found to be the most active agent against MTB H37Rv and INHR-MTB with minimum inhibitory concentration of <0.30 μΜ. When compared with INH, compound (4b) was found to be 3.32- and 33.4-fold more active against MTB and INHR-MTB, respectively. Following these compounds, 2-chlorophenyl (4h) and 2,6-dichlorophenyl (4i) substituents were found to be more active than INH against MTB H37Rv and INHR-MTB with MIC of 0.78 μΜ, 1.72 μΜ, and 1.28 μΜ, 3.96 μΜ, respectively. However, the electron withdrawing group such as 4-cholrophenyl, 2-chlorophenyl, 2,6-dichlorophenyl, thiophenyl, furyl, and 4-nitrophenyl substituted analogues showed good to excellent inhibitory activity against MTB H37Rv and moderate to good activity against INHR-MTB. On the other hand, the electron donating group containing analogues, such as (OCH3) group substituted 4-methoxy phenyl (4a), 3,4 dimethoxy phenyl (4e), 3,4,5 trimethoxy phenyl (4f), and 3-hydroxyl, 4-methoxy substituted compound (4o), and electron withdrawing group substituted 4-dimethylaminophenyl (4c) and 4-flurophenyl (4g) showed moderate anti-tubercular activity. These reports clearly show that the substitution of electron withdrawing group such as 4-chloro makes remarkable improvement in antimycobacterial activity.
All the compounds were tested for cytotoxicity (IC50) in VERO cells at concentrations of 62.5 μg/ml or 10×. After 72 h of exposure, viability was assessed on the basis of cellular conversion of 3-(4,5-dimethylthiazol-2-yl)2,5-diphenyl tetrazolium bromide (MTT) into a formazan product using the Promega Cell Titer 96 non-radioactive cell proliferation method.Citation15 Most of the active compounds were found to be nontoxic up to the concentration of 62.5 μg/ml.
Conclusion
The screening of all the isoxazole derivatives identified novel compounds that are endowed with antimycobacterial activity. It is conceivable that derivatives showing more potency, selectivity, and low toxicity make them excellent leads for synthesizing novel derivatives for antimycobacterial activity against MTB and INHR-MTB. Also, these derivatives can be further modified to exhibit better potency than the standard drugs. Further studies are ongoing in our laboratory to acquire more information about quantitative structure–activity relationships and MDR. The isoxazole derivatives discovered in this study may provide valuable therapeutic intervention for the treatment of anti-tubercular diseases.
Acknowledgements
The authors thank Institute for Research in Molecular Medicine, Universiti of Sains Malysia, Pennag, Malaysia and Alwar Pharmacy College, Alwar, Rajasthan, India, for providing research facilities.
Declaration of interest
The authors declare no conflicts of interest.
References
- Kamr AG, Abdel-Latif NA, Abdalla, MM. Synthesis and antiandrogenic activity of some new 3-substituted androstano[17,16-c]-5′-aryl-pyrazoline and their derivatives. Bioorg Med Chem 2007;15:373–384.
- Ruhoglu O, Ozdemir Z, Calis U, Gümüsel B, Bilgin AA. Synthesis of and pharmacological studies on the antidepressant and anticonvulsant activities of some 1,3,5-trisubstituted pyrazolines. Arzneimittelforschung 2005;55:431–436.
- Shafiee A, Bagheri M, Shekarchi M, Abdollahi M. The antinociceptive activities of 1-(4-aryl-2-thiazolyl)-3,5-disubstituted-2 pyrazolines in mouse writhing test. J Pharm Pharm Sci 2003;6:360–362.
- Berghot MA, Moawad EB. Convergent synthesis and antibacterial activity of pyrazole and pyrazoline derivatives of diazepam. Eur J Pharm Sci 2003;20:173–179.
- Palaska E, Aytemir M, Uzbay IT, Erol D. Synthesis and antidepressant activities of some 3,5-diphenyl-2-pyrazolines. Eur J Med Chem 2001;36:539–543.
- Turan-Zitouni G, Ozdemir A, Güven K. Synthesis of some 1-[(N, N-disubstituted thiocar bamoylthio)acetyl]-3-(2-thienyl)-5-aryl-2-pyrazoline derivatives and investigation of their antibacterial and antifungal activities. Arch Pharm (Weinheim) 2005;338:96–104.
- Jeong TS, Kim KS, An S, An SJ, Cho KH, Lee S et al. Novel 3,5-diaryl pyrazolines as human acyl-CoA:cholesterol acyltransferase inhibitors. Bioorg Med Chem Lett 2004;14:2715–2717.
- Foks H, Pancechowska-Ksepko D, Janowiec M, Zwolska Z, Augustynowicz-Kopec E. Synthesis and tuberculostatic activity of some (4-phenylpiperazin-1-ylmethyl)-1,3,4-oxadiazole and (4-phenylpiperazin-1-ylmethyl)-1,2,4-triazole derivatives. Acta Pol Pharm 2004;61:473–476.
- Bethge K, Pertz HH, Rehse K. New oxadiazole derivatives showing partly antiplatelet, antithrombotic and serotonin antagonistic properties. Arch Pharm (Weinheim) 2005;338:78–86.
- Ali MA, Shaharyar M, Siddiqui AA. Synthesis, structural activity relationship and anti-tubercular activity of novel pyrazoline derivatives. Eur J Med Chem 2007;42:268–275.
- Ali MA, Shaharyar M. Oxadiazole mannich bases: synthesis and antimycobacterial activity. Bioorg Med Chem Lett 2007;17:3314–3316.
- Yar MS, Ali MA, Bakht MA, Velmurugan S. Synthesis, antimycobacterial activity of 4-[5-(substituted phenyl)-4, 5-dihydro-3-isoxazolyl]-2-methylphenol. J Enzyme Inhib Med Chem 2008;23:432–436.
- Yar MS, Siddiqui AA, Ali MA, Murugan V, Chandrashekhar R. Synthesis and cytotoxic activity of novel pyrazoline derivatives against human lung tumor cell line (A549). J Chin Chem Soc 2007;54:81–86.
- Heifets LB, Flory MA, Lindholm-Levy PJ. Does pyrazinoic acid as an active moiety of pyrazinamide have specific activity against Mycobacterium tuberculosis? Antimicrob Agents Chemother 1989;33:1252–1254.
- Gundersen LL, Nissen-Meyer J, Spilsberg B. Synthesis and antimycobacterial activity of 6-arylpurines: the requirements for the N-9 substituent in active antimycobacterial purines. J Med Chem 2002;45:1383–1386.