Abstract
A new series of biologically active thienyl derived triazole Schiff bases and their oxovanadium(IV) complexes have been synthesized and characterized on the basis of physical (m.p., magnetic susceptibility and conductivity), spectral (IR, 1H and 13C NMR, electronic and mass spectrometry) and microanalytical data. All the Schiff base ligands and their oxovanadium(IV) complexes have been subjected to in vitro antibacterial activity against four Gram-negative (Escherichia coli, Shigella flexneri, Pseudomonas aeruginosa, Salmonella enterica serover typhi) and two Gram-positive (Staphylococcus aureus and Bacillus subtilis) bacterial strains and, for in vitro antifungal activity against Trichophyton longifucus, Candida albican, Aspergillus flavus, Microscopum canis, Fusarium solani and Candida glabrata. Brine shrimp bioassay was also carried out to check the cytotoxic nature of these compounds.
Introduction
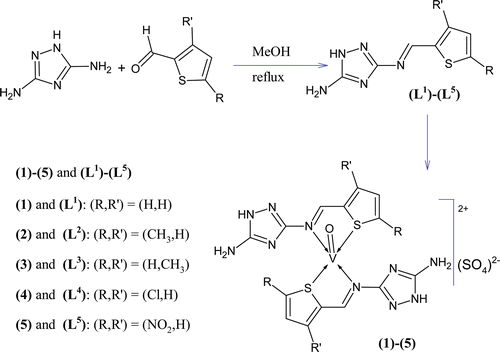
Compounds having S, N and O donor atoms play a vital role in antibacterialCitation1–3, antifungalCitation4,Citation5, antitumorCitation6, antitubercularCitation7,Citation8, anticancerCitation9 and antiviralCitation10 activities due to their significant biological properties. Presently, the vanadium metal chemistry is gaining attention for its remarkable activities such as antimicrobialCitation11, antitumorCitation12, and most recently as insulin mimeticCitation13. In continuation of our previous efforts to design and synthesize biologically active compounds, we have prepared a new series of thiophene derived triazole Schiff base ligands (L1)–(L5) and their oxovanadium(IV) complexes (1)–(5) () and wish to report in this paper their characterization and in vitro antibacterial, antifungal and cytotoxic properties.
Materials and methods
All the chemicals used for preparation of the triazole Schiff base ligands and their oxovanadium(IV) complexes were of analytical grades. Melting points were recorded on Fisher Johns equipment. IR spectra were recorded on Shimadzu FTIR spectrophotometer. Elemental analysis (C, N, H) was carried out on Perkin-Elmer (USA model). 1H and 13C NMR spectra were recorded on a Bruker Spectrospin Avance DPX spectrometer at 400 MHz, respectively. Electron impact mass spectra (EIMS) were recorded on JEOL MS Route instrument. Stanton SM12/S Gouy’s balance was used to measure magnetic susceptibility of the oxovanadium complexes at room temperature. Molar conductance was taken by Inolab Cond 720 Conductivity Bridge. In vitro antibacterial, antifungal and cytotoxic properties were studied at HEJ Research Institute of Chemistry, International Centre for Chemical Sciences, University of Karachi, Pakistan.
Synthesis of triazole derived Schiff base (L1)
An equimolar methanol solution of 3,5-diamino-1,2,4-triazole (0.99 g, 10 mmol, 20 mL) and thiophene-2-carboxaldehyde (0.94 mL, 10 mmol, 20 mL) was stirred at room temperature for 2 h and the reaction was monitored by TLC. Precipitation took place during stirring. It was then filtered, washed with methanol, then with diethyl ether and dried under vacuum. Recrystallization was carried out in a mixture of methanol:dioxane (1:4) to get required TLC pure product (L1) in 70% yield. The same method was applied for the preparation of other ligands (L2)–(L5) ().
N3-[(E)-thiophen-2-ylmethylidene]-1H-1,2,4-triazole-3,5-diamine (L1)
Yield (1.35 g, 70%); m.p. 172–174°C; IR (KBr, cm−1): 3345 (NH2), 3190 (NH), 1631 (HC=N), 1609 (C=N, triazole), 1570, 1546 (C=C), 1020 (N-N), 882 (C-S); 1H NMR (DMSO-d6, δ ppm): δ 6.05 (s, 2H, NH2), 7.20 (dd, 1H, J = 4.9, 3.8 Hz, thienyl C4-H), 7.71 (d, 1H, J = 4.9 Hz, thienyl C3-H), 7.81 (d, 1H, J = 3.8 Hz, thienyl C5-H), 8.32 (s, 1H, C6-H), 11.92 (s, 1H, NH); 13C NMR (DMSO-d6, δ ppm): δ 125.22 (C5), 133.54 (C4), 138.72 (C3), 144.31 (C2), 156.12 (C6), 160.97 (C8), 163.98 (C7); EIMS (70 eV) m/z (%): 193.2 ([M]+, 100), 177 (5), 137 (34), 122 (22), 110 (10), 96 (21), 84 (13); Anal. Calcd. for C7H7N5S (193.23): C, 43.51; H, 3.65; N, 36.24; S, 16.59; Found: C, 43.49; H, 3.61; N, 36.21; S, 16.55%.
N3- [(E)-(5-methylthiophen-2-yl)methylidene]-1H-1,2,4-triazole-3,5-diamine (L2)
Yield (1.59 g, 78%); m.p. 234–236°C; IR (KBr, cm−1): 3344 (NH2), 3187 (NH), 1633 (HC=N), 1609 (C=N, triazole), 1578, 1556 (C=C), 1018 (N-N), 884 (C-S); 1H NMR (DMSO-d6, δ ppm): δ 2.45 (s, 3H, CH3), 5.97 (s, 2H, NH2), 6.90 (d, 1H, J = 3.8 Hz, thienyl C4-H), 7.50 (d, 1H, J = 3.6 Hz, thienyl C3-H), 8.41 (s, 1H, C6-H), 11.86 (s, 1H, NH); 13C NMR (DMSO-d6, δ, ppm): δ 15.62 (CH3- thienyl), 126.97 (C4), 134.61 (C3), 140.15 (C5), 145.89 (C2), 156.39 (C6), 161.27 (C8), 163.54 (C7); EIMS (70 eV) m/z (%): 207 ([M]+, 100), 192 (5), 151 (22), 136 (12), 123 (9), 110 (13), 109 (9), 84 (7); Anal. Calcd. for C8H9N5S (207.26): C, 46.36; H, 4.38; N, 33.79; S, 15.47; Found: C, 46.36; H, 4.35; N, 33.76; S, 15.44%.
N3-[(E)-(3-methylthiophen-2-yl)methylidene]-1H-1,2,4-triazole-3,5-diamine (L3)
Yield (1.43 g, 69%); m.p. 239–240°C; IR (KBr, cm−1): 3343 (NH2), 3187 (NH), 1629 (HC=N), 1610 (C=N, triazole), 1575, 1562 (C=C), 1020 (N-N), 885 (C-S); 1H NMR (DMSO-d6, δ ppm): δ 2.22 (s, 3H, CH3), 6.04 (s, 2H, NH2), 7.0 (d, 1H, J = 3.8 Hz, thienyl C4-H), 7.68 (d, 1H, J = 3.7 Hz, thienyl C5-H), 8.42 (s, 1H, C6-H), 11.88 (s, 1H, NH); 13C NMR (DMSO-d6, δ, ppm): δ 16.78 (CH3- thienyl), 122.63 (C2), 126.39 (C4), 135.54 (C5), 143.77 (C3), 156.93 (C6), 160.85 (C8), 163.85 (C7); EIMS (70 eV) m/z (%): 207.2 ([M]+, 100), 192 (5), 174 (4), 165 (5), 151 (19), 136 (11), 134 (14), 124 (13), 115 (15), 109 (18), 84 (5); Anal. Calcd. for C8H9N5S (207.26): C, 46.36; H, 4.38; N, 33.79; S, 15.47; Found: C, 46.31; H, 4.36; N, 33.76; S, 15.41%.
N3-[(E)-(5-chlorothiophen-2-yl)methylidene]-1H-1,2,4-triazole-3,5-diamine (L4)
Yield (1.69 g, 74%); m.p. 224–225°C; IR (KBr, cm−1): 3346 (NH2), 3186 (NH), 1636 (HC=N), 1612 (C=N, triazole), 1567, 1544 (C=C), 1022 (N-N), 887 (C-S), 810 (C-Cl); 1H NMR (DMSO-d6, δ ppm): δ 6.07 (s, 2H, NH2), 7.33 (d, 1H, J = 2.8 Hz, thienyl C4-H), 7.73 (d, 1H, J = 2.6 Hz, thienyl C3-H), 8.53 (s, 1H, C6-H), 12.15 (s, 1H, NH); 13C NMR (DMSO-d6, δ, ppm): δ 124.43 (C3), 126.82 (C4), 132.36 (C5), 143.39 (C2), 157.22 (C6), 161.12 (C8), 164.12 (C7); EIMS (70 eV) m/z (%): 228.3 ([M]+, 100), 192 (31), 171 (24), 158 (6), 144 (5), 130 (10), 119 (13), 95 (8), 84 (16); Anal. Calcd. for C7H6ClN5S (227.67): C, 36.93; H, 2.66; N, 30.76; S, 14.08; Found: C, 36.91; H, 2.64; N, 30.71; S, 14.05%.
N3-[(E)-(5-nitrothiophen-2-yl)methylidene]-1H-1,2,4-triazole-3,5-diamine (L5)
Yield (1.61 g, 68%); m.p. 188–189°C; IR (KBr, cm−1): 3349 (NH2), 3194 (NH), 1637 (HC=N), 1611 (C=N, triazole), 1565, 1552 (C=C), 1355 (NO2), 1024 (N-N), 889 (C-S); 1H NMR (DMSO-d6, δ, ppm): δ 6.09 (s, 2H, NH2), 7.94 (d, 1H, J = 3.4 Hz, thienyl C3-H), 8.04 (d, 1H, J = 3.2 Hz, thienyl C4-H), 8.68 (s, 1H, C6-H), 12.20 (s, 1H, NH); 13C NMR (DMSO-d6, δ, ppm): δ 129.39 (C3), 134.43 (C4), 145.17 (C5), 149.97 (C2), 157.48 (C6), 161.88 (C8), 166.24 (C7); EIMS (70 eV) m/z (%): 238.2 ([M]+, 100), 192 (95), 182 (11), 157 (6), 150 (19), 136 (8), 123 (5), 109 (12), 95 (20); Anal. Calcd. for C7H6N6O2S (238.23): C, 35.29; H, 2.54; N, 35.28; S, 13.46; Found: C, 35.26; H, 2.51; N, 35.25; S, 13.41%.
Synthesis of oxovanadium(IV) complexes
Oxovanadium(IV) complex with (L1) (1)
To a hot magnetically stirred dioxane solution of N3-[(E)-thiophen-2-ylmethylidene]-1H-1,2,4-triazole-3,5-diamine (L1) (0.39 g, 2 mmol, 30 mL), a methanol solution of vanadyl(IV) sulphate (0.163 g, 1 mmol, 20 mL) was added. The mixture was refluxed for 3 h and then cooled to room temperature. During the refluxing, precipitation took place. The precipitated product thus formed was filtered off, washed with methanol, dioxane and then with diethyl ether. It was recrystallized in a mixture of water:dioxane (1:3) to obtain the pure required complex (1). All other complexes (2)–(5) were prepared following the same method using the same vanadyl salt with the respective Schiff base ligands ().
Pharmacology
The testing procedures for in vitro antibacterial, antifungal, minimum inhibitory concentration (MIC) and cytotoxicity bioassay have already been reportedCitation14,Citation15.
Results and discussion
Chemistry
Triazole derived Schiff base ligands (L1)–(L5) were formed by the equimolar reaction of 3,5-diamino-1,2,4-triazole with a variety of methyl-, chloro- and nitro-substituted thiophene-2-carboxaldehydes (). All of them were air and moisture stable and soluble in dioxane, DMF and DMSO. All the oxovanadium(IV) complexes (1)–(5) were prepared by the stoichiometric reaction of VOSO4 with respective triazole derived Schiff bases in molar ratio (M:L) 1:2 having a general formula, [M (L)2]SO4 where L = (L1)–(L5) and M = VIVO in a square-pyramidal geometry. All the synthesized oxovanadium(IV) complexes were air stable, nonhygroscopic and green-colored solids which decomposed on heating instead of melting. All oxovanadium(IV) complexes were only soluble in DMF and DMSO but not in common organic solvents. The elemental analysis and solubility data strongly recommended that these complexes are monomers and possessed 1:2 stoichiometry of the type ML2. The analytical data given in , also, agreed well with the proposed structure of the complexes.
Table 1. Physical and micronalytical data of the oxovanadium(IV) complexes (1)–(5).
Conductance and magnetic susceptibility
The molar conductance studies () were carried out in DMF (1 mmol solution at 25°C). The high molar conductance data (82–90 ohm−1 cm2 mol−1) of oxovanadium(IV) complexes (1)–(5) show them to have an electrolytic natureCitation16,Citation17. The observed magnetic moment values of the complexes at room temperature were found to be in the range of 1.72–1.77 B.M. consistent with the values reported for a square-pyramidal geometryCitation18,Citation19.
IR spectra
The most prominent IR frequencies of the triazole Schiff bases and their oxovanadium(IV) complexes are reported in the experimental part and . All the Schiff base ligands showed bands at 3343–3349, 3186–3194, 1609–1612 and 1018–1024 cm−1, respectively, due to NH2, N-H, C=N and N-N vibrations of triazoleCitation20,Citation21. Originally, a simple 3,5-diamino-1,2,4-triazole has two bandsCitation22,Citation23 at 3310 and 3350 cm−1 assigned to two amino groups. All Schiff base ligands demonstrated an absence of a band at 3310 cm−1 emerging into a new band of azomethine v(HC=N) linkageCitation3,Citation24,Citation25 at 1629–1637 cm−1 giving thus an evidence for condensation of one amino group of the triazole moiety; however, another band of amino group at 3350 cm−1 remained unchanged giving a clue of its non-participation in condensation reaction. The band appearing in all ligands at 882–889 cm−1 was assignedCitation26 to (C-S) vibrations. Moreover, the ligands (L4) and (L5) showed vibrations at 810 and 1355 cm−1 assigned to C-Cl and C-NO2 groups, respectively. On comparison of the spectra of ligands with their oxovanadium(IV) complexes, the following conclusions can be drawn providing strong evidences for the complexation reaction between the ligands and the oxovanadium metal atom:
An azomethine, ν(HC=N) band at 1629–1637 cm−1 in ligands shifted to lower frequency (10–20 cm−1) at 1617–1621 cm−1 in vanadyl complexes indicating coordination of azomethine-N with the vanadium (IV) metal ionCitation24.
The band at 882–889 cm−1 assigned to ν(C-S) also shifted to lower frequency (10–15 cm−1) at 866–875 cm−1 indicating the coordination of thiophene-S with the vanadium(IV) metal.
The appearance of weaker low-frequency new bands at 487–497 and 371–388 cm−1 attributed to v (V-N) and v(V-S), respectively, confirmed the coordination of triazole Schiff base ligand through azomethine-N and thiophene-S with the vanadium(IV) metal atom.
Two new bands which were not observed in the spectra of the ligands but appeared in the spectra of the complexes at 1082–1088 and 976–987 cm−1 were assigned to the presence of (SO4) outside the coordination sphere of the complexCitation27 and (V=O), respectivelyCitation28,Citation29.
1H NMR spectra
The 1H NMR spectra of the triazole Schiff bases (L1)–(L5) were recorded in DMSO-d6. The 1H NMR spectral data along with the possible assignments is recorded in the experimental part. All the heteroaromatic/aromatic protons were found as to be in their expected regionCitation30. All the Schiff bases (L1)-(L5) possessed a singlet because of amino (NH2) proton, azomethine (CH=N) proton and NH protons of triazole moiety at 5.97–6.09, 8.32–8.68 and 11.92–12.20, respectively. Also, the (CH3) protons of the Schiff bases (L2) and (L3) were found at 2.22–2.45. Moreover, the Schiff base (L1) displayed the C4-H proton of thiophene ring at 7.20 as a double doublet, C3-H and C5-H protons were observed at 7.71 and 7.81 as a doublet, respectively. In addition, the C3-H and C4-H protons of Schiff bases (L2), (L4) and (L5), also appeared as a doublet at 6.9–8.04. However, in case of the Schiff base (L3), the C5-H proton was found at 7.68 as a doublet. Thus, the number of proton calculated from the integration curvesCitation31 and obtained values of the expected CHN analysis agreed well with each others.
13C NMR spectra
The 13C NMR spectra of the triazole Schiff bases (L1)–(L5) were also recorded in DMSO-d6. The 13C NMR spectral data along with the possible assignments is recorded in the experimental part. The 13C NMR spectra of the Schiff bases (L1)–(L5) possessed triazole carbons (C7) and (C8) appeared in region at 160.85–166.24, respectively. The azomethine carbon (C6) found in all the Schiff bases at 156.12–157.48. All carbons (C2)–(C5) of thiophene ring found in the Schiff bases (L1)–(L5) were observed in the region at 122.63–149.97. In the Schiff bases (L2) and (L3), methyl carbons were observed in the region at 15.62–16.78. Moreover, the presences of the number of carbons are well in agreement with the expected values. Furthermore, the conclusions drawn from these studies present further support to the modes of bonding discussed in their IR and 1H NMR spectra.
Electronic spectra
The electronic spectra of all the oxovanadium(IV) complexes (1)–(40) in DMF displayed three () distinct low to high intensity bands (v1, v2 and v3) which were assigned to b2 (dxy) → eπ(dxz, dyz), b2 (dxy) → b1 (dx2 − y2) and b2 (dxy) → a1 (dz2) transitions, respectivelyCitation32,Citation33. The first band observed at 13269–13412 cm−1 was assigned to b2→ eπ d-d transitions. The second band was observed at 16473–16556 cm−1 which can be attributed to b2→ b1 and the presence of third band at 24934–25087 cm−1 can be assigned to the transitions b2→ a1. The fourth band of high intensity observed at 36175–37117 cm−1 was due to metal → ligand charge transfer (MLCT). All these observations provide an evidence for the complexes to show a square-pyramidal geometry.
Table 2. Conductivity, magnetic and spectral data of oxovanadium(IV) complexes.
Biological activity
Antibacterial bioassay (in vitro)
In vitro antibacterial results of triazole Schiff bases and their oxovanadium(IV) complexes are reported in . All compounds were tested against four Gram-negative (Escherichia coli, Shigella flexneri, Pseudomonas aeruginosa, Salmonella enterica serover typhi) and two Gram-positive (Staphylococcus aureus, Bacillus subtilis) bacterial strains according to literature protocolCitation34,Citation35. The obtained antibacterial results were compared with that of the standard drug imipenem, by considering its activity as 100%. The synthesized compounds showed different degree of inhibitory effects: low (up to 33%), moderate (up to 53%) and significant (above 53%). The (L1) showed a significant (54–58%) activity against bacterial strains (a), (b), (d) and (f), and moderate (46–48%) activity against (c) and (e). Similarly, the (L2) possessed significant (58–62%) activity against (b) and (e), moderate (38–50%) against (a), (c), (d) and (f). Also, the (L3) demonstrated significant (55%) activity against (b), moderate (37–50%) activity was observed against (a), (c), (d) and (f), and weaker (31%) against (e). In addition, (L4) exhibited significant (54–69%) activity against (a), (c), (d) and (e) except (b) and (f) which displayed moderate (48–50%) activity. Furthermore, (L5) demonstrated overall significant (54–72%) activity against all bacterial strains (a)–(e). Beside this, the oxovanadium(IV) complexes (1)–(5) exhibited overall a significant (57–90%) activity against all bacterial strains. The results of these studies pointed out that the antibacterial activity is overall improved upon chelation/complexation that confirmed our previous studiesCitation36–38. On comparison of the activity between Schiff base ligands and their oxovanadium(IV) complexes, higher activity was experienced by the compounds (4) and (5) owing to chloro and nitro substituents. “As these substituents are more electronegative therefore, they become involved in hydrogen bonding thus showing significant interaction with the bacterial proteins to enhance the bioactivity”. It is obvious from the above discussion that coordination makes the Schiff bases strongly antibacterial and inhibits the growth of bacteria more potentially rather than the uncomplexed Schiff bases.
Table 3. Antibacterial activity (concentration used 1 mg/mL of DMSO) of triazole Schiff bases and oxovanadium(IV) complexes.
MIC for antibacterial activity
Compounds which showed significant (above 80%) antibacterial activity were selected for MIC studies. The obtained results are reported in . The MIC was determined using the disc diffusion technique by preparing discs containing 10, 25, 50 and 100 µg/mL of the compounds. It was observed that oxovanadium(IV) complexes (4) and (5) were found to be the most active due to chloro and nitro substitution. Amongst the two, compound (5) was found to be the most active showing the highest inhibition (3.891 × 10−8 M) against B. subtilis bacterial strain.
Antifungal bioassay (in vitro)
In vitro antifungal screening of all the synthesized compounds was carried out against Trichophyton longifucus, Candida albican, Aspergillus flavus, Microscopum canis, Fusarium solani and Candida glabrata fungal strains and results of inhibition were compared with the standard drugs, miconazole and amphotericin B. The results are reported in and . Ligand, (L1) displayed significant (55%) activity against fungal strain (e), moderate (38–52%) against (a), (d) weaker (24%) against (b) and inactive against (c). Similarly, (L2) showed significant (54–57%) activity against (b) and (f), moderate (38–50%) against (a) and (c)–(e) strains. On the other hand, (L3) possessed significant (56%) activity against (b) and, moderate (41–47%) activity was experienced by (a), (d), (e) and (f) but (c) showed no activity. In the same way, (L4) showed significant (55%) activity against (a), moderate (38–49%) against (c)–(f) and no activity was observed against (b). Furthermore, (L5) showed moderate (45–48%) activity against (a), (e) and remaining possessed significant (54–65%) activity. The oxovanadium(IV) complex (1) exhibited significant (55–72%) activity against (a), (c)–(e) and moderate (42–48%) against (b) and (f). Besides this, compounds (2), (4), (5) possessed overall significant (54–78%) activity against all strains except (b) strain of complex (4) which showed moderate (38%) activity. The compound (3) displayed significant (56–70%) activity against (a), (b), (d) and (f), and moderate (35–49%) against (c) and (e), respectively. Complex (2) demonstrated significant (56–75%) activity against all strains except (d) which showed weaker (28%) activity. The complex (3) showed significant (56–70%) activity against (a), (b), (d) and (f), and moderate (35–42%) activity was observed against (c) and (e), respectively. It was generally observed that the activity of ligands was increased upon coordination with the vanadium metal.
Table 4. Antifungal activity (concentration used 200 µg/mL) of triazole Schiff bases and oxovanadium(IV) complexes.
Table 5. Minimum inhibitory concentration (M/mL) of the selected compounds (4) and (5) against selected bacteria.
Cytotoxic bioassay (in vitro)
All synthesized compounds were screened for their cytotoxic activity (brine shrimp bioassay) activity using the protocol of Meyer et al.Citation39,Citation40 The compound (2) only exhibited effective cytotoxicity (LD50 = 2.154 × 10−4) against Artemia salina, while all other compounds were almost inactive for this assay.
Conclusion
It has been demonstrated that the complexation of bioactive triazole ligands with oxovanadium(IV) form a new set of antimicrobial agents with an attractive range of efficiency against the aforesaid microorganisms. Particularly, the MIC values of compound (4) and (5) and level of cytotoxicity against compound (2) are significant and encouraging considering the current existence of drug-resistant strains in various clinical practices. Moreover, the present studies have also indicated that the formation of the oxovanadium(IV) complexes plays a key role in determining antimicrobial properties.
Acknowledgment
One of the authors (SHS) is grateful to Higher Education Commission (HEC), Government of Pakistan for the award of scholarship to carry out this research. We are also indebted to HEJ Research Institute of Chemistry, University of Karachi, Pakistan, for providing their help in taking the NMR, mass spectra, antibacterial, antifungal and brine shrimp bioassays.
Declaration of interest
The authors report no conflict of interest and are responsible for the contents and writing of the paper.
References
- Singh K, Singh DP, Barwa MS, Tyagi P, Mirza Y. Some bivalent metal complexes of Schiff bases containing N and S donor atoms. J Enzyme Inhib Med Chem 2006;21:749–755.
- Gülerman NN, Dogan HN, Rollas S, Johansson C, Celik C. Synthesis and structure elucidation of some new thioether derivatives of 1,2,4-triazoline-3-thiones and their antimicrobial activities. Farmaco 2001;56:953–958.
- Bagihalli GB, Avaji PG, Patil SA, Badami PS. Synthesis, spectral characterization, in vitro antibacterial, antifungal and cytotoxic activities of Co(II), Ni(II) and Cu(II) complexes with 1,2,4-triazole Schiff bases. Eur J Med Chem 2008;43:2639–2649.
- Rezaei Z, Khabnadideh S, Pakshir K, Hossaini Z, Amiri F, Assadpour E. Design, synthesis, and antifungal activity of triazole and benzotriazole derivatives. Eur J Med Chem 2009;44:3064–3067.
- Serdar M, Gumrukcuoglu N, Karaoglu SA, Demirbas N. Synthesis of some novel 3,5-diaryl-1,2,4-triazole derivatives and investigation of their antimicrobial activities. Turk J Chem 2007;31:315–26.
- Dabak K, Sezer O, Akar A, Anaç O. Synthesis and investigation of tuberculosis inhibition activities of some 1,2,3-triazole derivatives. Eur J Med Chem 2003;38:215–218.
- Ulusoy N, Gürsoy A, Otük G. Synthesis and antimicrobial activity of some 1,2,4-triazole-3-mercaptoacetic acid derivatives. Farmaco 2001;56:947–952.
- Joshi S, Khosla N, Tiwari P. In vitro study of some medicinally important Mannich bases derived from antitubercular agent. Bioorg Med Chem 2004;12:571–576.
- Shivarama Holla B, Veerendra B, Shivananda MK, Poojary B. Synthesis characterization and anticancer activity studies on some Mannich bases derived from 1,2,4-triazoles. Eur J Med Chem 2003;38:759–767.
- Kritsanida M, Mouroutsou A, Marakos P, Pouli N, Papakonstantinou-Garoufalias S, Pannecouque C et al. Synthesis and antiviral activity evaluation of some new 6-substituted 3-(1-adamantyl)-1,2,4-triazolo[3,4-b][1,3,4]thiadiazoles. Farmaco 2002;57:253–257.
- Muhammad N, Ali S, Shahzadi S, Khan AN. Oxovanadium(IV) complexes of non-steroidal anti-inflammatory drugs: Synthesis, spectroscopy, and antimicrobial activity. Russian J Coord Chem 2008;34:448–53.
- Noblía P, Vieites M, Parajón-Costa BS, Baran EJ, Cerecetto H, Draper P et al. Vanadium(V) complexes with salicylaldehyde semicarbazone derivatives bearing in vitro anti-tumor activity toward kidney tumor cells (TK-10): crystal structure of [VVO2(5-bromosalicylaldehyde semicarbazone)]. J Inorg Biochem 2005;99:443–451.
- Sakurai H, Kojitane Y, Yoshikawa Y, Kawabe K, Yasui H, Antidiabetic vanadium(IV),and zinc(II) complexes. Coord Chem Rev 2002;226:187–189.
- Chohan ZH. Synthesis of organometallic-based biologically active compounds: In vitro antibacterial, antifungal and cytotoxic properties of some sulfonamide incorporated ferrocences. J Enzyme Inhib Med Chem 2009;24:169–175.
- Chohan ZH, Sumrra SH. Some biologically active oxovanadium(IV) complexes of triazole derived Schiff bases: their synthesis, characterization and biological properties. J Enzyme Inhib Med Chem 2010;25:599–607.
- Maurya MR, Agarwal S, Abid M, Azam A, Bader C, Ebel M et al. Synthesis, characterisation, reactivity and in vitro antiamoebic activity of hydrazone based oxovanadium(IV), oxovanadium(V) and mu-bis(oxo)bis{oxovanadium(V)} complexes. Dalton Trans 2006;7: 947.
- Pandey OP. Oxovanadium(IV) complexes of carbohydrazones and thiocarbohydrazones. Polyhedron 1986;5:1587–91.
- Shashidhara GM, Goudar TR. Oxovanadium(IV) and niobium(V) complexes with some new Schiff bases. Synth React Inorg MetOrg Chem 2000;30:1581–1599.
- Raman N, Raja JD, Sakthivel A. Design, synthesis, spectroscopic characterization, biological screening, and DNA nuclease activity of transition complexes derived from a tridentate Schiff base. Russ J Coord Chem 2008;34:400–406.
- Maurya MR, Agarwal S, Abid M, Azam A, Bader C, Ebel M et al. Synthesis, characterisation, reactivity and in vitro antiamoebic activity of hydrazone based oxovanadium(IV), oxovanadium(V) and mu-bis(oxo)bis{oxovanadium(V)} complexes. Dalton Trans 2006;:937–947.
- Holla BS, Mahalinga M, Karthikeyan MS, Poojary B, Akberali PM, Kumari NS. Synthesis, characterization and antimicrobial activity of some substituted 1,2,3-triazoles. Eur J Med Chem 2005;40:1173–1178.
- Kumar VK, Keresztury G, Sundius T, Xavier RJ. Hydrogen bonding and molecular vibrations of 3,5-diamino-1,2,4-triazole. Spectrochim Acta a Mol Biomol Spectrosc 2005;61:261–267.
- Guennouna L, Eljastimi J, Guédiraa F, Marakchi K, Kabbaj OK, El Hajji A, Zaydouna S. Molecular geometry and vibrational studies of 3,5-diamino-1,2,4-triazole using quantum chemical calculations and FT-IR and FT-Raman spectroscopies. Spectrochimica Acta Part A 2011;78:347–353.
- Boghaei DM, Mohebi S. Non-symmetrical tetradentate vanadyl Schiff base complexes derived from 1,2-phenylene diamine and 1,3-naphthalene diamine as catalysts for the oxidation of cyclohexene. Tetrahedron 2002;58:5357–5366.
- Yadawe MS, Patil SA. Synthesis, characterization and biological studies of cobalt(II) and nickel(II) complexes with new Schif bases. Transition Met Chem 1997;22:220–224.
- Prashanthi Y, Raj S. Synthesis and characterization of transition metal complexes with N,O; N,N and S,N-donor Schifff base ligands. J Sci Res 2010;2:114–126.
- Stoilova D, Georgiev D, Marinova D. Infrared study of the vibrational behavior of SO42- guest ions matrix-isolated in metal (II) chromates (Me = Ca, Sr, Ba) Vibrational Spectroscopy 2005;39:46.
- Maurya MR, Singh H, Pandey A. (hydroxyalkyl/aryl]benzimidazolato) dioxovanadates(V) α Potassium bis(2-[through base assisted aerial oxidation of the corresponding oxovanadium(IV) complexes. Synth React Inorg Met-Org Chem 2002;32:231.
- Xie M, Gao L, Li L, Liu W, Yan S. A new orally active antidiabetic vanadyl complex–bis(alpha-furancarboxylato)oxovanadium(IV). J Inorg Biochem 2005;99:546–551.
- Nyquist RA. Interpreting Infrared, Raman, and Nuclear Magnetic Resonance Spectra, Published by Academic Press, 2001, p. 2.
- Freeman R, A Handbook of Nuclear Magnetic Resonance. Longman Publishing, 2nd edn, 1997.
- Raman N, Raja JD, Sakthivel A. Design, synthesis, spectroscopic characterization, biological screening, and DNA nuclease activity of transition metal complexes derived from a tridentate Schiff base. Russian J Coord Chem 2008;34:400.
- Bagihalli GB, Patil SA. Synthesis, physico-chemical investigations, and in vitro microbial, studies of VO(IV) complexes with novel ONON donor Schiff bases. Main Group Chem 2009;8:71.
- Rahman AU, Choudhary MI, Thomsen WJ. Bioassay techniques for drug development. Vol. 22, Harwood Academic Publishers, The Netherlands, 2001.
- McLaughlin JL, Chang CJ, Smith DL. Studies in natural products chemistry, “Bentch- Top” bioassays for the discovery of bioactive natural products: An update, structure and chemistry (part-B). 1991, Vol. 9: p. 363.
- Chohan ZH, Sumrra SH, Youssoufi MH, Hadda TB. Metal based biologically active compounds: design, synthesis, and antibacterial/antifungal/cytotoxic properties of triazole-derived Schiff bases and their oxovanadium(IV) complexes. Eur J Med Chem 2010;45:2739–2747.
- Chohan ZH, Sumrra SH. Synthesis, characterization and biological studies of oxovanadium(IV) complexes with triazole-derived Schiff bases Appl Organomet Chem 2010;24:122–130.
- Chohan ZH, Sumrra SH, Youssoufi MH, Hadda TB. Design and synthesis of triazole Schiff bases and their oxovanadium(IV) complexes as antimicrobial agents. J Coord Chem 2010;63:3981–3998.
- Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL. Brine shrimp: a convenient general bioassay for active plant constituents. Planta Med 1982;45:31–34.
- Finney DJ. Probit analysis. 3rd edn. Cambridge: Cambridge University Press, 1971.