Abstract
A series of 4-alkylamino-2-ethoxycyclobut-3en-1,2-diones has been synthesized, characterized and their inhibitory effect on pancreatic lipase (PL) was evaluated. The compound 1 has shown relatively high potency (IC50 = 0.11 mM) compared with the most effective anti-obesity drug, tetrahydrolipstatin (Orlistat) (IC50 value = 0.08 mM). The compounds have showed good selectivity toward PL and did not affect the activity of trypsin, another digestive enzyme.
Introduction
Obesity is a widespread nutritional disorder in Europe and United States, which is reaching epidemic proportions. As a result of imbalance of food intake and expenditure, the excess of energy is accumulated in adipose tissues.Citation1 Overweight is considered to be a major risk factor for development of many serious diseases such as type II diabetes, coronary heart diseases, metabolic disorders, osteoarthritis and so on.Citation2,Citation3 There are several approaches for treating the overweight people: suppression of dietary intake (drugs that reduce food intake, elicit the feeling of satiety), increasing of thermogenesis or altering metabolism.Citation4,Citation5 Unfortunately, many of the approved medications have serious undesirable effects on nervous and cardiovascular systems of the patients. This makes most of the preparations applicable only for a short term (<12 weeks).Citation6 The treatment of obesity is time-consuming, costly, and often the results obtained for few weeks are not considerable.
The most effective way to reduce body weight up-to-date is the regulation of fat absorption. The pancreatic lipase (PL) is a key enzyme for the lipid digestion. Orlistat (Xenical) is a hydrogenated derivative of lipostatin isolated from Streptomyces toxytricini. It is a reversible lipase inhibitor that reduces the amount of hydrolysed dietary fat and thus their absorption of respective calorie intake.Citation7,Citation8 It is the only one medication approved for a long-term management of obesity (up to 2 years). Satisfying results such as significant weight loss, improvement in blood pressure and blood glucose levels are obtained when Orlistat is applied to obese patients. Although few in numbers, this preparation has side effects such as abdominal cramps, diarrhoea and deficiency of the fat-soluble vitamins. Many isolates from medicinal plants or synthetic compounds inhibit PL.Citation9 They, however, are not selective against the lipase and affect the activity of the other digestive enzymes. There is still great demand for new effective and selective PL inhibitors.
In this article, we described the synthesis of a series of novel ester amides of squaric acid. All compounds were characterized and their inhibitory activity against PL was estimated. The results were compared with those obtained for Orlistat. We followed the effect of the substituent in the molecule of squaric acid on the selectivity of the compounds toward two digestive enzymes.
Materials and methods
Materials
3,4-Dihydroxy-3-cyclobutene-1,2-dione (squaric acid) (98% purity), 3,4-diethoxy-3-cyclobutene-1,2-dione (squaric acid diethyl ester) (98% purity), hexadecylamine (98% purity), octylamine (99.5% purity), butylamine (99% purity), dibutylamine (99% purity) and tert-butylamine (98% purity) were purchased from Aldrich (Germany). Orlistat (98% purity) and glyceryl trioleate (triolein, 99% purity), N-benzoyl-l-arginine-p-nitroanilide hydrochloride (98% purity) were obtained from (Sigma, Germany). Lipase from porcine pancreas (PL) (EC 3.1.1.3; 20.6 U/mg, olive oil as a substrate) and trypsin from hog pancreas (EC 3.4.21.4; 1473 U/mg, N-benzoyl-l-arginine ethyl ester as a substrate) was supplied by Fluka (Germany).
The progress of the reactions was monitored by thin-layer chromatography (TLC) on silica gel G 60 F254 plates (Merck, Germany). All solvents used in this study were of analytical grade.
Melting points of the synthesized compounds were determined on BÜCHI Melting Point B-540. IR spectra were recorded with IR spectrometer Bruker Tensor 27. 1H-NMR spectra were recorded on a Bruker Avance DRX-250 (250 MHz) spectrometer, chemical shifts were reported in ppm (δ) and referenced to TMS (δH = 0 ppm). The kinetic studies with trypsin were performed using Shimadzu UV-3000 spectrophotometer.
General procedure for the synthesis of 4-amino-3-ethoxy-3-cyclobutene-1,2-diones
Five novel ester amides of the squaric acid have been synthesized following the procedure described by Tietze et al.Citation10 To a solution of 3,4-diethoxy-3-cyclobutene-1,2-dione (1.7 mmol) in 10 mL ethanol was added the corresponding amine (1.9 mmol). The reaction mixture was magnetically stirred at room temperature until the complete conversion of the squaric acid diethyl ester (0.5–24 h). Then, the solvent was evaporated and the target product was purified either by crystallization or by solid phase extraction (SPE) on silica gel 60.
Structural characterization data for the synthesized compounds
3-Ethoxy-4-(hexadecylamino)-3-cyclobutene-1,2-dione (1)
White crystal (powder), Rf = 0.54 (chloroform/methanol; 9:0.5 v/v), yield 78%, mp 79.9–82.0°C (methanol/water). IR (cm−1) (KBr) ν = 3254; 3233 (N–H); νas = 2952 (CH3); νas = 2917 (CH2); νs = 2870 (CH3); νs = 2850 (CH2); νs = 1814 (C=O); νas = 1691 (C=O); ν = 1653 (C=C); 1539. 1H-NMR (CDCl3) (δ, ppm) 0.9–0.85 (t, J = 6.6 Hz, 3H, CH2CH3); 1.73–1.21 (m, 31H, 14CH2 + CH3); 3.76–3.67 (q, J = 7.0 Hz, 2H, CH2CH2NH); 4.81–4.73 (q, J = 7.0 Hz, 2H, CH2O); 8.63–8.62 (s, 1H, NHCH2).
C22H39NO3 (365.19) Calcd. % C 72.30 H 10.70 N 3.80; Found % C 72.90 H 10.68 N 3.75.
3-Ethoxy-4-(octylamino)-3-cyclobutene-1,2-dione (2)
White crystal (powder), Rf = 0.46 (chloroform/methanol; 9:0.5 v/v), yield 88%, mp 55.4–57.2°C (methanol/water). IR (cm−1) (KBr) ν = 3248; 3232 (N–H); νas = 2950 (CH3); νas = 2917 (CH2); νs = 2871 (CH3); νs = 2852 (CH2); νs = 1815 (C=O); νas = 1689 (C=O); ν = 1652 (C=C). 1H-NMR (CDCl3) (δ, ppm) 0.89–0.87 (t, J = 6.9 Hz, 3H, CH2CH3); 1.37–1.23 (m, 13H, 5CH2CH2 + CH3); 1.63–1.58 (quintet, 2H, CH2CH2CH2); 3.43–3.47 (q, J = 13.3 Hz, J = 6.9 Hz, 2H, CH2NH); 4.8–4.74 (q, J = 14 Hz, J = 6.9 Hz, 2H, CH2O); 8.63–8.62 (s, 1H, NHCH2).
C14H23NO3 (253.4) Calcd. % C 66.4 H 9.15 N 5.52; Found % C 66.7 H 8.91 N 5.55.
3-(Butylamino)-4-ethoxy-3-cyclobutene-1,2-dione (3)
White crystal, Rf = 0.60 (chloroform/methanol; 9:0.5 v/v), yield 75%, mp 48.0–50°C (methanol). IR (cm−1) (KBr) ν = 3259; 3236 cm−1 (N–H); νas = 2964 (CH3); νas = 2935 (CH2); νs = 2874 (CH3); νs = 1813 (C=O); νas = 1706; 1698 (C=O); ν = 1648; 1629 (C=C). 1H-NMR (d6-DMSO) (δ, ppm) 0.84–0.90 (t, J = 7.2 Hz, 3H, CH2CH3); 1.55–1.125 (m, 5H, CH2 + CH3); 2.58–2.48 (quintet, J = 3.6 Hz, J = 1.7 Hz, 2H, CH2CH2CH2); 3.50–3.24 (q, J = 13.3 Hz, J = 6.6 Hz, 2H, NHCH2); 4.69–4.61 (q, J = 13.9 Hz, J = 6.9 Hz, 2H, CH2O); 8.77–8.56 (s, 1H, NHCH2).
C10H15NO3 (197.23) Calcd. % C 60.89 H 7.66 N 7.10; Found % C 60.34 H 7.43 N 6.65.
3-(Dibutylamino)-4-ethoxy-3-cyclobutene-1,2-dione (4)
Yellowish oil, Rf = 0.81 (chloroform/methanol; 9:0.5 v/v), yield 55% (SPE, n-hexane/ethyl acetate; 15:0.5, v/v). IR (cm−1) (film on KBr) νas = 2960 (CH3); νas = 2933 (CH2); νs = 2874 (CH3); νs = 1797 (C=O); νas = 1710 (C=O); ν = 1653 (C=C). 1H-NMR (CDCl3) (δ, ppm) 0.91–1.0 (2t, J = 7.2 Hz, 6H, 2CH3); 1.67–1.32 (m, 11H, 4CH2 +CH3); 3.69–3.34 (2t, 4H, 2CH2); 4.8–4.72 (q, J = 7.1 Hz, 2H, CH2O).
C14H23NO3 (253.34) Calcd. % C 66.37 H 9.14 N 5.52; Found % 66.03 H 8.93 N 5.52.
3-(1,1-Dimethylethylamino)-4-ethoxy-3-cyclobutene-1,2-dione (5)
White crystal, Rf = 0.55 (chloroform/methanol; 9:0.5 v/v), yield 77%, mp 85.0–86.0°C (SPE, n-hexane/ethyl acetate; 15:0.5). IR (cm−1) (KBr) ν = 3215; 3145 (N–H); νas = 2988; 2974 (CH3); νs = 2939 (CH3); νs = 1794 (C=O); νas = 1702 (C=O); ν = 1607 (C=C). 1H-NMR (CDCl3) (δ, ppm) 1.78–0.91 (m, 14H, 4CH3 + 1CH2); 4.85 (s, 1H, NHCH2).
C10H15NO3 (197.23) Calcd. % C 60.89 H 7.66 N 7.10; Found % C 61.20 H 7.76 N 7.01.
Lipase inhibition assay
The inhibitory effect of the newly synthesized compounds on PL was estimated using titration method, previously reported by Karadzic et al. with modifications.Citation11
The substrate emulsion was prepared by 10-min sonication of a mixture consisted of 15 mL triolein, 50 mL emulsifying reagent (1.8 g NaCl, 0.04g KH2PO4, 50 mL glycerol, 0.6 g gum arabic in 100 mL deionized water) and 200 mL deionized water. Stock solutions (0.04 mol/L) of all studied inhibitors were prepared in dimethyl sulphoxide (DMSO). The concentration of DMSO in the reaction was 5% and did not affect the lipase activity.
The reaction mixture containing 1.5 mL substrate emulsion, 0.1 mL PL (1 U/mL stock solution) and 0.1 mL of the studied compounds (various concentrations) was incubated at 37°C for 30 min.
The lipase reaction was terminated by addition 10 mL ethanol–acetone mixture (1:1, v/v). The liberated oleic acid was titrated with 0.05 N sodium hydroxide using phenolphthalein as an indicator. Lipase inhibition was calculated from the residual activity detected in the presence of the compound with respect to that of the sample without additive. IC50, the concentration of the tested compound required to decrease the lipase activity by 50%, was determined graphically by a plot of percent of inhibitions versus log concentrations of the test sample. All experiments were done in triplicate the value reported referred to means. Standard deviation was determined by Microsoft Excel Program.
Trypsin activity test
The trypsin activity was determined by monitoring the hydrolysis of a chromogenic substrate N-α-benzoyl-l-arginine-p-nitroanilide (BAPNA).Citation7 All tested compounds and BAPNA were dissolved in DMSO.
To 1.8 mL 0.05 M Tris–HCl buffer (pH 7.5) were added 0.1 mL BAPNA (9.3 mM stock solution) and 0.1 mL of the studied compounds (various concentrations). The reaction started by adding 0.1 mL trypsin from hog pancreas (0.5 mg/mL stock solution in Tris–HCl buffer, pH 7.5). The temperature was maintained 25°C. The quantity of the released 4-nitroaniline was followed spectrophotometrically at 410 nm. All experiments were done in triplicate. The inhibitory activity (%) was calculated as (1−A/B) × 100, where A and B represent the activity of the compound with and without inhibitor.
Results and discussion
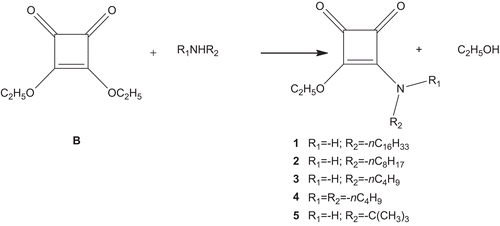
The design of the compounds was made considering the structure of the active site of the human PL and the molecule of the most effective anti-obesity drug. They all contain four-membered ring with ethoxy group in third position and different chain-length substituents in fourth position of the cycle, as well as two keto functional groups. The title compounds (1–5) were synthesized by reaction of squaric acid diethyl ester with an appropriate alkyl amine at equimolar ratio in ethanol () as described in the ‘Materials and methods’ section. The isolated and purified products were analysed by NMR and IR spectroscopy.
In a test reaction of hydrolysis of triolein, we have found that all studied compounds inhibit PL (). The substitution of the two hydroxyl groups in the molecule of squaric acid (A) with ethoxy groups (B) reduced six times the concentration required for a 50% inhibition of lipase activity. Among monoamino-derivatives with straight alkyl chains (1–3), the inhibitory potential of the compounds increased with the increase of the chain length of the substituent. The hexadecylamino-derivative (1) was one order of magnitude more active than the octylamino- (2) and butylamino-derivatives (3). Probably, this is due to stronger hydrophobic interactions between the inhibitor and the lipid-binding subsite of the enzyme. The introduction of a second substituent at the nitrogen atom improves the inhibitory activity of the compound. The dibutylamino-derivative (4) was five times more active than monobutylamino-derivative (3). Within the series, the compound with the bulk hydrophobic residue (5) showed the weakest inhibitory activity against the lipase. We assumed that due to a steric hindrance of the substituent the molecule could not be properly positioned in the active site of the enzyme. The compound 1 showed inhibitory activity (IC50 value: 0.11 mM), which is of the same order of magnitude as the positive control, Orlistat (IC50 value: 0.08 mM). As shown in , the inhibition of PL with compound 1 is not time-dependent in comparison with squaric acid (A).
Figure 1. IC50 for dihydroxy-(A), diethoxy-(B) derivatives of cyclobutendione, the newly synthesized compounds (1)–(5), and Orlistat. IC50 is the concentration of tested compound required for a 50% inhibition of the lipase activity. Data are presented ±standard deviation.
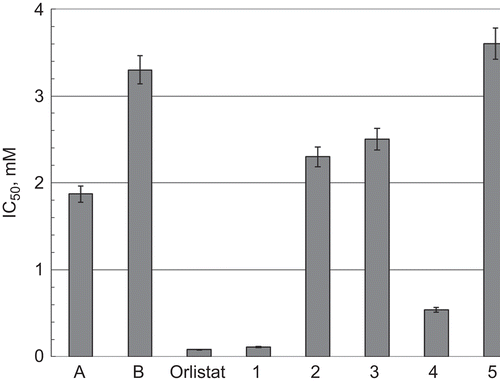
Figure 2. The dependence of the inhibitory activity of 3-ethoxy-4-(hexadecylamino)-3-cyclobutene-1,2-dione (1) and squaric acid from the incubation time. Solid squares, squaric acid; solid circles, compound 1.
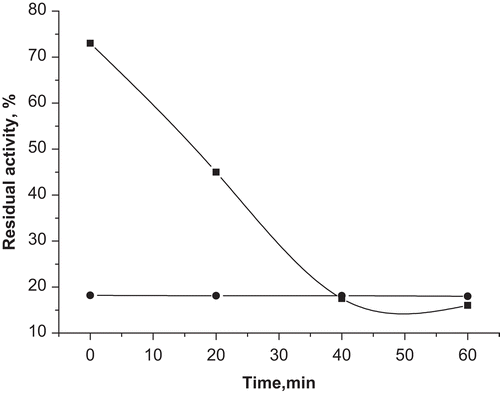
Probably, in the case of squaric acid, the rate of formation of the enzyme–inhibitor complex was comparable with the rate of the hydrolytic reaction, which means that A bound slowly to PL active site. The enzyme has showed higher affinity to 1.
Based on the crystallographic studies, many authors proposed similar way of binding of the substrates for most of the lipases.Citation12 It was found that the ester carbonyl groups in triacylglycerols, the natural substrate of lipases, are of great importance for its binding in the enzyme active centre. They interact with the amino acid residues forming the ‘oxyanion hole’, thus ensure appropriate binding of the molecule of the substrate and facilitate the nucleophile attack of the Oγ of the serine from the active site. It is proposed that two of the fatty acid residues of triacylglycerols are bound to hydrophobic ‘tunnels’ inside the enzyme and the third residue points into the solvent.
We assumed that the molecules of the studied compounds are attached similarly to the enzyme. With their carbonyl groups and hydrophobic substituents, they interact via hydrogen bonding and hydrophobic interactions, respectively, with the amino acid residues from the substrate-binding subsites and hinder the access of the substrate to the active site.
The catalytic site of trypsin and chymotrypsin, other digestive enzymes, is composed of the same residues as the active centre of PL. The three enzymes possess a catalytic triad Asp(Glu)-Ser-His and share a common catalytic mechanism although their substrates are different.Citation13 This makes difficult the discovery of selective inhibitors that affect only lipase activity and do not influence the proteolytic enzymes.
We estimated the effect of the studied compounds on the activity of trypsin using a spectrophotometric test with N-α-benzoyl-l-arginine-p-nitroanilide as a substrate.Citation7 In the test reaction, some of the compounds demonstrated poor solubility. The inhibitory activity of compounds (2), (3) and (5) against trypsin was studied in the concentration range up to IC50 of these compounds calculated for the PL. No inhibition by these compounds was detected. Squaric acid strongly deactivated trypsin and IC50 value was calculated to be 1.45 mM. The compounds with the highest anti-lipase activity (1 and 4) have been well-soluble in the test reaction mixture and were studied in a broader range reaching concentration twice higher than IC50 values estimated for the PL. The two compounds proved to be very selective for the lipase and did not suppress the trypsin activity.
The achieved results indicate that the ring structure and the carbonyl groups of the studied compounds is of importance for their correct binding in the active site of the PL and trypsin; however, the type of substituents determines the selectivity.
In conclusion, all compounds under investigation have been good lipase inhibitors. The hexadecylamino-derivative (1) has shown the highest selectivity and activity against PL. It has exhibited the activity as high as that of Orlistat. Further investigations on the safety and efficacy in vitro are needed to reveal its potential for anti-obesity drug.
Declaration of interest
The authors declare no conflict of interest.
References
- Redinger RN. Fat storage and the biology of energy expenditure. Transl Res 2009;154:52–60.
- Culebras A. A review of frontiers in clinical sleep medicine. Rev Neurol Dis 2010;7:9–18.
- Wannamethee SG, Shaper AG, Whincup PH, Walker M. Overweight and obesity and the burden of disease and disability in elderly men. Int J Obes 2004;28:1374–1382.
- Li M, Cheung BM. Pharmacotherapy for obesity. Br J Clin Pharmacol 2009;68:804–810.
- Bray GA. Drug treatment of obesity. Baillieres Best Pract Res Clin Endocrinol Metab 1999;13:131–148.
- Adan RA, Vanderschuren LJ, la Fleur SE. Anti-obesity drugs and neural circuits of feeding. Trends Pharmacol Sci 2008;29:208–217.
- Hadváry P, Lengsfeld H, Wolfer H. Inhibition of pancreatic lipase in vitro by the covalent inhibitor tetrahydrolipstatin. Biochem J 1988;256:357–361.
- Tiss A, Lengsfeld H, Carrièrec F, Verger R. Inhibition of human pancreatic lipase by tetrahydrolipstatin: further kinetic studies showing its reversibility. J Mol Catal B-Enzym 2009;58:41–47.
- Birari RB, Bhutani KK. Pancreatic lipase inhibitors from natural sources: unexplored potential. Drug Discov Today 2007;12:879–889.
- Tietze L, Arlt M, Beller M, Glüsenkamp K-H, Jähde E, Rajewsky M. Squaric acid diethyl ester: a new coupling reagent for the formation of drug biopolymer conjugates. Synthesis of squaric acid ester amides and diamides. Chem Ber 1991;124:1215–1221.
- Karadzic I, Masui A, Zivkovic LI, Fujiwara N. Purification and characterization of an alkaline lipase from Pseudomonas aeruginosa isolated from putrid mineral cutting oil as component of metalworking fluid. J Biosci Bioeng 2006;102:82–89.
- Mannesse ML, Boots JW, Dijkman R, Slotboom AJ, van der Hijden HT, Egmond MR et al. Phosphonate analogues of triacylglycerols are potent inhibitors of lipase. Biochim Biophys Acta 1995;1259:56–64.
- Polgár L. The catalytic triad of serine peptidases. Cell Mol Life Sci 2005;62:2161–2172.