Abstract
In attempt to make significant pharmacologically active molecule, we report here the synthesis and in vitro antimicrobial and antitubercular activity of various series of 3-(3-pyridyl)-5-(4-nitrophenyl)-4-(N-substituted-1,3-benzothiazol-2-amino)-4H-1,2,4-triazole. The antimicrobial activity of title compounds were examined against two Gram-positive bacteria (Staphylococcus aureus, Streptococcus pyogenes), two Gram-negative bacteria (Escherichia coli, Pseudomonas aeruginosa), and three fungi (Candida albicans, Aspergillus niger, Aspergillus clavatus) using the broth microdilution method and antitubercular activity H37Rv using Lowenstein-Jensen agar method.
Introduction
Tuberculosis (TB) is the leading infectious cause of death in the world today, with ∼3 million deceasing every year. An increase in the global burden of TB with the worldwide mortality rate of 23% is a major concern in the socioeconomic and health sectors.Citation1–5 The synergy of this disease with HIV infection and the emergence of multidrug resistance and extensively drug resistance tuberculosis (MDRTB and XDRTB) pose a threatening global challenge.Citation6–8 Although a number of lead molecules exist today to develop new drugs, no new chemical entity has emerged for clinical use for over the last 45 years in the treatment of this disease.Citation9,Citation10 Therefore, there is an urgent need to develop new drugs, acting through a novel mechanism of action for the chemotherapy of TB.
Recently, certain triazole-based compounds were reported to possess antimicrobial activities.Citation11–13 It is believed that aryl-azolyl-ethane moiety, present in many azole antifungal drugs, serves as pharmacophore in compounds having Mycobacterium killing activity.Citation14,Citation15 Many azole derivatives have also been shown to possess interesting antimycobacterial activity in addition to antifungal activity.Citation16–18 In addition, closer analogues of our oxadiazole were reported to possess antitubercular activity.Citation19,Citation20 It is established that these compounds target the sterol demethylase, a mixed-function oxidase involved in sterol synthesis in eukaryotic organisms.Citation21 The unraveling of Mycobacterium genome sequence has revealed that a protein having homology to one of the above mixed oxidase function is present in Mycobacterium tuberculosis.Citation22 In view of this data, we aimed the synthesis, antimicrobial, and antitubercular evaluation of new substituted 1,2,4-triazole derivatives. We have incorporated triazoles with pyridine and benzothiazole derivatives, which possess wide variety of biological activity.
Experimental section
Chemistry
All chemicals were of analytical grade and use directly. All melting points were determined in PMP-DM scientific melting point apparatus and are uncorrected. The completion of the reaction was checked by TLC using Merck silica gel 60 F254 and spots were visualized under UV radiation. IR spectra were recorded on Perkin-Elmer RX 1 FT-IR spectrophotometer in KBr (γmax in cm−1). 1H NMR spectra were recorded in CDCl3 on a Bruker Avance II 400 NMR spectrometer (400 MHz) using TMS as internal standard (δ in ppm). 13C NMR spectra were recorded in CDCl3 on a Bruker Avance II 400 NMR spectrometer operating at 400 MHz (δ in ppm). The microanalyses were performed on a Heraeus Carlo Erba 1180 CHN analyzer. The mass spectra were recorded on micromass Q-T of micro (TOF MS ES+).
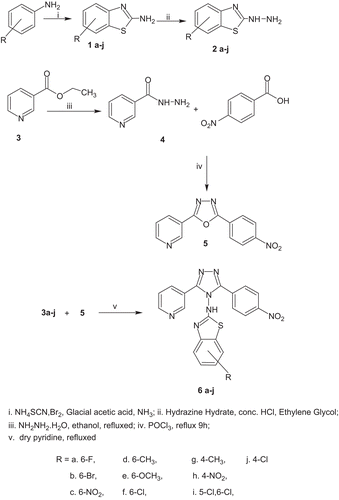
Substituted 2-hydrazino-1,3-benzothiazole (2a–j) was prepared by the literature procedure.Citation23,Citation24
General procedure for synthesis of 2-hydrazino benzothiazoles
(2a–j). Concentrated hydrochloric acid (0.067 mol) was added drop wise with stirring to hydrazine hydrate (0.12 mol) at 5–6°C followed by ethylene glycol (30 mL); thereafter substituted 2-amino-1,3-benzothiazole (1a–j) (20 mmol) was added in portions and the resultant mixture was refluxed for 2–3 h and cooled at room temperature. The reaction progress was monitored by TLC using toluene:ethylacetate (75:25) as mobile phase. The reaction mixture was filtered and resulting precipitate was washed with distilled water. The resulting crude was crystallized from ethanol. The other compounds of the series were prepared by similar procedure.
(2a). Yield 70%: m.p. 204–206°C. IR (KBr): 3435 (NH2), 3200 (NH), 1631 (C=N), 1442 (thiazole) cm−1; 1H NMR (400 MHz, CDCl3) δ 4.83 (s, 2H, NH2, disappeared on D2O exchange), 8.94 (s, 1H, NH, disappeared on D2O exchange), 7.28–7.64 (m, 3H, 3CH) ppm. Anal. calcd. for C7H6N3FS: C, 45.89; H, 3.30; N, 22.94; Found: C, 45.91; H, 3.31; N, 22.98%.
(2b). Yield 61%; m.p. 200–202°C; IR (KBr): 3449 (NH2), 3212 (NH), 1623 (C=N), 1451 (thiazole) cm−1; 1H NMR (400 MHz, CDCl3) δ 4.83 (s, 2H, NH2, disappeared on D2O exchange), 8.90 (s, 1H, NH, disappeared on D2O exchange), 7.63–7.93 (m, 3H, 3CH) ppm. Anal. calcd. for C7H6N3SBr: C, 34.44; H, 2.48; N, 17.21; Found: C, 34.40; H, 2.45; N, 17.19%.
(2c). Yield 67%; m.p. 210–212°C; IR (KBr): 3445 (NH2), 3220 (NH), 1640 (C=N), 1448 (thiazole) cm−1; 1H NMR (400 MHz, CDCl3) δ 4.81 (s, 2H, NH2, disappeared on D2O exchange), 8.92 (s, 1H, NH, disappeared on D2O exchange), 7.04–7.79 (m, 3H, 3CH) ppm. Anal. calcd. for C7H6N4O2S: C, 40.00; H, 2.88; N, 26.65; Found: C, 39.97; H, 2.90; N, 26.68%.
(2d). Yield 62%; m.p. 198–200°C; IR (KBr): 3449 (NH2), 3222 (NH), 1620 (C=N), 1439 (thiazole) cm−1; 1H NMR (400 MHz, CDCl3) δ 4.82 (s, 2H, NH2, disappeared on D2O exchange), 8.94 (s, 1H, NH, disappeared on D2O exchange), 2.45 (s, 3H, CH3), 7.26–7.71 (m, 3H, 3CH) ppm. Anal. calcd. for C8H9N3S: C, 53.61; H, 5.06; N, 23.44; Found: C, 53.57; H, 5.08; N, 23.47%.
(2e). Yield 65%; m.p. 193–195°C; IR (KBr): 3439 (NH2), 3208 (NH), 1628 (C=N), 1448 (thiazole) cm−1; 1H NMR (400 MHz, CDCl3) δ 4.80 (s, 2H, NH2, disappeared on D2O exchange), 8.94 (s, 1H, NH, disappeared on D2O exchange), 3.86 (s, 1H, OCH3), 7.16–7.65 (m, 3H, 3CH) ppm. Anal. calcd. for C8H9N3OS: C, 49.21; H, 4.65; N, 21.52; Found: C, 49.25; H, 4.62; N, 21.48%.
(2f). Yield 68%; m.p. 198–200°C; IR (KBr): 3445 (NH2), 3218 (NH), 1624 (C=N), 1428 (thiazole) cm−1; 1H NMR (400 MHz, CDCl3) δ 4.79 (s, 2H, NH2, disappeared on D2O exchange), 8.90 (s, 1H, NH, disappeared on D2O exchange), 7.82–8.21 (m, 3H, 3CH) ppm. Anal. calcd. for C7H6N3ClS: C, 42.11; H, 3.03; N, 17.76; Found: C, 42.15; H, 3.07; N, 17.79%.
(2g) Yield 69%; m.p. 167–169°C; IR (KBr): 3439 (NH2), 3220 (NH), 1638 (C=N), 1435 (thiazole) cm−1; 1H NMR (400 MHz, CDCl3) δ 4.83 (s, 2H, NH2, disappeared on D2O exchange), 8.92 (s, 1H, NH, disappeared on D2O exchange), 2.83 (s, 3H, CH3), 7.31–7.69 (m, 3H, 3CH) ppm. Anal. calcd. for C8H9N3S: C, 53.61; H, 5.06; N, 23.44; Found: C, 53.65; H, 5.01; N, 23.38%.
(2h). Yield 60%; m.p. 199–201°C; IR (KBr): 3440 (NH2), 3200 (NH), 1631 (C=N), 1445 (thiazole) cm−1; 1H NMR (400 MHz, CDCl3) δ 4.88 (s, 2H, NH2, disappeared on D2O exchange), 8.95 (s, 1H, NH, disappeared on D2O exchange), 7.06–8.82 (m, 3H, 3CH) ppm. Anal. calcd. for C7H6N4O2S: C, 40.00; H, 2.88; N, 26.65; Found: C, 40.04; H, 2.85; N, 26.61%.
(2i). Yield 68%; m.p. 248–250°C; IR (KBr): 3449 (NH2), 3212 (NH), 1640 (C=N), 1439 (thiazole) cm−1; 1H NMR (400 MHz, CDCl3) δ 4.86 (s, 2H, NH2, disappeared on D2O exchange), 8.94 (s, 1H, NH, disappeared on D2O exchange), 7.81 (s, 1H, CH), 7.98 (s, 1H, CH) ppm. Anal. calcd. for C7H5N3Cl2S: C, 35.91; H, 2.15; N, 17.95; Found: C, 35.88; H, 2.19; N, 17.92%.
(2j). Yield 63%; m.p. 239–241°C; IR (KBr): 3449 (NH2), 3220 (NH), 1640 (C=N), 1445 (thiazole) cm−1; 1H NMR (400 MHz, CDCl3) δ 4.82 (s, 2H, NH2, disappeared on D2O exchange), 8.92 (s, 1H, NH, disappeared on D2O exchange), 7.55–7.87 (m, 3H, 3CH) ppm. Anal. calcd. for C7H6N3ClS: C, 42.11; H, 3.03; N, 17.76; Found: C, 42.07; H, 3.01; N, 18.00%.
2-(3-Pyridyl)-5-(4-nitrophenyl)-1,3,4-oxadiazole (5)
A mixture of nicotinoyl hydrazide (4) (5 mmol) and 4-nitro benzoic acid (5 mmol) in phosphorus oxychloride (5 mL) was refluxed on water bath for 9 h. The progress of the reaction was monitored by TLC using toluene:ethylacetate:methanol (70:20:10) as mobile phase. After the completion of reaction, it was cooled and poured onto crushed ice with continuous stirring. The solid mass separated was neutralized with sodium bicarbonate solution (10% w/v). The resulting solid thus obtained was collected by filtration, washed well with cold water, dried, and crystallized from absolute ethanol.
(5). Yield 65%; m.p. 121–123°C; IR (KBr): 1667 (C=N), 1284, 1078 (C-O-C) cm−1; 1H NMR (400 MHz, CDCl3) δ 9.38 (s, 1H, CH), 8.83 (dd, 1H, J = 3.48 Hz, CH), 8.39–8.48 (m, 5H, 5CH), 7.61 (t, 1H, CH) ppm; 13C NMR (400 MHz, CDCl3) δ 160.22 (C2-oxadiazole), 159.58 (C5-oxadiazole), 149.50, 146.88, 145.38, 135.83, 129.65, 128.68, 124.68, 124.29, 123.34 (aromatic ring) ppm; MS (m/z): 268 (M+); Anal. calcd. for C13H8N4O3: C, 58.21; H, 3.01; N, 20.89; Found: C, 58.17; H, 3.04; N, 20.85%.
General procedure for the synthesis of 3-(3-pyridyl)-5-(4-nitrophenyl)-4-(N-substituted-1,3-benzothiazol-2-amino)-4H-1,2,4-triazole (6a–j)
A mixture of 2-(3-pyridyl)-5-(4-nitrophenyl)-1,3,4-oxadiazole (5) (5 mmol) and substituted 2-hydrazino-1,3-benzothiazole (2a–j) (5 mmol) in dry pyridine (10 mL) was refluxed for 18–24 h. The reaction was monitored by TLC on silica gel using ethyl acetate:toluene (2.5:7.5). It was then cooled and poured on to crushed ice. The reaction mass was neutralized by dilute hydrochloric acid and resulting solid was washed with cold water, dried, and crystallized from absolute ethanol. The other compounds of the series were prepared by similar procedure ().
(6a). Yield 66%; m.p. 210–212°C; IR (KBr): 3434 (NH), 1649 (C=N) cm−1; 1H NMR (400 MHz, CDCl3) δ 7.73 (s, 1H, NH), 9.38 (s, 1H, CH), 8.84 (dd, 1H, J = 4.0 Hz, CH), 8.39–8.49 (m, 5H, 5CH), 7.60 (t, 1H, CH), 7.06–7.26 (m, 3H, benzothiazole-H); 13C NMR (400 MHz, CDCl3) δ 163.48 (C3-triazole), 163.35 (C5-triazole), 152.91, 149.66, 147.94, 145.66, 138.43, 134.42, 132.66, 131.12, 129.03, 129.12, 128.15, 124.55, 124.19, 121.91, 113.66, 109.85 (aromatic ring) ppm; MS (m/z): 433 (M+); Anal. calcd. for C20H12N7O2FS: C, 55.42; H, 2.79; N, 22.62; Found: C, 55.38; H, 2.81; N, 22.71%.
(6b). Yield 62%; m.p. 199–201°C; IR (KBr): 3432 (NH), 1646 (C=N) cm−1; 1H NMR (400 MHz, CDCl3) δ 7.79 (s, 1H, NH), 9.37 (s, 1H, CH), 8.82 (dd, 1H, J = 4.0 Hz, CH), 8.40–8.51 (m, 5H, 5CH), 7.56 (t, 1H, CH), 7.61-7.76 (m, 3H, benzothiazole-H); 13C NMR (400 MHz, CDCl3) δ 163.28 (C3-triazole), 162.69 (C5-triazole), 153.03, 149.82, 147.69, 145.60, 136.69, 134.28, 133.15, 131.43, 129.03, 128.15, 127.78, 124.49, 124.16, 123.09, 118.99, 111.63 (aromatic ring) ppm; MS (m/z): 494 (M+), 496 (M+2); Anal. calcd. for C20H12N7O2BrS: C, 48.59; H, 2.45; N, 19.83; Found: C, 48.63; H, 2.41; N, 19.79%.
(6c). Yield 69%; m.p. 186–188°C; IR (KBr): 3449 (NH), 1652 (C=N) cm−1; 1H NMR (400 MHz, CDCl3) 7.80 (s, 1H, NH), 9.38 (s, 1H, CH), 8.83 (dd, 1H, J = 3.8 Hz, CH), 8.36–8.47 (m, 5H, 5CH), 7.62 (t, 1H, CH), 7.33 (d, 1H, J = 8.16 Hz, CH), 7.56 (d, 1H, J = 7.85 Hz, CH), 8.74 (s, 1H, CH); 13C NMR (400 MHz, CDCl3) δ 162.94 (C3-triazole), 162.53 (C5-triazole), 152.96, 148.83, 147.74, 146.61, 141.77, 134.48, 132.86, 132.32, 129.03, 129.53, 129.31, 124.61, 124.19, 121.98, 110.58, 119.72 (aromatic ring) ppm; MS (m/z): 460 (M+); Anal. calcd. for C20H12N8O4S: C, 52.17; H, 2.63; N, 24.34; Found: C, 52.21; H, 2.59; N, 24.31%.
(6d). Yield 70%; m.p. 179–180°C; IR (KBr): 3447 (NH), 1661 (C=N) cm−1; 1H NMR (400 MHz, CDCl3) δ 2.34 (s, 3H, CH3), 7.82 (s, 1H, NH), 9.37 (s, 1H, CH), 8.82 (dd, 1H, J = 4.0 Hz, CH), 8.39–8.52 (m, 5H, 5CH), 7.56 (t, 1H, CH), 7.05-7.50 (m, 3H, benzothiazole-H); 13C NMR (400 MHz, CDCl3) δ 163.07 (C3-triazole), 162.61 (C5-triazole), 22.91 (CH3), 152.93, 149.57, 148.01, 144.75, 135.28, 135.07, 131.31, 129.05, 129.92, 128.79, 128.52, 124.61, 124.21, 124.02, 121.27, 121.38 (aromatic ring) ppm; MS (m/z): 429 (M+); Anal. calcd. for C21H15N7O2S: C, 58.73; H, 3.52; N, 22.83; Found: C, 58.77; H, 3.47; N, 22.78%.
(6e). Yield 71%; m.p. 189–191°C; IR (KBr): 3449 (NH), 1655 (C=N) cm−1; 1H NMR (400 MHz, CDCl3) δ 3.82 (s, 3H, OCH3), 7.76 (s, 1H, NH), 9.36 (s, 1H, CH), 8.81 (dd, 1H, J = 4.0 Hz, CH), 8.39–8.48 (m, 5H, 5CH), 7.59 (t, 1H, CH), 6.84–7.28 (m, 3H, benzothiazole-H); 13C NMR (400 MHz, CDCl3) δ 163.38 (C3-triazole), 162.82 (C5-triazole), 52.59 (OCH3), 152.91, 149.99, 147.76, 147.35, 143.02, 134.36, 132.60, 132.44, 131.13, 129.13, 128.42, 124.23, 124.08, 119.15, 113.98, 106.25 (aromatic ring) ppm; MS (m/z): 445 (M+); Anal. calcd. for C21H15N7O3S: C, 56.62; H, 3.39; N, 22.01; Found: C, 56.66; H, 3.41; N, 21.98%.
(6f). Yield 65%; m.p. 204–206°C; IR (KBr): 3442 (NH), 1657 (C=N) cm−1; 1H NMR (400 MHz, CDCl3) δ 7.80 (s, 1H, NH), 9.36 (s, 1H, CH), 8.82 (dd, 1H, J = 3.8 Hz, CH), 8.37–8.48 (m, 5H, 5CH), 7.52 (t, 1H, CH), 7.59–7.76 (m, 3H, benzothiazole-H); 13C NMR (400 MHz, CDCl3) δ 163.34 (C3-triazole), 162.85 (C5-triazole), 152.87, 148.97, 147.32, 144.44, 134.08, 131.52, 131.05, 129.09, 127.81, 127.62, 126.85, 125.47, 124.33, 123.84, 121.95, 121.52 (aromatic ring) ppm; MS (m/z): 449 (M+), 451 (M+2); Anal. calcd. for C20H12N7O2ClS: C, 53.40; H, 2.69; N, 21.79; Found: C, 53.42; H, 2.73; N, 21.83%.
(6g). Yield 65%; m.p. 192–194°C; IR (KBr): 3435 (NH), 1660 (C=N) cm−1; 1H NMR (400 MHz, CDCl3) δ 2.61 (s, 3H, CH3), 7.76 (s, 1H, NH), 9.36 (s, 1H, CH), 8.82 (dd, 1H, J = 3.8 Hz, CH), 8.38–8.49 (m, 5H, 5CH), 7.59 (t, 1H, CH), 7.09–7.28 (m, 3H, benzothiazole-H); 13C NMR (400 MHz, CDCl3) δ 163.18 (C3-triazole), 162.65 (C5-triazole), 20.82 (CH3), 152.88, 148.93, 146.98, 143.47, 134.28, 132.47, 132.25, 131.07, 129.18, 128.96, 128.82, 127.57, 124.53, 123.98, 122.04, 120.96 (aromatic ring) ppm; MS (m/z): 429 (M+); Anal. calcd. for C21H15N7O2S: C, 58.73; H, 3.52; N, 22.83; Found: C, 58.81; H, 3.57; N, 22.87%.
(6h). Yield 67%; m.p. 171–173°C; IR (KBr): 3442 (NH), 1659 (C=N) cm−1; 1H NMR (400 MHz, CDCl3) δ 7.75 (s, 1H, NH), 9.37 (s, 1H, CH), 8.82 (dd, 1H, J = 4.0 Hz, CH), 8.39–8.48 (m, 5H, 5CH), 7.60 (t, 1H, CH), 8.22 (d, 1H, J = 8.16 Hz, CH), 8.56 (d, 1H, J = 8.14 Hz, CH), 6.63 (t, 1H, CH); 13C NMR (400 MHz, CDCl3) δ 162.94 (C3-triazole), 162.45 (C5-triazole), 152.21, 148.83, 147.74, 144.36, 138.06, 134.67, 132.10, 131.98, 131.81, 129.23, 128.07, 127.60, 124.20, 123.91, 122.55, 120.65 (aromatic ring) ppm; MS (m/z): 460 (M+); Anal. calcd. for C20H12N8O4S: C, 52.17; H, 2.63; N, 24.34; Found: C, 52.21; H, 2.67; N, 24.37%.
(6i). Yield 68%; m.p. 205–207°C; IR (KBr): 3449 (NH), 1649 (C=N) cm−1; 1H NMR (400 MHz, CDCl3) δ 7.88 (s, 1H, NH), 9.36 (s, 1H, CH), 8.81 (dd, 1H, J = 3.8 Hz, CH), 8.38–8.50 (m, 5H, 5CH), 7.59 (t, 1H, CH), 7.59 (s, 1H, CH), 7.66 (s, 1H, CH); 13C NMR (400 MHz, CDCl3) δ 163.34 (C3-triazole), 162.89 (C5-triazole), 152.35, 149.74, 147.82, 144.86, 134.29, 133.72, 133.50, 129.69, 129.41, 128.02, 128.75, 124.46, 124.02, 123.37, 123.78, 121.74 (aromatic ring) ppm; MS (m/z): 484 (M+), 486 (M+2), 488 (M+4); Anal. calcd. for C20H11N7O2Cl2S: C, 49.60; H, 2.29; N, 20.24; Found: C, 49.64; H, 2.26; N, 20.21%.
(6j). Yield 65%; m.p. 191–193°C; IR (KBr): 3438 (NH), 1661 (C=N) cm−1; 1H NMR (400 MHz, CDCl3) δ 7.76 (s, 1H, NH), 9.37 (s, 1H, CH), 8.81 (dd, 1H, J = 4.0 Hz, CH), 8.38–8.51 (m, 5H, 5CH), 7.61 (t, 1H, CH), 6.91–7.30 (m, 3H, benzothiazole-H); 13C NMR (400 MHz, CDCl3) δ 163.42 (C3-triazole), 162.96 (C5-triazole), 152.59, 148.96, 146.89, 144.86, 134.12, 131.65, 131.32, 131.59, 128.02, 127.81, 124.47, 124.08, 123.18, 120.29, 120.18, 118.79 (aromatic ring) ppm; MS (m/z): 449 (M+), 451 (M+2); Anal. calcd. for C20H12N7O2ClS: C, 53.40; H, 2.69; N, 21.79; Found: C, 53.34; H, 2.72; N, 21.83%.
Antimicrobial activity
The minimum inhibitory concentrations (MICs) of synthesized compounds were carried out by broth microdilution method as described by Rattan.Citation25 Antibacterial activity was screened against two Gram-positive bacteria (Staphylococcus aureus MTCC 96 and Streptococcus pyogenes MTCC 442) and two Gram-negative bacteria (Escherichia coli MTCC 443 and Pseudomonas aeruginosa MTCC 2488). Ampicillin was used as a standard antibacterial agent. Antifungal activity was screened against three fungal species Candida albicans MTCC 227, Aspergillus niger MTCC 282, and Aspergillus clavatus MTCC 1323. Griseofulvin was used as a standard antifungal agent.
All MTCC cultures were collected from Institute of Microbial Technology, Chandigarh and tested against above mentioned known drugs. Mueller hinton broth was used as nutrient medium to grow and dilute the drug suspension for the test. Inoculum size for test strain was adjusted to 108 colony-forming unit (CFU) per millilitre by comparing the turbidity. Dimethyl sulphoxide (DMSO) was used as diluents to get desired concentration of drugs to test upon standard bacterial strains. Serial dilutions were prepared in primary and secondary screening. The control tube containing no antibiotic was immediately subcultured (before inoculation) by spreading a loopful evenly over a quarter of plate of medium suitable for the growth of the test organism and put for incubation at 37°C overnight. The tubes were then incubated overnight. The MIC of the control organism was read to check the accuracy of the drug concentrations. The lowest concentration inhibiting growth of the organism was recorded as the MIC. All the tubes not showing visible growth (in the same manner as control tube described earlier) was subcultured and incubated overnight at 37°C. The amount of growth from the control tube before incubation (which represents the original inoculum) was compared. Subcultures might show similar number of colonies indicating bacteriostatic, a reduced number of colonies indicating a partial or slow bactericidal activity and no growth if the whole inoculum has been killed. The test must include a second set of the same dilutions inoculated with an organism of known sensitivity. Each synthesized drug was diluted obtaining 2000 μg/mL concentration, as a stock solution. In primary screening, 500, 250, and 125 μg/mL concentrations of the synthesized drugs were taken. The active synthesized compounds found in this primary screening were further tested in a second set of dilution against all microorganisms. The drugs found active in primary screening were similarly diluted to obtain 100, 50, 25, 12.5, 6.250, 3.125, and 1.5625 μg/mL concentrations. The highest dilution showing at least 99% inhibition is taken as MIC.
Antitubercular activity
Drug susceptibility and determination of MIC of the test compounds against M. tuberculosis H37Rv were performed by L.J. agar (MIC) methodCitation25–28 where primary 1000, 500, 250 and secondary 200, 100, 62.5, 50, 25, 12.5, 6.25, 3.25 μg/mL dilutions of each test compound were added; liquid L.J. medium and then media were sterilized by inspissation method. A culture of M. tuberculosis H37Rv growing on L.J. medium was harvested in 0.85% saline in bijou bottles. All test compounds that make first stock solution of 2000 μg/mL concentration of compounds were prepared in DMSO. These tubes were then incubated at 37°C for 24 h followed by streaking of M. tuberculosis H37Rv (5 × 104 bacilli per tube). These tubes were then incubated at 37°C. Growth of bacilli was seen after 12 days, 22 days, and finally 28 days of incubation. Tubes having the compounds were compared with control tubes where medium alone was incubated with M. tuberculosis H37Rv. The concentration at which no development of colonies occurred or <20 colonies was taken as MIC concentration of test compound. The standard strain M. tuberculosis H37Rv was tested with known drug rifampicin.
Results and discussion
Chemistry
2-Amino-6-flouro-1,3-benzothiazole 1a on treatment of hydrazine hydrate, concentrated hydrochloric acid, and ethylene glycol yields 2-hydrazino-6-flouro-1,3-benzothiazole 2a. IR spectra of 2a showed broad stretching band around 3425 and 3200 cm−1 for NH and NH2. 1H NMR spectrum showed a singlet at δ 4.83 and δ 8.93, which were accounted for NH2 and NH, which vanished on D2O exchange. Ethyl nicotinate 3 on treatment with hydrazine hydrate yields nicotinoyl hydrazide 4; the IR spectra of 4 showed stretching band around 3335 and 3278 cm−1 due to amine/amide NH, whereas strong stretching band at 1610 cm−1 was attributed to amide carbonyl. 1H NMR spectrum showed a singlet at δ 4.51 and δ 9.81, which were accounted for NH2 and NH, which vanished on D2O exchange. Intermolecular cyclization of nicotinoyl hydrazide 4 with 4-nitrobenzoic acid in presence of phosphorus oxy chloride affords 2-(3-pyridyl)-5-(4-nitrophenyl)-1,3,4-oxadiazole 5. Disappearance of 1H NMR resonances observed with NH and NH2 functions in the 1H NMR spectrum of 5 proved that ring closure starting from 4 resulted in the formation of 1,3,4-oxadiazole ring. This was further substantiated by the 13C NMR data of 5, which showed a peak at δ 160.22 and δ 159.58 due to C2 and C5 of oxadiazole. Mass spectrum of 5 displayed a molecular ion peak at m/z 268 that confirmed its molecular weight. Condensation of 5 with various substituted 2-hydrazino-1,3-benzothiazole 2a–j in pyridine results in 3-(3-pyridyl)-5-(4-nitrophenyl)-4-(N-substituted-1,3-benzothiazol-2-amino)-4H-1,2,4-triazole 6a–j. Absence of 1H NMR resonances observed with NH2 function of 2a and appearance of signal at δ 7.73 for NH was observed in 1H NMR of 6a proved the condensation of 2 and 5 resulted in the formation of 1,2,4-triazole ring. This was substantiated by 13C NMR data of 6a that showed a peak at δ 163.48 and δ 163.35 due to C3 and C5 of triazole. Mass spectrum of 6 displayed a molecular ion at m/z 433 that confirmed its molecular weight.
Antibacterial activity
The MICs of the tested compounds are shown in and . The results revealed that substituted 2-hydrazino benzothiazoles were moderately active against bacteria except 2e, which showed good activity against S. aureus and E. coli while 1,3,4-oxadiazole 5 exhibited quite good activity to some extent. Most of 1,2,4-triazole derivative were found good activity (62.5–250 μg/mL) against S. aureus. Compounds 6b, 6c, 6f, 6g, and 6j exhibited pronounced activity (62.5–125 μg/mL) against S. aureus. All the compounds exhibited moderate activity (150–250 μg/mL) except 6b and 6j (62.5 μg/mL) against S. pyogenes. Compounds 6b, 6f, 6g, 6h, and 6j possessed good activity (100–125 μg/mL) except 6c showed pronounced activity (62.5 μg/mL) while others displayed moderate activity (150–250 μg/mL) against E. coli. Compounds 6c and 6d showed good activity (100 μg/mL) except 6i showed very good activity (62.5 μg/mL) while others possessed moderate activity (150–250 μg/mL) against P. aeruginosa. Compounds 6b, 6c, 6f, 6g, and 6j exhibited good activity against Gram-positive bacteria, whereas 6c, 6g, 6i, and 6j showed good activity toward Gram-negative bacteria. Compounds 2e, 6c, 6g, and 6j were found active against Gram-positive and Gram-negative bacteria.
Table 1. Minimum inhibitory concentrations (MICs, μg/mL) for the title compounds.
Table 2. Minimum inhibitory concentrations (MICs, μM) for the title compounds.
Antifungal activity
In vitro antifungal activities (MICs) of the synthesized compounds are shown in and . The results showed that 2-hydrazino benzothiazoles 2a–i possessed good activity (250–500 μg/mL) against C. albicans except 2j (1000 μg/mL). Compounds 2a–j displayed moderate to weak activity (250–500 μg/mL) against A. niger and A. clavatus, whereas 1,3,4-oxadiazole 5 exhibited weak activity against all three fungi. Compounds 6c, 6d, 6e, 6f, 6i, and 6j showed good activity (250–500 μg/mL), whereas 6a and 6h exhibited pronounced activity (100 μg/mL) against C. albicans. Compounds 6a, 6h, and 6i exhibited moderate activity (200–250 μg/ml), whereas remaining compounds showed weak activity against A. niger. Compounds 6d and 6h displayed moderate activity (200–250 μg/mL), whereas rest of the compounds showed weak activity against A. clavatus. Compounds 2f, 2g, 2h, 6a, 6d, and 6h were found active against all the three fungal species.
Table 3. Minimum inhibitory concentrations (MICs, μg/mL) for the title compounds.
Table 4. Minimum inhibitory concentrations (MICs, μM) for the title compounds.
Antitubercular activity
The encouraging results from the antibacterial studies impelled us to go for preliminary screening of synthesized compounds against M. tuberculosis are summarized in . From the preliminary examination of the antitubercular activity results, compound 2e containing hydrazide group showed better activity (50 μg/mL) against M. tuberculosis and compounds 6a, 6e, and 6j showed good activity (50–62.5 μg/mL). Due to the better activity against tested microorganisms and mycobacteria, compound 6j has been selected for further development, and studies to acquire more information about structure–activity relationships are in progress in our laboratories.
Table 5. Minimum inhibitory concentrations (MICs, μg/mL and μM) for the title compounds.
Conclusion
A series of newer analogues 1,2,4-triazoles were synthesized by the introduction of 2-hydrazino benzothiazoles to 1,3,4-oxadiazoles and accessed for antimicrobial and antitubercular activity. Modification of substituents on benzothiazoles ring with various electron-releasing and electron-withdrawing substituents improved the activity. The analogues with halogen, methyl, and nitro substituents emerged as promising antibacterials showing better to moderate activity, whereas analogues bearing nitro substituent showed better antifungal activity. It was also observed that the promising antimicrobials have proved to be better antituberculars. Specially, compound 6j due to their better activity against H37Rv strain, is the best choice for the preparation of new derivatives in order to improve antitubercular activity in future.
Acknowledgements
The authors are thankful to Veer Narmad South Gujarat University for providing necessary facilities and research fellowship. We also thank SAIF Lucknow for elemental analysis and SAIF Chandigarh for spectral analysis of the compounds and D. Rajani, Microbial Laboratory, Surat, for antimicrobial and antimycobacterial activity.
Declaration of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the article.
References
- Stokstad, E. Infectious disease. Drug-resistant TB on the rise. Science 2000, 287, 2391.
- WHO Global Tuberculosis Programme—Tuberculosis Fact Sheet, 2002. World Health Organization. Global Tuberculosis Control, WHO Report 2001, 2002.
- World Health Organization, Geneva, Switzerland, WHO/CDS/TB/2001, 287. http://www.who.int/mediacentre/factsheets/who104/en/index.html
- Mooran, N. WHO issues another gloomy tuberculosis report. Nat. Med. 1996, 2, 377.
- Dye, C., Scheele, S., Dolin, P., Pathania, V., Raviglione, M.C. Global burden of tuberculosis: estimated incidence, prevalence and mortality by country. J. Am. Med. Assoc. 1999, 282, 677–686.
- Dooley, S.W., Jarvis, W.R., Martone, W.J., Snider, D.E. Jr. Multidrug-resistant tuberculosis. Ann. Intern. Med. 1992, 117, 257–259.
- Raviglione, M.C., Snider, D.E. Jr, Kochi, A. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA 1995, 273, 220–226.
- Farmer, P., Bayona, J., Becerra, M., Furin, J., Henry, C., Hiatt, H., Kim, J.Y., Mitnick, C., Nardell, E., Shin, S. The dilemma of MDR-TB in the global era. Int. J. Tuberc. Lung Dis. 1998, 2, 869–876.
- Hudson, A., Imamura, T., Gutteridge, W., Kanyok, T., Nunn, P. The Current Anti-TB Drug Research and Development Pipeline. WHO TDR/PRD/03.1W Geneva, 2003.
- Tripathi, R.P., Tewari, N., Dwivedi, N., Tiwari, V.K. Fighting tuberculosis: an old disease with new challenges. Med. Res. Rev. 2005, 25, 93–131.
- Banfi, E., Scialino, G., Zampieri, D., Mamolo, M.G., Vio, L., Ferrone, M., Fermeglia, M., Paneni, M.S., Pricl, S. Antifungal and antimycobacterial activity of new imidazole and triazole derivatives. A combined experimental and computational approach. J. Antimicrob. Chemother. 2006, 58, 76–84.
- Ulusoy, N., Gürsoy, A., Otük, G. Synthesis and antimicrobial activity of some 1,2,4-triazole-3-mercaptoacetic acid derivatives. Farmaco 2001, 56, 947–952.
- Muhi-Eldeen Z, Nadir M, Aljobory NR, Husseen F, Stohs SJ. Synthesis and antimicrobial evaluation of 3-(4-tert-amino-2-butynyl)thio and alkyl/alkenylthio-4,5-disubstituted-4H-1,2,4-triazoles. Eur. J. Med. Chem. 1991, 26, 237–241.
- Küçükgüzel, I., Tatar, E., Küçükgüzel, S.G., Rollas, S., De Clercq, E. Synthesis of some novel thiourea derivatives obtained from 5-[(4-aminophenoxy)methyl]-4-alkyl/aryl-2,4-dihydro-3H-1,2,4-triazole-3-thiones and evaluation as antiviral/anti-HIV and anti-tuberculosis agents. Eur. J. Med. Chem. 2008, 43, 381–392.
- Banfi, E., Mamolo, M.G., Zampieri, D., Vio, L., Monti Bragadin, C. Antimycobacterial activity of N1-[1-[3-aryl-1-(pyridin-2-, 3- or 4-yl)-3-oxo] propyl]-2-pyridinecarboxamidrazones. J. Antimicrob. Chemother. 2001, 48, 705–707.
- Jackson, C.J., Lamb, D.C., Kelly, D.E., Kelly, S.L. Bactericidal and inhibitory effects of azole antifungal compounds on Mycobacterium smegmatis. FEMS Microbiol. Lett. 2000, 192, 159–162.
- Guardiola-Diaz, H.M., Foster, L.A., Mushrush, D., Vaz, A.D. Azole-antifungal binding to a novel cytochrome P450 from Mycobacterium tuberculosis: implications for treatment of tuberculosis. Biochem. Pharmacol. 2001, 61, 1463–1470.
- McLean, K.J., Marshall, K.R., Richmond, A., Hunter, I.S., Fowler, K., Kieser, T., Gurcha, S.S., Besra, G.S., Munro, A.W. Azole antifungals are potent inhibitors of cytochrome P450 mono-oxygenases and bacterial growth in mycobacteria and streptomycetes. Microbiology 2002, 30, 314–318.
- Dabiri, M., Salehi, P., Baghbanzadeh, M., Bahramnejad, M. A facile procedure for the one-pot synthesis of unsymmetrical 2,5-disubstituted 1,3,4-oxadiazoles. Tetrahedron Lett. 2006, 47, 6983–6986.
- Navarrete-Vázquez, G., Molina-Salinas, G.M., Duarte-Fajardo, Z.V., Vargas-Villarreal, J., Estrada-Soto, S., González-Salazar, F., Hernández-Núñez, E., Said-Fernández, S. Synthesis and antimycobacterial activity of 4-(5-substituted-1,3,4-oxadiazol-2-yl)pyridines. Bioorg. Med. Chem. 2007, 15, 5502–5508.
- Podust, L.M., Poulos, T.L., Waterman, M.R. Crystal structure of cytochrome P450 14alpha-sterol demethylase (CYP51) from Mycobacterium tuberculosis in complex with azole inhibitors. Proc. Natl. Acad. Sci. U.S.A. 2001, 98, 3068–3073.
- Bellamine, A., Mangla, A.T., Nes, W.D., Waterman, M.R. Characterization and catalytic properties of the sterol 14alpha-demethylase from Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U.S.A. 1999, 96, 8937–8942.
- Barnett, C.J. Exchange Amination Process for Preparing 2-Hydrazinobenzothiazoles. U.S. Patent 3,937,714.
- Kumar, V., Roy, R.K., Kumar, V., Kukshal, A., Yadav, V.P. Synthesis and antimicrobial activity of 2-(6-substituted-1,3-benzothiazol-2-yl)-N-(4-halophenyl)hydrazinecarbothiomide. J. Indian Chem. Soc. 2008, 85B, 333–335.
- Rattan, A. Antimicrobials in Laboratory Medicine. New Delhi: Churchill BI Livingstone, 2000, pp. 85–108.
- Anargyros, P., Astill, D.S., Lim, I.S. Comparison of improved BACTEC and Lowenstein-Jensen media for culture of mycobacteria from clinical specimens. J. Clin. Microbiol. 1990, 28, 1288–1291.
- Shah, R.R., Mehta, R.D., Parikh, A.R. Studies on isoniazide derivatives: preparation and antimicrobial activity of 2-aryl-3-(pyridylcarbomyl)-5-carboxymethyl-4-thiazolidinones. J. Indian Chem. Soc. 1985, 62B, 255–257.
- Desai, N.C., Shukla, H.K., Tahker, K.A. Some new 2-aryl-3-isonicotamido-4-thiazolidinones and their 5-carboxymethyl homologues as potential antitubercular and antibacterial agent. J. Indian Chem. Soc. 1984, 61B, 239–240.