Abstract
A series of 2-{4-[4-(2,5-disubstituted thiazolyl)phenylethyl] piperazin-1-yl}-1,8-naphthyridine-3-carbonitriles were synthesized in an effort to prepare novel atypical antipsychotic agents. The compounds were synthesized either by microwave irradiation technique or by conventional synthesis and were characterized by spectral data (IR, 1H NMR, and MS) and the purity was ascertained by microanalysis. The D2 and 5-HT2A affinity of the synthesized compounds was screened in vitro by radioligand displacement assays on membrane homogenates isolated from rat striatum and rat cortex, respectively. Furthermore, all the synthesized compounds were screened for their in vivo pharmacological activity in Swiss albino mice. The D2 antagonism studies were performed using climbing mouse assay model and 5-HT2A antagonism studies were performed using quipazine-induced head twitches in mice. It was observed that none of the new chemical entities exhibited catalepsy and 10f is the most active among the synthesized compounds with 5-HT2A/D2 ratio of 1.1286 although the standard drug risperidone exhibited 5-HT2A/D2 ratio of 1.0989.
Introduction
Schizophrenia is a devastating mental disorder with high morbidity and mortality, affecting about 1% of the population worldwide.Citation1 Symptoms of schizophrenia emerge during adolescence and are classified into positive (hallucinations, delusions, and severe thought disorganization), negative (alogia, anhedonia, avolition, and flattened affect), and cognitive symptoms (slow thinking, poor concentration, poor memory, and difficulty in understanding).Citation2
Classical antipsychotics, which act as D2 antagonists in the limbic forebrain, are useful for the treatment of the positive symptoms, but failed to manage the negative symptoms of schizophreniaCitation3 and their use is frequently associated with serious side effects, such as extrapyramidal syndrome (EPS), tardive dyskinesia, and hyperprolactinaemia.Citation4 This led to the development of improved “atypical” antipsychotic agents, like the prototype antipsychotic drug clozapine, which are in general effective for positive, negative, and cognitive symptoms of schizophrenia. These drugs are more efficacious than classical antipsychotics in treatment-refractory patients and have a low incidence of EPS.Citation5,Citation6
Over the past two decades, much attention regarding the treatment for schizophrenia has focused on this new class of antipsychotic medications and led to the proliferation of “atypical” antipsychotics, including risperidone, olanzapine, quetiapine, ziprasidone, sertindole, iloperidone, aripiprazole, amisulpride, and zotepine.Citation7 Meltzer et al. suggested that in the efficacy of clozapine and other atypical antipsychotics such as risperidone and olanzapine the most important factor is their relative affinities for the D2 and 5-HT2A receptors.Citation8–10 Antagonism at 5-HT2A and D2 receptors by these molecules is responsible for alleviating the negative and positive symptoms of the disorder, respectively.Citation11,Citation12 But these compounds are also not completely devoid of side effects. Side effects caused by “atypical antipsychotics” are a result of their significant binding affinity to numerous receptors other than required for atypical antipsychotic activity. Side effects associated with these drugs include weight gain (serotonergic 5-HT2C and histaminic H1 receptors blockade),Citation13 postural or orthostatic hypotension, sedation, dizziness (α1-adrenergic blockade), somnolence (histaminic H1 receptor blockade), seizures (muscarinic receptor blockade),Citation14 new-onset type 2 diabetes mellitus,Citation15 hyperlipidaemia, atropine-like side effects such as dry mouth, constipation, urinary retention (muscarinic M1 receptor blockade), cardiac ventricular arrhythmias (prolongation of QT interval due to the blockade of Ikr channels), myocarditis, insomnia, headache, and other possible secondary cardiovascular complications.Citation16
The rationale for development of the antipsychotic drugs recently introduced, and currently under development is predominantly based on dopamine and serotonin hypotheses of schizophrenia. In continuation of our quest for novel atypical antipsychotics,Citation17,Citation18 we followed the strategy employed by Pfizer group,Citation19 synthesized the title compounds, and evaluated for the atypical antipsychotic activity in animal models.
The strategy of Ariens has been employed for the design of the compounds.Citation20 Ariens strategy, in brief, involves modification of the structure of a receptor agonist, in this case dopamine, with a large lipophilic group on the amino position, which binds to the accessory binding site adjacent to the agonist binding site and transforms the agonist into an antagonist. Using this strategy, the current marketed drug ziprasidone was developed.Citation19 We adopted this strategy and employed 2-(piperazin-1-yl)-1,8-naphthyridine-3-carbonitrile, which has affinity towards serotonin receptorsCitation21 as one portion of the molecule and chloroethylphenylthiazoles was selected as other portion as Pfizer group has come up with potent atypical antipsychotics using this heterocyclic system.Citation22
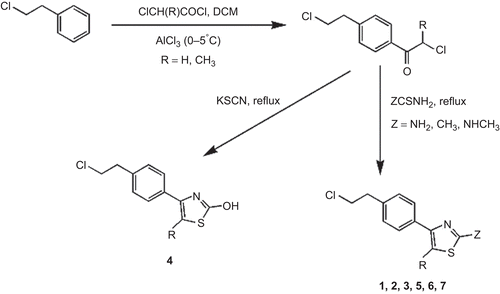
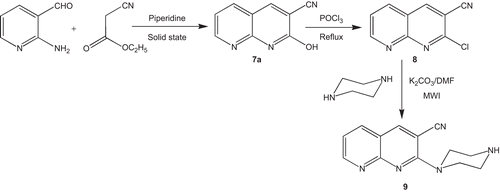
We synthesized compounds in which 2- and 5-substituted chloroethylphenylthiazoles (1–7) have been incorporated at the piperazinyl nitrogen atom of 2-(piperazin-1-yl)-1,8-naphthyridine-3-carbonitrile looking for 5-HT2A and D2 antagonism. Seven new compounds have been synthesized and the synthetic schemes are illustrated in . illustrates the synthesis of 2- and 5-substituted chloroethylphenylthiazoles (1–7), whereas illustrates the synthesis of 2-(piperazin-1-yl)-1,8-naphthyridine-3-carbonitrile (9) and depicts the coupling of these two fragments to yield the title compounds.
Experimental
Materials
The starting compounds, 2- and 5-substituted chloroethylphenylthiazoles (1–7) and 2-(piperazin-1-yl)-1,8-naphthyridine-3-carbonitriles (9), were prepared according to the reported method.Citation21,Citation22 All other starting materials and solvents were obtained from commercially available sources and used without additional purification.
Methods
Melting points were determined in open capillaries using Büchi 530 melting point apparatus and are uncorrected. The reactions were monitored and the purity of the compounds checked by ascending thin-layer chromatography (TLC) on silica gel-coated aluminium plates (Merck 60 F254, 0.25 mm) using mixture of chloroform and methanol and the spots were visualized under ultraviolet light at 254 and 366 nm. The microwave-assisted procedures were carried out in a LG microwave oven specially designed for organic synthesis operating at a maximum power of 1000 W. Infrared (IR) spectra were recorded in KBr pellets on Schimadzu IR Prestige-21 FT-IR spectrophotometer (cm−1). 1H NMR spectra were obtained from Bruker DRX300 spectrometer using tetramethylsilane as internal standard [chemical shifts (δ) in parts per million (ppm)], mass spectra on a VG-70-S mass spectrometer, and elemental analysis on a Perkin Elmer 2400 CHN elemental analyzer.
2-Hydroxy-1,8-naphthyridine-3-carbonitrile (7a): Literature procedureCitation21 was used to synthesize 7a. Yield: 90% (1.54 g); m.p.: > 300°C (Lit. m.p. > 300°C).Citation21
2-Chloro-1,8-naphthyridine-3-carbonitrile (8): Compound 8 was prepared according to the reported method.Citation21 Yield: 79% (1.5 g); m.p.: > 300°C (Lit. m.p. > 300°C).Citation21
2-Piperazin-1-yl-1,8-naphthyridine-3-carbonitrile (9): Compound 9 was prepared using the literature protocol.Citation21 Yield: 74% (0.90 g); m.p.: 225–226°C (Lit. m.p. 226–227°C).Citation21
General procedure for 2-{4-[4-(2,5-disubstituted thiazolyl)phenylethyl] piperazin-1-yl}-1,8-naphthyridine-3-carbonitriles (10a–g)
The procedure described by Lowe and coworkersCitation22 was adapted for this preparation. In a 10-mL round bottom flask equipped with a reflux condenser and N2 inlet were placed equimolar amounts (0.05 mM) of 2-piperazin-1-yl-1,8-naphthyridine-3-carbonitrile (9) and respective chloroethylphenylthiazoles (1–7), 0.1174 g (1.11 mM) of sodium carbonate, and 2 mg of potassium iodide in 2 mL of dimethylformamide (DMF). The reaction mixture was refluxed for 2 days. After completion of reaction, as indicated by TLC (9:1 chloroform:methanol as mobile phase), the cooled reaction mixture was poured into ice–water mixture and the precipitate was filtered, washed with water, and recrystallized in DMF–water mixture to afford the pure final compounds 10a–g.
2-{4-[4-(2-Aminothiazol-4-yl)phenethyl]piperazin-1-yl}-1,8-naphthyridine-3-carbonitrile (10a). Yield: 77% (0.17 g); m.p.: 260–262°C. 1H NMR (CDCl3) (δ) ppm: 2.65–2.69 (m, 4H, (CH2)2); 2.76 (t, 4H, J = 4.8 Hz, N4(CH2)2); 3.17 (t, 4H, J = 4.8 Hz, N1(CH2)2); 3.87 (s, 2H, NH2); 6.92 (s, 1H, thiazole); 7.15–7.29 (m, 4H, Ph); 7.34 (dd, 1H, J = 8.2 Hz, J = 1.4 Hz, C6-H; 1,8-naphthyridine); 7.84 (d, 1H, J = 5.1 Hz, C5-H; 1,8-naphthyridine); 8.35 (s, 1H, C4-H; 1,8-naphthyridine); 9.09 (d, 1H, J = 5.1 Hz, C7-H; 1,8-naphthyridine). IR (KBr) cm−1: 3428 and 3400 (NH2 stretch); 3037, 3018 (aromatic C-H stretch); 2955, 2890 (aliphatic C-H stretch); 2243 (C-N stretch); 1651 (C=N ring stretch); 1602 (aromatic C=C stretch); 1264 (aliphatic C-N stretch); 808 (para disubstituted benzene); 706 (C-S-C stretch). FAB-MS m/z: 441.1710 calculated: 441.1712; Anal. calculated for C24H23N7S: C 65.28, H 5.25, N 22.21, S 7.26; found: C 65.26, H 5.21, N 22.17, S 7.19.
2-{4-[4-(2-(Methylamino)thiazol-4-yl)phenethyl]piperazin-1-yl}-1,8-naphthyridine-3-carbonitrile (10b). Yield: 66% (0.15 g); m.p.: 148–150°C. 1H NMR (CDCl3) (δ) ppm: 2.47 (s, 3H, NHCH3); 2.68-2.71 (m, 4H, (CH2)2); 2.78 (t, 4H, J = 4.8 Hz, N4(CH2)2); 3.17 (t, 4H, J = 4.8 Hz, N1(CH2)2); 4.08 (s, 1H, NHCH3); 6.79 (s, 1H, thiazole); 7.18–7.34 (m, 4H, Ph); 7.46 (dd, 1H, J = 8.4 Hz, J = 1.6 Hz, C6-H; 1,8-naphthyridine); 7.87 (d, 1H, J = 5.2 Hz, C5-H; 1,8-naphthyridine); 8.44 (s, 1H, C4-H; 1,8-naphthyridine); 9.11 (d, 1H, J = 5.2 Hz, C7-H; 1,8-naphthyridine). IR (KBr) cm−1: 3400 (NH stretch); 3030, 3005 (aromatic C-H stretch); 2985, 2875 (aliphatic C-H stretch); 2250 (C-N stretch); 1640 (C=N ring stretch); 1258 (aliphatic C-N stretch); 1610, 1590 (aromatic C=C stretch); 815 (para disubstituted benzene); 706 (C-S-C stretch). FAB-MS m/z: 455.1670, calculated: 455.1677; Anal. calculated for C25H25N7S: C 65.91, H 5.33, N 21.52, S 7.04; found: C 65.81, H 5.31, N 21.47, S 7.01.
2-{4-[4-(2-Amino-5-methylthiazol-4-yl)phenethyl]piperazin-1-yl}-1,8-naphthyridine-3-carbonitrile (10c). Yield: 81% (0.184 g); m.p.: 210–212°C. 1H NMR (CDCl3) (δ) ppm: 2.29 (s, 3H, CH3); 2.57 (t, 4H, J = 4.8 Hz, N4(CH2)2); 2.61–2.66 (m, 4H, (CH2)2); 3.21 (t, 4H, J = 4.8 Hz, N1(CH2)2); 3.92 (s, 2H, NH2); 7.19–7.31 (m,4H, Ph); 7.37 (dd, 1H, J = 8.3 Hz, J = 1.5 Hz, C6-H; 1,8-naphthyridine); 7.89 (d, 1H, J = 5.1 Hz, C5-H; 1,8-naphthyridine); 8.52 (s, 1H, C4-H; 1,8-naphthyridine); 9.31 (d, 1H, J = 5.1 Hz, C7-H; 1,8-naphthyridine). IR (KBr) cm−1: 3430 and 3415 (NH2 stretch); 3045, 3020 (aromatic C-H stretch); 2980, 2875 (aliphatic C-H stretch); 2240 (C-N stretch); 1637 (C=N ring stretch); 1608 (aromatic C=C stretch); 1255 (aliphatic C-N stretch); 825 (para disubstituted benzene); 712 (C-S-C stretch). FAB-MS m/z: 455.2111, calculated: 455.2114; Anal. calculated for C25H25N7S: C 65.91, H 5.33, N 21.52, S 7.04; found: C 65.94, H 5.31, N 21.47, S 6.98.
2-{4-[4-(2-Hydroxythiazol-4-yl)phenethyl]piperazin-1-yl}-1,8-naphthyridine-3-carbonitrile (10d). Yield: 76% (0.167 g); m.p.: 232–234°C. 1H NMR (CDCl3) (δ) ppm: 2.62 (t, 4H, J = 4.8 Hz, N4(CH2)2); 2.68–2.79 (m, 4H, (CH2)2); 3.19 (t, 4H, J = 4.8 Hz, N1(CH2)2); 5.42 (br s, 1H, OH); 7.04–7.19 (m, 4H, Ph); 7.23 (s, 1H, thiazole); 7.43 (dd, 1H, J = 8.0 Hz, J = 1.4 Hz, C6-H; 1,8-naphthyridine); 7.87 (d, 1H, J = 5.4 Hz, C5-H; 1,8-naphthyridine); 8.37 (s, 1H, C4-H; 1,8-naphthyridine); 9.14 (d, 1H, J = 5.4 Hz, C7-H; 1,8-naphthyridine). IR (KBr) cm−1: 3600 (OH stretch, broad); 3030, 3010 (aromatic C-H stretch); 2960, 2925 (aliphatic C-H stretch); 2241 (C-N stretch); 1645 (C=N ring stretch); 1587 (aromatic C=C stretch); 1260 (aliphatic C-N stretch); 818 (para disubstituted benzene); 705 (C-S-C stretch). FAB-MS m/z: 442.1413, calculated: 442.1415; Anal. calculated for C24H22N6OS: C 65.14, H 5.01, N 18.99, S 7.25; found: C 65.10, H 4.99, N 18.91, S 7.19.
2-{4-[4-(2,5-Dimethylthiazol-4-yl)phenethyl]piperazin-1-yl}-1,8-naphthyridine-3-carbonitrile (10e). Yield: 57% (0.13 g, oil). 1H NMR (CDCl3) (δ) ppm: 2.31 (s, 3H, 5-CH3); 2.64 (t, 4H, J = 4.9 Hz, N4(CH2)2); 2.69–2.75 (m, 4H, (CH2)2); 2.79 (s, 3H, 2-CH3); 3.16 (t, 4H, J = 4.9 Hz, N1(CH2)2); 7.07-7.26 (m, 4H, Ph); 7.45 (dd, 1H, J = 8.2 Hz, J = 1.4 Hz, C6-H; 1,8-naphthyridine); 7.91 (d, 1H, J = 5.1 Hz, C5-H; 1,8-naphthyridine); 8.28 (s, 1H, C4-H; 1,8-naphthyridine); 9.14 (d, 1H, J = 5.1 Hz, C7-H; 1,8-naphthyridine). IR (Neat) cm−1: 3070, 3045 (aromatic C-H stretch); 2980, 2955 (aliphatic C-H stretch); 2249 (C-N stretch); 1648 (C=N ring stretch); 1620, 1602 (aromatic C=C stretch); 1256 (aliphatic C-N stretch); 820 (para disubstituted benzene); 710 (C-S-C stretch). FAB-MS m/z: 454.1920, calculated: 454.1926; Anal. calculated for C26H26N6S: C 68.69, H 5.76, N 18.49, S 7.05; found: C 68.62, H 5.71, N 18.46, S 7.02.
2-{4-[4-(5-Methyl-2-(methylamino)thiazol-4-yl)phenethyl]piperazin-1-yl}-1,8-naphthyridine-3-carbonitrile (10f). Yield: 41% (0.16 g); m.p.: 198–200°C. 1H NMR (CDCl3) (δ) ppm: 2.36 (s, 3H,CH3); 2.51 (s, 3H, NHCH3); 2.62 (t, 4H, J = 4.9 Hz, N4(CH2)2); 2.66–2.69 (m, 4H, (CH2)2); 3.18 (t, 4H, J = 4.9 Hz, N1(CH2)2); 4.21 (s, 1H,NHCH3); 7.15–7.40 (m, 4H, Ph); 7.45 (dd, 1H, J = 8.4 Hz, J = 1.2 Hz, C6-H; 1,8-naphthyridine); 7.87 (d, 1H, J = 5.3 Hz, C5-H; 1,8-naphthyridine); 8.47 (s, 1H, C4-H; 1,8-naphthyridine); 9.09 (d, 1H, J = 5.3 Hz, C7-H; 1,8-naphthyridine). IR (KBr) cm−1: 3400 (NH stretch); 3025, 3010 (aromatic C-H stretch); 2917, 2895 (aliphatic C-H stretch); 2245 (C-N stretch); 1652 (C=N ring stretch); 1618, 1586 (aromatic C=C stretch); 1256 (aliphatic C-N stretch); 828 (para disubstituted benzene); 705 (C-S-C stretch). FAB-MS m/z: 469.2138, calculated: 469.2142; Anal. calculated for C26H27N7S: C 66.50, H 5.80, N 20.88, S 6.83; found: C 66.43, H 5.69, N 20.79, S 6.86.
2-{4-[4-(2-Methylthiazol-4-yl)phenethyl]piperazin-1-yl}-1,8-naphthyridine-3-carbonitrile (10g). Yield: 57% (0.13 g, oil). 1H NMR (CDCl3) (δ) ppm: 2.59 (t, 4H, J = 4.9 Hz, N4(CH2)2); 2.65-2.71 (m, 4H, (CH2)2); 2.74 (s, 3H, CH3); 3.17 (t, 4H, J = 4.9 Hz, N1(CH2)2); 7.11–7.28 (m, 4H, Ph); 7.43 (s, 1H, thiazole); 7.51 (dd, 1H, J = 8.4 Hz, J = 1.6 Hz, C6-H; 1,8-naphthyridine); 7.82 (d, 1H, J = 5.2 Hz, C5-H; 1,8-naphthyridine); 8.32 (s, 1H, C4-H; 1,8-naphthyridine); 9.21 (d, 1H, J = 5.2 Hz, C7-H; 1,8-naphthyridine). IR (Neat) cm−1: 3065, 3015 (aromatic C-H stretch); 2976, 2952 (aliphatic C-H stretch); 2247 (C-N stretch); 1638 (C=N ring stretch); 1616, 1591 (aromatic C=C stretch); 1261 (aliphatic C-N stretch); 827 (para disubstituted benzene); 708 (C-S-C stretch). FAB-MS m/z: 440.1831, calculated: 440.1836; Anal. calculated for C25H24N6S: C 68.16, H 5.49, N 19.08, S 7.28; found: C 68.12, H 5.48, N 18.99, S 7.26.
In vitro radioligand displacement studies
The affinity and specificity of the NCEs were estimated in radioligand displacement studies on rat 5-HT2A and D2 receptors obtained from rat cortical (5-HT receptors) and striatal (D2 receptors) membrane preparations. Test compounds were dissolved in dimethyl sulphoxide (DMSO) (10 mM stock solution), aliquoted, and stored at −25°C. For competitive binding experiments, the membrane preparations were thawed, diluted with assay buffer, 50 mM Tris–HCl, pH 7.4, and washed twice. The particular receptor preparation was incubated with the respective radioligand (5-HT2A: [3H]ketanserine, Perkin Elmer Life and Analytical Sciences, Inc., Rodgau - Jügesheim, Germany and GE Healthcare (former Amersham): GE Healthcare Europe GmbH, Freiburg, Germany Aspec: 67 Ci/mmol; D2: [3H]spiperone, Amersham GE, Aspec: 101 Ci/mmol) and up to six concentrations of the NCEs. Dilutions of the NCEs were made with assay buffer. Nonspecific binding of the radioligands was determined with 100 µM mianserin for the 5-HT2A ligand [3H]ketanserine and 100 µM haloperidol for D2 ligand [3H]spiperone. To block the 5-HT affinity of the D2 radioligand [3H]spiperone, 10 µM ketanserin was added to the respective assays. The assay samples were incubated at ambient temperature for 60 min (D2) or 90 min (5-HT2A), rapidly filtered through Whatman GF/B glass-fibre filters, and washed four times with ice-cold assay buffer. Filter-bound radioactivity was determined by liquid scintillation counting. All test compounds were assayed in at least three independent experiments. The IC50 values were estimated using iterative nonlinear curve fitting.
In vivo pharmacological studies
The Institutional Animal Ethics Committee of the Birla Institute of Technology and Science, Pilani, Rajasthan, India approved experimentation on animals (Protocol No. IAEC/RES/11/2). Swiss albino mice (25–30 g) of either sex obtained from Hissar Agricultural University, Hissar, Haryana, India were used for the pharmacological studies. Pharmacokinetic studies showed that the exposure at 10 mg/kg dose was similar to the exposure of several atypical antipsychotics at therapeutically relevant doses, and hence this dose was chosen to carry out the in vivo pharmacological tests.
D2 receptor antagonism studies in nigrostriatal pathway (climbing mouse assay)
Apomorphine hydrochloride (1 mg/kg) solution (as per the base calculations) was prepared in triple distilled water containing 0.1% w/v sodium metabisulphite and was injected s.c. 1 h before testing.
Risperidone (0.6 mg/kg) and new chemical entities (NCEs) (10 mg/kg) were prepared as suspension in 0.25% w/v sodium carboxymethylcellulose in triple distilled water and were injected i.p. 30 min before testing.
Inhibition or reversal of apomorphine-induced cage-climbing behaviour in mice by a test molecule is an indication of mesolimbic dopaminergic D2 receptor antagonism.Citation21,Citation23 During the experimentation, mice were placed individually in separate aluminium cages, measuring 20 × 15 × 15 cm3, with walls lined with 1 cm2 aluminium wire mesh (diameter 2 mm). They were placed in the above cages 30 min for adaptation before the experiment. Groups of mice were administered with either the test molecule (10 mg/kg) or vehicle or risperidone i.p. 1 h prior to the apomorphine challenge (1 mg/kg, s.c.). Mice were then observed for the climbing behaviour after 10, 20, and 30 min and the scoring was done as below.
“0”, when all the four feet were placed on the cage floor
“1”, when three feet were placed on the cage floor
“2”, when two feet were placed on the cage floor
“3”, when one foot was placed on the cage floor
“4”, when all the four feet were off the cage floor.
The percentage inhibition or reversal of climbing behaviour of apomorphine hydrochloride was calculated by the difference from the score of treated subjects to the score of control animals and referring it to score of control group set to 100%. Haloperidol (1.0 mg/kg, i.p.) was used as standard as it completely inhibited the climbing induced by apomorphine.
5-HT2A receptor antagonism studies (quipazine-induced head twitches)
Quipazine maleate (5 mg/kg) solution (as per the base calculations) was prepared in triple distilled water containing 0.1% w/v sodium metabisulphite and was injected i.p. 30 min before testing. Risperidone (0.6 mg/kg) and NCEs (10 mg/kg) were prepared as suspension in 0.25% w/v sodium carboxymethylcellulose in distilled water and were also injected i.p. 30 min before testing.
Inhibition or reversal of quipazine-induced head twitches in mice by the test molecule is an indication of central serotonergic 5-HT2A receptor antagonism.Citation24 During the experimentation, mice were placed individually in separate plastic translucent cages, measuring 20 × 15 × 15 cm3. They were placed in the above cages 30 min for adaptation before the experiment. Groups of mice were administered i.p. with either the test molecule (10 mg/kg) or vehicle or risperidone 1 h prior to the quipazine maleate challenge (5 mg/kg, i.p.). Risperidone (0.6 mg/kg, i.p.) was used as standard as it completely inhibits quipazine-induced head twitches in mice. The head twitches were then counted between 30 and 40 min. The percentage inhibition or reversal of head twitches was calculated by the difference from the count of treated subjects to the count of control animals and referring it to count of control group set to 100%.
D2 receptor antagonism studies in nigrostriatal pathway (catalepsy test)
NCEs (10 mg/kg) were prepared as suspension in 0.25% w/v sodium carboxymethylcellulose in triple distilled water and were injected i.p. 30 min before testing.
Induction of catalepsy by the test molecules is an indication of antagonism at nigrostriatal dopaminergic D2 receptors leading to EPS.Citation25,Citation26 During the experimentation, mice were placed individually in separate plastic translucent cages, measuring 20 × 15 × 15 cm3. They were placed in the above cages 30 min for adaptation before the experiment. Groups of mice were administered i.p. with either the test molecule (10 mg/kg) or vehicle. The mice were then tested for catalepsy by placing both the front paws on a 4-cm high wooden block (6 × 4× 4 cm3) and measuring the time taken for it to come back to the normal posture. The scoring was done in accordance with literature.Citation26 If the animal maintained the imposed posture for at least 20 sec, then it was said to be cataleptic and given one point. For every further 20 sec it continued to maintain the imposed posture, an extra point was given, thus the animal was given a score of 2 points if it maintained the posture for 40 sec, 3 points for 60 sec, and so on. The mice were tested for cataleptic behaviour 1.0, 2.0, 3.0, 4.0, and 5.0 h after treatment with the test molecule. Average cataleptic times and scores were calculated at each time of measurement of cataleptic behaviour per molecule. The maximum of all average cataleptic scores/times were noted per molecule and then conclusions were drawn with respect to which test molecule is cataleptic and the degree of catalepsy.
Results and discussion
Substituted chloroethylphenylthiazoles were prepared as per the literature protocol with modifications in some steps.Citation22 2-(Piperazin-1-yl)-1,8-naphthyridine-3-carbonitrile was also prepared as defined using microwave irradiation technique.Citation21 Equimolar amounts of 2-(piperazin-1-yl)-1,8-naphthyridine-3-carbonitrile (9) and 2- and 5-substituted chloroethylphenylthiazoles (17) along with 2.125 equivalents of anhydrous Na2CO3 and catalytic amount of KI (2 mg) in DMF as solvent when refluxed for 48 h afforded the title compounds. All the synthesized compounds were characterized by spectral (IR, 1H NMR, and MS) and elemental analysis data. IR spectral analysis of the final compounds (10a–g) showed strong peaks at ~ 2245, ~ 1650, and ~ 825 cm−1 due to C≡N, C=N, and para disubstituted benzene functions, respectively. In 1H NMR spectra, methylene protons (cyclic) adjacent to N1 nitrogen of piperazine showed triplet in the range of δ 3.16–3.22, whereas methylene protons (cyclic) adjacent to N4 nitrogen of piperazine showed triplet in the range of δ 2.55–2.81. The final compounds showed the following 1H NMR signals for 1,8-naphthyridinyl moiety; C4-H: δ ~ 8.28–8.52 (s), C5-H: δ ~ 7.80–7.96 (d), C6-H: δ ~ 7.30–7.55 (dd), C7-H: δ ~ 9.07–9.34 (d). PMR signal corresponding to four protons of the ethyl linker was observed at δ 2.65–2.79 as multiplet. Elemental (CHNS) analysis indicated that the calculated and observed values were within the acceptable limits (±0.4%).
Although up to 1 µM, none of the NCEs tested inhibited either the specific binding of the 5-HT2A ligand [3H]ketanserine or the D2 ligand [3H]spiperone in vitro (), in vivo pharmacological data clearly indicate an atypical antipsychotic efficacy of the NCEs. Because the compounds obviously possess efficacy at micromolar concentrations in vivo, we assume that the observed slight increase in specific binding of the two radioligands in in vitro assays reflects cooperativity, that is, the binding of one ligand to one binding site changes the affinity of another ligand, because G-protein-coupled receptors such as 5-HT2A and D2 are characterized by this phenomenon. In particular, this has been shown for the binding of [3H]ketanserine in rabbit retinaCitation23 and in [3H]spiperone in cell culture.Citation24,Citation25 Furthermore, the inconsistency between in vitro and in vivo data might be attributed to the difference in the extent of uptake or distribution of NCEs into the target cells. The difference in the uptake or distribution into the cells could be because of the mechanism involved in the transportation of the NCEs such as carrier-mediated transport in the in vivo studies, although such mechanism might be absent in case of in vitro studies.
Table 1. Summary of in vitro radioligand displacement studies of final compounds (n = 3) (10a–g).
The effect of pretreatment with 10 mg/kg dose of the test compounds on apomorphine (0.5 mg/kg s.c.)-induced cage-climbing behaviour was studied by the literature method.Citation26 Haloperidol (1.0 mg/kg i.p.) was used as standard as it completely inhibited the climbing induced by apomorphine. Inhibition or reversal of quipazine-induced head twitches in mice by the test molecule (10 mg/kg dose) is an indication of central serotonergic 5-HT2A receptor antagonism and this behaviour was studied by the literature method.Citation27 Risperidone (0.6 mg/kg i.p.) was used as standard as it completely inhibits quipazine-induced head twitches in mice. Cataleptic effect of NCEs was evaluated by the literature methodCitation28 and scoring was done as per literature.Citation29
The initial trial compound, 10a, mimicking both the position and acidity of one of the phenol groups in dopamine, gratifyingly possesses the desired antagonistic activity at D2 receptor as it exhibited ≥60% inhibition in the in vivo studies. Among the synthesized compounds, 10c and 10d exhibited highest D2 antagonistic activity. On increasing the steric bulk of amino group (N-methyl derivatives), as seen in 10b and 10f, the D2 antagonistic activity decreased. Compound 10e, 2,5-dimethyl derivative, exhibited better D2 antagonistic activity than the monosubstituted derivative 10g. Compound 10f, N,5-dimethylthiazol-2-amine derivative, exhibited highest 5-HT2A antagonism. Except 10a, the remaining amino derivatives (10b, 10c, 10f) exhibited moderate to good 5-HT2A antagonism.
Overall seven compounds have been synthesized in the present series and 10f was the most active compound showing 79% and 70% 5-HT2A and D2 inhibition, respectively.
Conclusions
In summary, we have demonstrated the synthesis and pharmacological activity of novel 2-{4-[4-(2,5-disubstituted thiazolyl)phenylethyl]piperazin-1-yl}-1,8-naphthyridine-3-carbonitriles (10a–g) as atypical antipsychotic agents. The results () clearly indicate that all the NCEs have the capability of antagonizing mesolimbic dopaminergic D2 receptors with % inhibition varying between 45% and 95% at the dose level studied (10 mg/kg). A maximum of 95% inhibition was observed in 10c and 10d, whereas a minimum of 45% inhibition was noticed in 10b. The capability of antagonizing central serotonergic 5-HT2A receptors varied between 31% and 79% at the dose level studied (10 mg/kg). A maximum of 79% inhibition was evidenced in 10f, whereas a minimum of 31% inhibition was evidenced in 10a. The results tabulated in clearly indicate that the maximum average cataleptic score observed is 0 (as maximum average cataleptic time is <20 sec) for the NCEs at dose level studied (10 mg/kg) indicating that all compounds are noncataleptic. From , it is evident that 10f is the most active among the synthesized compounds with 5-HT2A/D2 ratio of 1.1286 and an average cataleptic score of zero (Risperidone exhibited 5-HT2A/D2 ratio of 1.0989). Hence 10f satisfies all the criteria required for a compound to be atypical antipsychotic according to Meltzer’s classification.Citation30
Table 2. Results of D2 and 5-HT2A antagonism studies of final compounds (10a–g).
Table 3. Results of D2 and 5-HT2A antagonism studies, catalepsy test of final compounds (10a–g).
Acknowledgements
Sincere thanks are due to UGC, New Delhi, India for providing financial assistance. The authors are grateful to Head, RSIC, CDRI, Lucknow for providing 1H NMR, elemental analysis, and mass spectral data.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Jablensky, A. The 100-year epidemiology of schizophrenia. Schizophr. Res. 1997, 28, 111–125.
- Mueser, K.T., McGurk, S.R. Schizophrenia. Lancet 2004, 363, 2063–2072.
- Lewine, R.R., Fogg, L., Meltzer, H.Y. Assessment of negative and positive symptoms in schizophrenia. Schizophr. Bull. 1983, 9, 368–376.
- Marder, S.R., Wirshing, W.C., Van Putten, T. Drug treatment of schizophrenia. Overview of recent research. Schizophr. Res. 1991, 4, 81–90.
- Tandon, R., Jibson, M.D. Efficacy of newer generation antipsychotics in the treatment of schizophrenia. Psychoneuroendocrinology 2003, 28(Suppl. 1), 9–26.
- Miyamoto, S., Duncan, G.E., Marx, C.E., Lieberman, J.A. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol. Psychiatry 2005, 10, 79–104.
- Horacek, J., Bubenikova-Valesova, V., Kopecek, M., Palenicek, T., Dockery, C., Mohr, P., Höschl, C. Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia. CNS Drugs 2006, 20, 389–409.
- Meltzer, H.Y., Matsubara, S., Lee, J.C. The ratios of serotonin2 and dopamine2 affinities differentiate atypical and typical antipsychotic drugs. Psychopharmacol. Bull. 1989, 25, 390–392.
- Roth, B.L., Meltzer, H.Y., Khan, N. Binding of typical and atypical antipsychotic drugs to multiple neurotransmitter receptors. Adv. Pharmacol. 1998, 42, 482–485.
- Lowe, J.A., III. Atypical antipsychotics based on the D2/5-HT2 ratio hypothesis. Curr. Med. Chem. 1994, 1, 50–60.
- Meltzer, H.Y., Li, Z., Kaneda, Y., Ichikawa, J. Serotonin receptors: their key role in drugs to treat schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2003, 27, 1159–1172.
- Meltzer, H.Y. What’s atypical about atypical antipsychotic drugs? Curr. Opin. Pharmacol. 2004, 4, 53–57.
- Wirshing, D.A., Wirshing, W.C., Kysar, L., Berisford, M.A., Goldstein, D., Pashdag, J., Mintz, J., Marder, S.R. Novel antipsychotics: comparison of weight gain liabilities. J. Clin. Psychiatry 1999, 60, 358–363.
- Owens, D.G. Adverse effects of antipsychotic agents. Do newer agents offer advantages? Drugs 1996, 51, 895–930.
- Cohen, D. Atypical antipsychotics and new onset diabetes mellitus. An overview of the literature. Pharmacopsychiatry 2004, 37, 1–11.
- Tamminga, C.A. The promise of new drugs for schizophrenia treatment. Can. J. Psychiatry 1997, 42, 265–273.
- Chandra Sekhar, K.V.G., Rao, V.S., Vyas, D.R.K., Kumar, M.M.K. Synthesis and preliminary pharmacological evaluation of N-2-(4-(4-(2-substituted thiazol-4-yl)piperazin-1-yl)-2-oxoethyl)acetamides as novel atypical antipsychotic agents. Bioorg. Med. Chem. Lett. 2008, 18, 6054–6057.
- Chandra Sekhar, K.V.G., Rao, V.S., Vyas, D.R.K., Kumar, M.M.K. Synthesis and preliminary screening of novel N-{2-[4-(substituted)piperazin-1-yl]-2-oxoethyl}acetamides as potential atypical antipsychotic agents. J. Enzyme Inhib. Med. Chem. 2009, 24, 871–875.
- Howard, H.R., Lowe, J.A. 3rd, Seeger, T.F., Seymour, P.A., Zorn, S.H., Maloney, P.R., Ewing, F.E., Newman, M.E., Schmidt, A.W., Furman, J.S., Robinson, G.L., Jackson, E., Johnson, C., Morrone, J. 3-Benzisothiazolylpiperazine derivatives as potential atypical antipsychotic agents. J. Med. Chem. 1996, 39, 143–148.
- Ariens, E.J., Beld, A.J., Rodrigues, J.M.F., Simonis, A.M. In The Receptors: A Comprehensive Treatise. O’Brien (Ed.), Plenum Press, New York, 1979, Vol. 1, p. 33.
- Mahesh, R., Perumal, R.V., Pandi, P.V. Microwave assisted synthesis of 2-(4-substituted piperazin-1-yl)-1,8-naphthyridine-3-carbonitrile as a new class of serotonin 5-HT3 receptor antagonists. Bioorg. Med. Chem. Lett. 2004, 14, 5179–5181.
- Lowe, J.A. 3rd, Seeger, T.F., Nagel, A.A., Howard, H.R., Seymour, P.A., Heym, J.H., Ewing, F.E., Newman, M.E., Schmidt, A.W., Furman, J.S. 1-Naphthylpiperazine derivatives as potential atypical antipsychotic agents. J. Med. Chem. 1991, 34, 1860–1866.
- Schmeer, C., Lima, L. Modulation of outgrowth from goldfish retinal explants by a 5-HT2 receptor agonist and [3H]ketanserin binding sites in goldfish and rabbit retina. Vision Res. 2000, 40, 33–40.
- Vivo, M., Lin, H., Strange, P.G. Investigation of cooperativity in the binding of ligands to the D(2) dopamine receptor. Mol. Pharmacol. 2006, 69, 226–235.
- Armstrong, D., Strange, P.G. Dopamine D2 receptor dimer formation: evidence from ligand binding. J. Biol. Chem. 2001, 276, 22621–22629.
- Costall, B., Naylor, R.J., Nohria, V. Climbing behaviour induced by apomorphine in mice: a potential model for the detection of neuroleptic activity. Eur. J. Pharmacol. 1978, 50, 39–50.
- Malick, J.B., Doren, E., Barnett, A. Quipazine-induced head-twitch in mice. Pharmacol. Biochem. Behav. 1977, 6, 325–329.
- Costall, B., Naylor, R.J. Mesolimbic involvement with behavioural effects indicating antipsychotic activity. Eur. J. Pharmacol. 1974, 27, 46–58.
- Joshi, V.V., Muley, M.P., Balsara, J.J., Chandorkar, A.G. Effect of l-histidine pretreatment on haloperidol induced catalepsy and methamphetamine stereotypy in mice. Indian J. Pharmacol. 1979, 11, 293–300.
- Meltzer, H.Y., Matsubara, S., Lee, J.C. The ratios of serotonin2 and dopamine2 affinities differentiate atypical and typical antipsychotic drugs. J. Pharmacol. Exp. Ther. 1989, 251, 238–246.
![Scheme 3. Synthesis of 2-{4-[4-(2,5-disubstituted thiazolyl)phenylethyl]piperazin-1-yl}-1,8-naphthyridine-3-carbonitriles (10a–g).](/cms/asset/02724428-bcd9-44d2-8ad1-6337baba8701/ienz_a_537658_f0003_b.gif)