Abstract
Four mixed ligand gold(I) complexes with the thioamides 2-mercapto-thiazolidine (mtzdH), 2-mercapto-benzothiazole (mbztH) and 5-chloro-2-mercapto-benzothiazole (ClmbztH) and triphenylphosphine (tpp) of formulae [Au(tpp)Cl] (1) [Au(tpp)(mtzd)] (2), [Au(tpp)(mbzt)] (3) and [Au(tpp)(Clmbzt)] (4), already known, were used to study their mechanism of inhibition activity towards the catalytic oxidation of linoleic acid to hydroperoxylinoleic acid by the enzyme lipoxygenase (LOX), kinetically and theoretically. The results are compared to those of cisplatin. In addition, the anticancer cell screening results against leimyosarcoma (LMS) cells have shown that 2-4 complexes were more active than cisplatin. The uptake of complexes in LMS cells were also studied with electrospray ionisation mass spectrometry spectroscopy.
Introduction
Gold thiolates are used clinically against rheumatoid arthritisCitation1. Auronofin (triethylphosphine-gold(I) thioglugose (Et3PAuTg)) and its second generation drug Ridaura TM (triethylphosphine(2,3,4,6-tetra-acetyl-glycopyrasonato-S-)gold(I)), an extensively gold(I) anti-arthritic drug in use, were found to be highly cytostatic to tumour cells and active against i.p. P388 leukaemiaCitation2. Many gold(III) and gold(I) compounds were tested for their anti-tumour activity against various cancerous cell linesCitation3 and the results also have been, reviewedCitation4. A reason for the anti-tumour investigation of gold compounds is related to the square-planar geometry, also found for platinum in cisplatin, since gold(III) is isoelectronic with platinum(II)Citation2–4. Given the generally reducing mammalian environment, compounds containing gold(III) may be expected to be reduced in vivo to gold(I) and this led to the investigation of possible anti-tumour properties of gold(I) compoundsCitation2–4.
The relationship between polyunsaturated fatty acid (such as linoleic or arachidonic acids) metabolism by enzymes like lipoxygenase (LOX) or cyclooxygenase (COX), inflammation and carcinogenesis, has been extensively examined in numerous pharmacological studiesCitation5–7. The lipid peroxides derived from fatty acids by this metabolism can regulate cellular proliferation or apoptosisCitation5. Thus LOX or COX inhibition provides a potential novel targets for the treatment and chemoprevention for a number of different cancersCitation5–7.
In the course of our studies on metalotherapeuticsCitation8–11 we have previouslyCitation1 synthesised and structurally characterised new gold(I) mixed ligand complexes of [Au(tpp)Cl] (1) (triphenylphosphine (tpp)) with the thioamides 2-mercapto-thiazolidine (mtzdH), 2-mercapto-benzothiazole (mbztH) and 5-chloro-2-mercapto-benzothiazole (ClmbztH) of formulae [Au(tpp)(mtzd)] (2), [Au(tpp)(mbzt)] (3) and [Au(tpp)(Clmbzt)] (4)Citation1. The mechanism of inhibition activity of complexes 1–4 towards the catalytic oxidation of linoleic acid to hydroperoxylinoleic acid by the enzyme LOX was subsequently studied by means of kinetic and theoretic studies. A comparison with the corresponding data of cisplatin is also made. In addition, the anticancer cell screening results against leimyosarcoma (LMS) cells have shown that 2–4 complexes were more active than cisplatinCitation1. The uptake of complexes in LMS cells was also studied utilising electrospray ionisation mass spectrometry (ESI-MS) spectroscopy.
Experimental
Synthesis of compounds [Au(tpp)Cl] (1), [Au(tpp)(mtzd)] (2), [Au(tpp)(mbzt)] (3) and [Au(tpp)(Clmbzt)] (4)
The synthesis of complexes 1–4 were achieved by the method described previouslyCitation1. In summary, Au(I) complexes 2, 3, and 4 were synthesised by reacting 1 mmol of [Au(tpp)Cl]Citation12 (1) with 1 mmol of the appropriate thioamide 2-mercapto-thiazolidine (mtzdH), 2-mercapto-benzothiazole (mbztH) or ClmbztH in the presence of an equimolar amount of KOH, in methanol/water solution. The precursor [Au(tpp)Cl] (1) was derived from the reaction between 1 mmol tpp with 0.5 mmol of HAuCl4 solution (1 mL, 0.5 M) in 10 mL methanolCitation1. The elemental analysis, proton nuclear magnetic resonance (1H NMR) and Fourier transform infrared (IR) spectroscopic data of these complexes were identical with those given previouslyCitation1.
[1] m.p. 236–240°C. Found: C, 43.14%; H, 2.98%. Calc. for C18H18AuPCl: C, 43.70%; H, 3.06%. IR (KBr): 3433.22 w, 3053.33, 1479.10, 1435.44 s, 1179.39, 1101.89 s, 998.65, 748.00 s, 712.70, 692.90 vs. 546.65 s, 502.31 s. Far-IR (polyethylene): 330.12 vs.171.89 s. 1H NMR (CDCl3) δ 7.55 m, H(Ar).
[2] m.p. 160–161°C. Found: C, 43.98%; H, 3.26%; N, 2.64%; S, 10.91%. Calc. for C21H17AuNPS2: C, 43.83%; H, 2.98%; N, 2.43%; S, 11.14%. IR (KBr): 3053.33, 2915.55 m, 1554.96 vs. 1478.99, 1435.57 vs. 1303.02, 1181.67, 1100.53 s, 1022.00, 996.65 s, 963.11 s, 917.49 s, 750.24 s, 709.30, 692.08 vs. 631.68, 538.10 vs. 502.53 s. Far-IR (polyethylene): 279.29 vs. 245.94 s, 151.08 s, 179.64, 99.43 vs. 75.48 vs. 1H NMR (CDCl3) δ 7.50 m, H(Ar(Ph3P)); 4.28 m H4(CH2), 3.38 m, H5(CH2).
[3] m.p. 157–161°C. Found: C, 48.33%; H, 3.18%; N, 2.39%; S, 9.90%. Calc. for C25H19AuNPS2: C, 48.00%; H, 3.06%; N, 2.24%; S, 10.25%. IR (KBr): 3048.88, 1478.62, 1433.85 s, 1417.03 vs. 1308.59, 1272.59, 1234.89, 1099.85 s, 1073.00, 993.17, 971.66 vs. 757.12 s, 709.30, 691. 50 vs. 538.59 vs. 501.65 s. %. Far-IR (polyethylene): 279.94 s, 178.48, 89.30 m, 38.95 vs.1H NMR (CDCl3) δ 7.60 m, H(Ar(Ph3P)); 7.25 m, H(Ar(BZT)).
[4] m.p. 88–91°C. Found: C, 45.33%; H, 2.98%; N, 2.13%; S, 9.74%. Calc. for C25H18AuNClPS2: C, 45.50%; H, 2.75%; N, 2.12%; S, 9.72%. IR (KBr): 3051.54, 1479.24, 1435.50 s, 1408.97 vs. 1308.08 m, 1101.22 s, 1065.82, 998.38 vs. 790.99, 750.13 s, 693.40 vs. 537.85 s, 503.93 s. Far-IR (polyethylene): vs. 279.11 s, 170.88 s, 88.85 s, 70.09 s, 54.29 s. 1H NMR (CDCl3) δ 7.56 m, H(Ar(Ph3P)); 7.14 m, H(Ar(CBZT)).
Colony efficiency capacity
Cells were seeded into 24-well plates at a density of 2 × 104 cells per well. After 24 h of incubation the cells were treated with media containing various concentrations of complexes 1 and 2 in dimethyl sulfoxide (DMSO). Control plates had only the DMSO in the same doses to those added to the test well. After 48 h of incubation, the cells were washed with phosphate buffer saline (PBS) and they were treated by brief trypsinisation (250 μL of trypsin—incubation at 37°C, 95% O2, 5% CO2). Then, 750 μL of Dulbecco’s modified eagle medium (DMEM) were added, cells were counted using the Neubauer cytometer and 5 × 103 cells from each well were seeded in Petri dishes. DMEM was added at a total volume of 10 mL. After 7 days of incubation, cells were washed with 10 mL of PBS and 10 mL of methanol/acetic acid (3:1) solution was added. Petri dishes were put at −20°C for 10 min. The methanol/acetic acid solution was removed and dishes were left to dry. Ten millilitre of Giemsa dye (diluted in water at a ratio 1:10) were added and left for 20 min in order to stain the colonies. Dishes were washed at least twice and left to dry. Finally, the stained blue colonies were counted in all Petri dishes.
Tumour cell screening
Measurements of in vitro cells toxicity have been carried out in preliminary repetitions. Cell proliferation/survival was measured (%) at 24 and 48 h of exposure. Cell lines were maintained in DMEM with 10% foetal calf serum, incubated at 37°C, 5% CO2. The tested compounds were each weighted and dissolved to equal a 0.01 M solution. From this solution a dilution was made into the test range concentration of 0.5 μM to 30 μM. Exponentially growing cancer cells were seeded in 3.5 cm, six well culture plates at 50 000/mL and allowed a 24-h doubling time then treated with 100 μL of various concentrations of tested compounds. After 24 and 48 h the cells were washed with buffer solutions, detached from support with trypsin-ethylenediaminetetraacetic and counted on haemocytometer (Bauer slide).
Cellular uptake studies
Cells were grown until at least 70% confluency in Petri dishes. Stock solutions of the gold complexes in DMSO were freshly prepared and diluted with cell culture medium to the desired concentration (1: 8, 20 and 30 μM 2: 0.6, 1.2 and 2 μM). The cell culture mediums were incubated at 37°C/5% CO2 for 48 h. DMEM solution was removed and the culture was washed by PBS. The cell pellets were isolated by trypsinisation and centrifugation (room temperature, 3000 rpm, 10 min), in DMEM (initially) and in PBS twice afterwards and lysed by ultrasonic in 30% actetonitrile solution in double distil water which contains formic acid (0.7% v/v). The aliquot was isolated by centrifugation. The determination of the gold content of the samples was performed by ESI-MS. Results were calculated from the data of 2–3 independent experiments.
Study of LOX inhibition mechanism and computational details
The procedure is similar to that previously reportedCitation9,Citation13 and includes the following protocol:
Solutions
The buffer used for all experiments was 0.2 M Boric Acid at pH 9: 0.1 mol boric acid (H3BO3, 6.18 gr) was added to 300 cm3 distilled water. The pH was adjusted to 9 with 50% w/v NaOH. Finally the solution was diluted to 500 cm3. Substrate solution of linoleic acid was prepared as described below: 0.05 cm3 of linoleic acid was dissolved in 0.05 cm3 95% ethanol in a volumetric flask (50 cm3). The appropriate volume of H2O was gradually added in the flask. 5 cm3 of the solution prepared was added to 30 cm3 of the borate buffer. Enzyme solution (LOX from soybean type I-B, lyophilised powder, 48 000 units/mg solid (Aldrich)): a solution of 10 000 units of enzyme for per cm3 of borate buffer (1.93 μM) was prepared in ice cold bath. An amount of 500 units for every 3 cm3 of reaction mixture is used in every experiment. A unit of LOX causes and increase in absorption at 234 nm equal to 0.001 per minute.
Procedure
Enzyme activity was monitored by UV analysis. 0.05 cm3 of enzyme solution was added to a cuvette containing 2 cm3 linoleic acid solution and the appropriate amounts of buffer and inhibitor solutions in thermostatic water bath at 25°C. There was no pre-incubation time of the enzyme with inhibitor solution. The activity of the enzyme was determined by monitoring the increase in the absorption caused by the oxidation of linoleic acid at 234 nm and 25°C (ϵ = 25 000 M−1 cm−1). One standard solution of complexes in DMSO (10−2 M) was used for the inhibition activity experiments (three sets). In this case, the substrate concentration was kept constant (0.3 mM), while the amounts of buffer and inhibitor solutions varied according to the inhibitor final concentration needed (0.5–30 μM or 0.15–9 μL from standard solutions). The total experiment volume was 3 cm3.
For Km and Vmax determination experiments (three sets of experiments), the concentration of inhibitor was kept constant (at concentrations equal with their IC50 values from a standard solution of 10−2 M in DMSO) and the substrate concentrations used were 0.35, 0.30, 0.25, 0.2, 0.15, 0.10, 0.05, 0.01 mM. All solutions were kept at thermostatic water bath at 25°C, except from the enzyme solution that was kept at ice cold bath (0°C).
Computational methods—docking study
The three dimensional coordinates of LOX were obtained through the internet at the Research Collaboratory for Structural Bioinformatics Protein Data BankCitation14 and of the complexes by X-ray diffraction analysis.
All solvent molecules within the protein structure were removed prior to the docking procedure. Molecular docking was performed with the grid based version of the MolDock Score functionCitation15 as this is implemented in Molegro Virtual Docker software (http://www.molegro.com). Ground state geometry optimisations based on the X-ray structures of the complexes were carried out using the PM3 parametrisation schemeCitation16 in order to confirm that no significant divergence in the conformations of the complexes due to crystal packing effects. The docked derivatives were scored and reranked using the ‘reranking score’ scheme.
Results and discussion
General aspects
Based on the crystal structures of the complexesCitation1 the molecular diagrams of the 1–4 are shown in .
Tumour cells screening
Complexes 1–4 and HAuCl4 were previously tested for cytotoxic activity against LMS cells from the Wistar rats, polycyclic aromatic hydrocarbons (benzo[a]pyrene) carcinogenesisCitation1. The IC50 values of complexes 1–4 were: 1: inactive at the concentrations tested, 2: 0.8 ± 0.4, 3: 2.1 ± 1.3 and 4: 1.1 ± 0.5 μM, while HAuCl4 was found to be inactive against leiomyosarcoma cellsCitation1. The corresponding activity of cisplatin towards LMS cells is 4.6 μMCitation17. Thus, complexes 2–4 were more active than cisplatin.
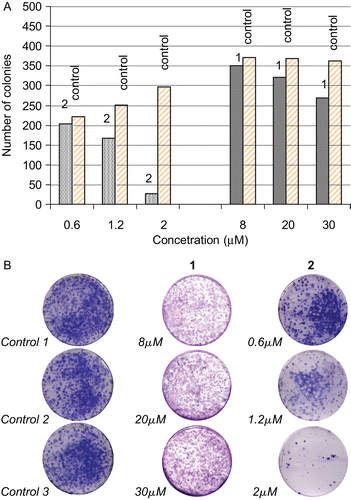
Colony efficiency capacity of LMS cells in the absent (control) and after treatment with various concentrations of complexes 1 and 2 shows that only 2 exhibit the ability to inhibit malignant cells growth proliferation, while no such activity was observed by complex 1 (). shows that LMS cells lose their colony growth efficiency after treatment with 2 at concentration of 2 μM, while they still keep this efficiency when they treated with 1 at concentration higher than 30 μM. This might be due to the non-permeability of LMS cells membrane by complex 1.
Figure 2. (A) Numbers of leimyosarcoma (LMS) cells colonies growth after their treatment with various concentrations of 1 or 2 in contrast to numbers of LMS cells colonies growth from the untreated ones. (B) Colony efficiency of LMS cells in the absent (control) and after treatment with various concentrations of complexes 1 and 2.
Inhibition of LOX
LOX are non-haeme iron dioxygenases that catalyse the incorporation of dioxygen into polyunsaturated fatty acids containing one or several cis,cis-pentadiene systems (e.g., linoleic acid). Oxidative stress and carcinogenesis are associated with uncontrolled LOX activityCitation5–7, making these enzymes important pharmacological targets. Linoleic acid on the other hand, was proven to be a potential mutagen inhibitorCitation18. LOX inhibitory activity of complexes 1–4 were determined spectrophotometrically by measuring the increase in absorbance at 234 nm for the oxidation of linoleic acid. The reaction mixture contained the test compounds, dissolved in DMSO at concentrations of 0.5–30 μM, or the solvent (control), linoleic acid 300 μM and enzyme solution (50 μL), in borate buffer (pH = 9.0) with a total volume of 3 mL. shows LOX activity inhibitions caused by 4 in various concentrations. compares the inhibitory effect of complexes 1–4 in various concentrations.
Figure 3. (A) lipoxygenase (LOX) activity inhibition caused by 4 at concentrations of 0, 5, 15, and 20 μM. (B) Activity of LOX after 10 min of the oxidation of linoleic acid in the presence of different concentrations of complexes 1–4 used to determine IC50 values graphically.
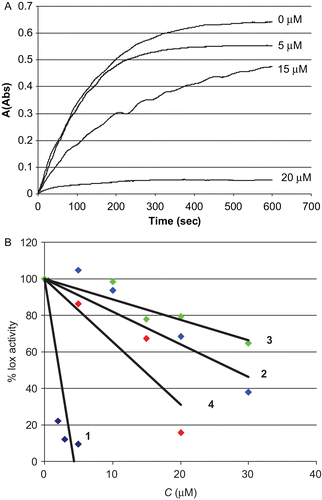
It is shown that the catalytic activity of LOX decreased significantly in the presence of low concentrations (about 1–30 μM) of these complexes in contrast to cisplatin which shows no such activityCitation9. Surprisingly, among gold(I) complexes 1–4, complex 1 with no cytotoxic activity in the concentrations tested, exhibits the strongest inhibitory activity against LOX. (IC50 values found: 1.2 (1), 18.8 (2), 39.4 (3) and 16 (4) μM, respectively ).
A series of experiments were performed in order to determine the reversibility of inhibition. Reversible type of inhibition was proved by steady-state kinetics at various concentrations of enzyme (25, 50, 75 μL) and substrate (0.01, 0.20, 0.35 mM) in the presence of the complex 1 in constant concentration (IC50 = 1.2 μM). The plot of Vmax vs. [E] shows reversible inhibition type of complex 1 towards LOX.
The respective kinetic parameters (Km and Vmax) were evaluated by performing series of experiments with various substrate concentrations (0.35, 0.30, 0.25, 0.2, 0.15, 0.10, 0.05, 0.01 mM) in the presence of the complexes at constant concentrations equal with their IC50 valuesCitation19. The experimental data were processed by a graphical method in Lineweaver–Burk coordinates (double reciprocal method). The kinetic parameters were estimated from the slope and intercept of the line. The Km of the enzyme is 0.035 mM with υmax of 27.5 mM/ sCitation19, while the corresponding apparent Km values for complexes 1–3 are found to be higher (0.06 (1), 0.18 (2) and 0.45 (3) mM/s, respectively) with υmax values to be lower than the corresponding ones of the free enzyme (0.7 (1), 3.4 (2) and 9.7 (3) mM/s, respectively). Thus, the compounds studied inhibit the enzyme with a mixed inhibition mechanismCitation20. In this mechanism either enzyme-inhibitor (EI) or enzyme-substrate-inhibitor (ESI) complex could be formedCitation16.
Computational methods—docking study
In order to investigate the complex–protein interactions we performed computational molecular docking studies for the complexes. The binding energy (E) of the substrate (S = linoleic acid) to its binding site in the enzyme LOX (E) when enzyme-subtrate (ES) complex formed, is E = −23.94 kcal/mol. The corresponding binding energies of inhibitors (I), in EI are calculated to be −17.92 (1), −24.14 (2), −24.05 (3) and −24.62 (4) kcal/mol, respectively, while the binding energies of ESI are found to be −17.62 (1), −17.50 (2), −19.86 (3) and −23.68 (4) kcal/mol. Thus, according to the E values of ES in contrast to those of EI and ESI, only EI complexes in case of inhibitors 2, 3, and 4 are more stable than ES. This is in accordance with the mixed mechanism found experimentally for 2–3 (see above) where at least one of ESI or EI complexes could be formed. However, in case of 1 the energies of both complexes ESI and EI are higher than that of ES indicating instability of both ESI and EI complexes, in contrast to the high experimental inhibitory activity shown by 1 towards LOX (IC50 = 1.6 μM; see above).
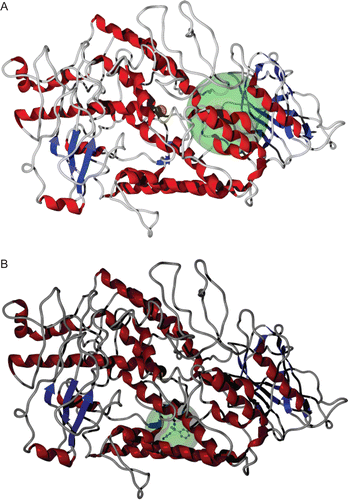
shows binding sites of LOX for inhibitors 2–4 in ESI complexes, while the docking area of 1. Complexes 2–4 docked in the binding pocket calculated for the inhibitors of LOXCitation9, in contrast to 1 which docked away from this area.
Cellular uptake studies
The LOX inhibitory activity of the complexes is directly correlated with their cytotoxic activity in case of complexes 2–4Citation5–7. However, an inconsistency is observed for 1. This prompted us to investigate the cellular uptake of complexes 1 and 2. Stock solutions of the gold complexes in DMSO were freshly prepared and diluted with cell culture medium to the desired concentration (1: 8, 20, and 30 μM 2: 0.6, 1.2, and 2 μM). The cell pellets were isolated by trypsinisation and centrifugation and lysed. The determination of the gold species was performed by ESI-MS spectroscopy. Gold species were detected only in case of the intracellular sample of 2 at 2 μM (m/z: 577.9 [Au(tpp)(mtzd)]+), while no such species were detected in case of 1. Therefore, in case of 1 the lower cytotoxic activity in contrast to the high LOX inhibitory activity observed migth be attributed to the non-permeability of cell membrane by 1 towards 2, which is also concluded by collony efficiency results (see above).
Conclusions
Since the metabolism of polyunsaturated fatty acid is known to be related with inflammation and carcinogenesisCitation5–7, inhibition of this mechanism is expected to be a potential novel target for metallotherapeutics. This is further supported by the biological activities found for complexes studied. Complexes 1–4 were tested for their LOX inhibitory activity on the oxidation of linoleic acid to its hyperoxo derivative. Thus, 1–4 inhibit LOX activity with low IC50 values (1.6–38 μM, while complexes 2–4 were found to inhibit strongly the LMS cells proliferation. However, 1 which shows high LOX inhibitory activity exhibit very low cytotoxicity against LMS. Cellular uptake studies of 1 and 2 shows that gold species were detected only in case of the intracellular sample of 2, while no such species were detected in case of 1. This might be attributed to the non-permeability of cell membrane by 1 towards 2 which results in the low inhibitory activity of 1 towards LMS cells proliferation although it is also exhibit high LOX inhibitory activity.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Kouroulis KN, Hadjikakou SK, Kourkoumelis N, Kubicki M, Male L, Hursthouse M, Skoulika S, Metsios AK, Tyurin VY, Dolganov AV, Milaeva ER, Hadjiliadis N. Synthesis, structural characterization and in vitro cytotoxicity of new Au(III) and Au(I) complexes with thioamides. Dalton Trans 2009:10446–10456.
- Vicente J, Chicote MT, Rubio C. Complexes with S-donor ligands, 4. Mono- and di-homo- and -hetero-nuclear gold(I), silver(I), and palladium(II) complexes with 2-(methylthio)pyridine, pyridine-2(1h)-thione, and benzoxazole-2(3h)-thione. Some examples of the use of complexes as ligands. Chem Ber 1996;129:327–330.
- Ronconi L, Giovagnini L, Marzano C, Bettìo F, Graziani R, Pilloni G, Fregona D. Gold dithiocarbamate derivatives as potential antineoplastic agents: design, spectroscopic properties, and in vitro antitumor activity. Inorg Chem 2005;44:1867–1881.
- Tiekink ERT. Gold derivatives for the treatment of cancer. Cr Rev Oncol-Hem 2002;42:225–248.
- Pidgeon GP, Lysaght J, Krishnamoorthy S, Reynolds JV, O’Byrne K, Nie D, Honn KV. Lipoxygenase metabolism: roles in tumor progression and survival. Cancer Metastasis Rev 2007;26:503–524.
- Cuendet M, Pezzuto JM. The role of cyclooxygenase and lipoxygenase in cancer chemoprevention. Drug Metabol Drug Interact 2000;17:109–157.
- Klurfeld DM, Bull AW. Fatty acids and colon cancer in experimental models. Am J Clin Nutr 1997;66(Suppl 6):1530S–1538S.
- Lazarou K, Bednarz B, Kubicki M, Verginadis II, Charalabopoulos K, Kourkoumelis N, Hadjikakou SK. Structural, photolysis and biological studies of the bis(μ2-chloro)-tris(triphenylphosphine)-di-copper(I) and chloro-tris(triphenylphosphine)-copper(I) complexes. Study of copper(I)–copper(I) interactions. Inorg Chim Acta 2010;363:763–772.
- Xanthopoulou MN, Hadjikakou SK, Hadjiliadis N, Milaeva ER, Gracheva JA, Tyurin V-Y Kourkoumelis, N, Christoforidis KC, Metsios AK, Karkabounas S, Charalabopoulos K. Biological studies of new organotin(IV) complexes of thioamide ligands. Eur J Med Chem 2008;43:327–335.
- Balas VI, Hadjikakou SK, Hadjiliadis N, Kourkoumelis N, Light ME, Hursthouse M, Metsios AK, Karkabounas S. Crystal structure and antitumor activity of the novel zwitterionic complex of tri-n-butyltin(IV) with 2-thiobarbituric acid bioinorganic chemistry and applications. Bioinorg Chem Appl 2008:654137.
- Abdellah MA, Hadjikakou SK, Hadjiliadis N, Kubicki M, Bakas T, Kourkoumelis N, Simos YV, Karkabounas S, Barsan M, Butler IS. Synthesis, characterization, and biological studies of organotin(IV) derivatives with o- or p-hydroxybenzoic acids bioinorganic chemistry and applications. Bioinorg Chem Appl 2009:542979.
- Al-Sa’ady AK, McAuliffe CA, Parish RV, Sandbank JA. A general synthesis for gold(I) Complexes. Inorg Synth 1985;23:191–194.
- Ozturk I, Filimonova S, Hadjikakou SK, Kourkoumelis N, Dokorou V, Manos MJ, Tasiopoulos AJ, Barsan MM, Butler IS, Milaeva ER, Balzarini J, Hadjiliadis N. Structural motifs and biological studies of new antimony(III) iodide complexes with thiones. Inorg Chem 2010; 49:488–501.
- Protein Data Bank. http://www.rcsb.org/pdb/
- Thomsen R, Christensen MHJ. MolDock: A new technique for high-accuracy molecular docking. J Med Chem 2006;49:3315–3321.
- Stewart JJP. Optimization of parameters for semiempirical methods I. Method. J Comput Chem 1989;10:209–220.
- Hadjikakou SK, Ozturk II, Xanthopoulou MN, Zachariadis PC, Zartilas S, Karkabounas S, Hadjiliadis N. Synthesis, structural characterization and biological study of new organotin(IV), silver(I) and antimony(III) complexes with thioamides. J Inorg Biochem 2008; 102:1007–1015.
- Kreich M, Claus P. Direct conversion of linoleic acid over silver catalysts in the presence of H2: an unusual way towards conjugated linoleic acids. Angew Chem Int Ed Engl 2005; 44:7800–7804.
- Xanthopoulou MN, Hadjikakou SK, Hadjiliadis N, Kubicki M, Karkabounas S, Charalabopoulos K, Kourkoumelis N, Bakas T. Synthesis and characterization of a new chloro-di-phenyltin(IV) complex with 2-mercapto-nicotinic acid: Study of its influence upon the catalytic oxidation of linoleic acid to hydroperoxylinoleic acid by the enzyme lipoxygenase. J Organomet Chem 2006;691:1780–1789.
- Segel IH. Biochemical Calculations, 2nd edn. New York: John Wiley & Sons, Inc., 1976.
![Figure 1. Molecular diagrams of complexes [Au(tpp)Cl] (1) [Au(tpp)(mtzd)] (2), [Au(tpp)(mbzt)] (3) and [Au(tpp)(Clmbzt)] (4).](/cms/asset/a7c20104-bae2-4a2e-abaf-43ad832d82b5/ienz_a_529807_f0001_b.gif)