Abstract
A series of 1,2,4-trisubstituted 5-imidazolinone derivatives were synthesized by Erlenmeyer condensation of benzoylglycine (hippuric acid) with different aldehydes in the presence of sodium acetate and acetic anhydride. The derivatives of the compounds were prepared by condensation of some known sulpha drugs with 5-oxazolone derivatives. The anticonvulsant activity of the compounds was determined by the protection of pentylenetetrazole-induced convulsions that was ranged from 10 to 60%. The compounds with p-OCH3, p-OH and o-Cl substitutions in the phenyl ring on 4th position of the imidazolinone ring exhibited good anticonvulsant activity. In silico metabolic and toxicity studies showed that all the compounds in the series are not likely to exhibit toxicity except the compounds IIIa, IIIb, VIa and VIb, that is predicted to show 29% mutagenicity and 53% irritation in comparison to the other compounds. The predicted lethal effect and hERG toxicity of the compounds showed that IIa, IVa, Va and Vb might be toxic at higher concentrations. The results successfully establish the synthesized imidazolinone derivatives as novel compounds with anticonvulsant properties, low predicted cardiotoxicity and lethal effects thus can be promising leads for further development as novel anticonvulsants.
Introduction
The development of a new bioactive molecule is complex, time consuming and very expensive and also the developed drugs may suffer from unwanted side effects, toxicity, resistance, etc. It is the primary thought of the scientist in the medicinal chemistry field to develop safe and potent molecules. The last two decades is called in neuroscience as “decade of the brain” and it has brought advances to the treatment of neurological disabilities, including epilepsyCitation1. Epilepsy, a group of disorders of the CNS characterized by paroxysmal cerebral dysrhythmia manifesting as brief episodes (seizures) of loss of or disturbances of consciousnessCitation2,Citation3. Although several classes of anti-epileptic agents with different nucleus are available for treating epilepsy, these agents have a number of shortcomings which limit their utilityCitation4–6.
Imidazolinone is an important scaffold possessing a spectrum of pharmacological actions, which includes anticonvulsant, antiparkinsonism and monoamino-oxidase inhibitory activitiesCitation7–12. A careful scrutiny of structure activity relationship studies of anticonvulsant agents reported in literature indicates that ureido moiety is responsible for the anticonvulsant actionCitation13–15. Precedence exist in literature on anticonvulsant properties of 1,2,4-trisubstituted 5-imidazolinonesCitation12, the N arylation of imide NH of the imidazolinone could serve two functions; increases the partition coefficient and prevents the dissociation of imido hydrogen, three both cases favored more effective distribution of the central nervous systemCitation16. The multiple biological properties of sulfonamides are well documented in literatureCitation17,Citation18. The present study aims to introduce two sulfa drugs sulfamethoxazole and sulfadoxin into 4-arylidene-2-phenyl-5-(4H)-oxazolones to formulate 1,2,4-trisubstituted 5-imidazolinones bearing sulfonamide moiety with the hope that combination of these active groups in the new molecular design would lead to better anticonvulsant agents. Based on the aforementioned, we have designed and synthesized trisubsituted imidazolinone compounds incorporating sulfa drugs (sulfamethoxazole and sulfadoxin) and evaluated them in vivo for ascertaining their anticonvulsant efficacy.
ADME-Tox property of molecules is an important parameter for the success of some compounds in clinic. In silico methods to predict toxicity and ADME properties even before a drug candidate was synthesized is widely used in drug discovery to understand the properties that are necessary to convert leads into good medicines, which increase the success rateCitation19,Citation20. Drug metabolism and clearance determine the success of a drug, as these properties have a significant impact on bioavailability (oral or intravenous) and give an idea about how a drug behave in the human body. The recognition of the metabolic site could be of great help in designing new compounds with better pharmacokinetic profile as well as to avoid the presence of toxic metabolites by chemically protecting the metabolic labile moieties in the drug candidateCitation21–24. Related to the foregoing, the synthesized compounds were subjected to in silico toxicity and metabolism studies in order to predict their metabolic and toxicity profile. A preliminary idea of the metabolic and toxicity profile of a compound could be of great help in assessing the suitability of the proposed molecule as a probable anticonvulsant drug candidate and as well as to avoid the presence functionalities that might lead to toxic metabolites.
Materials and methods
Materials used
Melting points were determined on electrothermal capillary melting point apparatus and were uncorrected. The progress of the reactions was monitored by thin layer chromatography (TLC) using silica gel G on glass plate as stationary phase and benzene: ethylacetate (80:20) as mobile phase. IR (KBr) (cm−1) spectra were recorded on FT/IR-470 Plus Jasco spectrophotometer. 1H NMR spectra were recorded on Brucker DRx (300 MHz) spectrometers and the chemical shift values are reported in parts per million using tetramethylsilane as internal standard. The samples were dissolved in CDCl3.
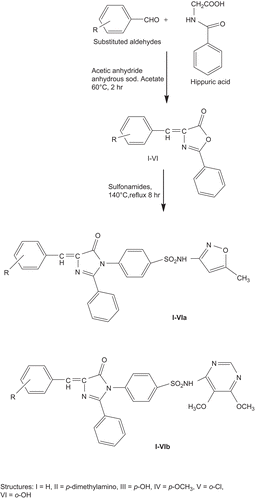
Synthesis of 4-arylidene-2-phenyloxazol-5(4H)-one derivatives
A mixture of 0.025 mol of redistilled benzaldehyde derivatives, 4.48 g (0.025 mol) of benzoyl glycine (hippuric acid), 7.66 g (0.075 mol) of acetic anhydride and 2.05 g (0.025 mol) of anhydrous sodium acetates were placed in a 100 ml were placed in a conical flask and heated on an electric hotplate with constant stirring till liquified. As soon as the mixture had liquefied completely, the flask was transferred on to a water bath and heated at 60°C for 2 h. Then 100 ml of ethanol was added slowly to the content of the flask and allowed the mixture to stand overnight. The crystalline product was filtered with suction and washed with two 25 ml portion of ice cold alcohol and then washed with two 25 ml portion of boiling water and dried at 100°C. The yield of almost pure oxazolone, m.p. 165–166°C was 4 g (64%). Recrystallization from benzene raises the m.p. to 167–168°C.
Synthesis of 1-N-substituted 4-(4-benzylidene-5-oxo-2-phenyl-4,5dihydro-1H-imidazol-1-yl)benzenesulfonamide
4-Arylidene-2-phenyloxazol-5(4H)-one derivatives (0.004 mol) was heated with an equimolar quantity of sulfonamide (sulfamethoxazole and sulfadoxin) in an oil bath at 140°C for 1 h. The resulting jelly like mass was taken in an organic solvent (acetone) and refluxed for 8 h with continuous removal of water and cooled, the excess solvent removed under vacuum and the resultant solid was collected. Crude solid product was recrystallized from ethyl acetate to get flakes of 5-imidazolinones and found chromatographically homogenous when detected with iodine.
Determination of anticonvulsant activity
Anticonvulsant activity was determined against pentylenetetrazole-induced seizures in mice, 25–30 g of either sexCitation11,Citation12,Citation14. The mice were divided into groups of five mice and keeping the group weight as near as possible. All compounds were suspended in 5% aqueous gum acacia (have devoid of anticonvulsant activity) to give a concentration of (0.025% w/v). An arbitary dose of 100 mg/kg i.p. of imidazolinone was administered to five mice. The mice were then injected with pentylenetetrazole (90 mg/kg i.p.) 30 min after the administration of the test compounds. The pentylenetetrazole has been shown to produce convulsions in almost all untreated mice and to exhibit 100% mortality during 24 h. No mortality of animals was observed during 24 h treatment with 100 mg/kg of the imidazolinones alone. The mice were observed 60 min for the occurrence of seizures and an episode of clonic spasm persisting for a minimum of 5 s was considered a threshold convulsion. Transient intermittent jerks and tremulousness were not counted. Mice devoid of threshold convulsions during 60 min were considered protected. The number of mice protected in each group was recorded and the anticonvulsant activity of these imidazolinone was represented as percent protection.
In silico metabolites and toxicity prediction
The metabolites and the toxicity profile of the compounds were predicted by computational method using Pallas 3.1.1.2. ADME-Tox prediction softwareCitation25. The LD50 and the hERG (pIC50) were predicted using q-Tox and q-hERG softwares, respectivelyCitation26.
Results and discussion
Chemistry
The oxazolone derivatives were synthesized by the condensation of benzaldehyde and hippuric acid in acetic anhydride and anhydrous sodium acetate. 1,2,4-trisubstituted 5-imidazolinones derivatives were synthesized by the condensation of some known sulpha drugs (sulfamethoxazole and sulfadoxin) with 5-oxazolone derivatives. The imidazolinone derivatives were synthesized employing a two step procedure as outlined in . The physicochemical characterization was performed in order to confirm the progress of reaction and the purity of compounds. TLC was carried out on silica gel G using benzene: ethyl acetate (80:20) as eluent (). The constitutions of the synthesized compounds were confirmed by IR and NMR. The functional groups on the compounds were confirmed by IR spectroscopy and the number and the environment of hydrogen atoms in the compounds were confirmed by NMR spectrum (). The anticonvulsant activity of the compounds (as per ethical guidelines) was determined by ascertaining the percentage protection against pentylenetetrazole-induced convulsions in mice and the activity of the synthesized compounds ranged from 10 to 60%. Amongst the compounds tested, maximal protection was observed for compounds with p-OCH3 (IVa), p-OH (IIIa) substitution in phenyl ring on the 4th positions of the imidazolinone ring of series bearing sulfamethoxazole moiety, and p-OCH3 (IVb), o-Cl (Vb), substitutions in the phenyl ring on the 4th positions of the imidazolinone ring of series bearing sulfadoxin moiety. In case of the unsubstituted compounds, the compound Ib with sulfadoxin moiety showed better protection (20%) compared to the compound Ia with sulfamethoxazole moiety suggesting that incorporation of sulfadoxin in the nucleus is most favoured for anticonvulsant activity exhibited by the title compounds. In sulfamethoxazole series, p-dimethyl amino substitution (IIa) and o-OH substitution (VIa) in the phenyl ring resulted in two fold increase in the protection whereas o-Cl substitution (Va) caused a fourfold increase in protection against pentylenetetrazole-induced convulsions in comparison to the unsubstituted compound Ia. In sulfadoxin series, p-dimethyl amino substitution (IIb) in phenyl ring did not improve the anticonvulsant activity whereas, p-OH (IIIb) and o-OH (VIb) substitution in phenyl ring improved anticonvulsant activity two-fold in comparison to the unsubstituted compound Ib. It is worth mentioning that the majority of the compounds have shown significant activity.
Table 1. Anticonvulsant activity of the synthesized compound.
Table 2. Analytical spectra data of the synthesized compounds.
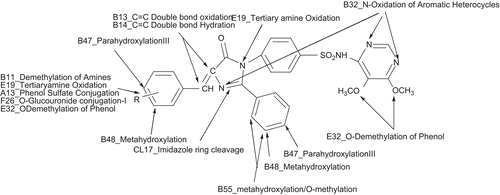
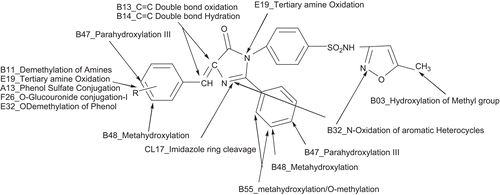
In silico metabolites and toxicity of the synthesized compounds were predicted by using Pallas 3.1.1.2., q-Tox and q-hERG software and the result obtained are tabulated in . The possible position of metabolism of the synthesized compounds is given in and . All the compounds appear to undergo hydroxylation at the para and meta position of the phenyl ring. N-oxidation of the aromatic heterocycles in the oxazole and pyrimidine ring occurred alongwith oxidation of the tertiary nitrogen atom in the imidazolinone ring. The imidazolinone ring has cleaved in all compounds. The methoxy compounds especially pyrimidine substituents containing compounds (Ib to VIb) and p-methoxy phenyl substituents (IVa) of the compounds undergo demethylation to give a free phenolic group. The results show that all the compounds in the series are expected to undergo phase I metabolism such as oxidation, hydration, ring cleavage, etc. ( and ). Only hydroxyl group containing compound IIIa,b and VIa,b are expected to undergo phase II metabolism of O-glucouronide conjugation and phenol sulfate conjugation.
Table 3. Predicted toxicities of the synthesized compounds.
The compounds except IIIa,b and VIa,b shows toxicity. All the compounds in this series show 0% toxicity in terms of oncogenicity and sensitivity. The teratogenic properties of the compounds are <50% which shows the probability of this toxicity is less. The compounds IIIa,b and VIa,b have >50% (53%) overall toxicity compared to other compounds (34%) and these four compounds exhibit irritation 53% and mutragenicity 29%. It shows that these compounds are more toxic than all other compounds. These compounds only give phase II metabolites due to the presence of OH group in the benzene ring as phenolic OH.
The predicted LD50 and the pIC50 (hERG toxicity) of the compounds show that the compounds IIa, IVa, Va and Vb might have lethal effect at high concentration and may also possess the cardiotoxicity in high concentration compared with other compounds. These compounds also exhibit significant anticonvulsant activities than other compounds. In comparison with the other toxicity studies as mentioned earlier, the compound with −OH groups in phenyl rings of their structure are more toxic than others. Unfortunately the −OH substitution at the para position on the phenyl ring of the molecule has considerably significant anticonvulsant activity.
Summarizing the above, it can be concluded that we have successfully synthesized 12 newly designed derivatives of 1,2,4-trisubstituted 5-imidazolinones incorporating two sulfa drugs. All the title compounds have been investigated for their anticonvulsant activity in mice models. Interestingly, the majority of the compounds showed moderate to very good anticonvulsant activity. The best results in terms of the percentage protection against pentylenetetrazole-induced convulsions in mice was achieved for compounds with p-OCH3 and p-OH substitution in the phenyl ring on the 4th positions of the imidazolinone ring for sulfamethoxazole series and p-OCH3 and o-Cl substitution in the phenyl ring on the 4th positions of the imidazolinone ring for sulfadoxin series. In silico metabolite and toxicity studies were also performed for the synthesized molecules to assess their suitability in terms of their susceptibility to metabolism and toxicity profile. Overall, the studies establish 1,2,4-trisubstituted 5-imidazolinones as novel leads for further exploration as anticonvulsant agents.
Acknowledgments
The author is thankful to the Vice Chancellor, RGPV, Bhopal for providing laboratory facility. Authors would like to give sincere thanks to AICTE for providing Scholarship and Career Award grant during this project. One of the authors, C. Karthikeyan, wishes to thank CSIR, New Delhi, for providing a Senior Research Fellowship.
Declaration of interest
The authors would like to give sincere thanks to AICTE for providing scholarship and career award grant during this project. One of the authors, C. Karthikeyan, wishes to thank CSIR, New Delhi, for providing a Senior Research Fellowship.
References
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med 2000;342:314–319.
- Carroll BJ, Curtis GC, Mendels J. Neuroendocrine regulation in depression. II Discrimination of depressed from non-depressed patients. Arch Gen Psychol 1973;33:1051–1057.
- Ellison G, Eison MS, Huberman HS, Daniel F. Long-term changes in dopaminergic innervation of caudate nucleus after continuous amphetamine administration. Science 1978;201:276–278.
- Worthen DR, Bence AK, Stables JP, Crooks PA. In vivo evaluation of diaminodiphenyls: anticonvulsant agents with minimal acute neurotoxicity. Bioorg Med Chem Lett 2009;19:5012–5015.
- Johannessen Landmark C, Johannessen LC. Pharmacological management of epilepsy: recent advances and future prospects. Drugs 2008;68:1925–1939.
- Snead OC, Donner EJ. A new generation of anticonvulsants for the treatment of epilepsy in children. Paediatr Child Health 2007;12:741–744.
- Verma M, Chaturvedi AK, Chaudhari A, Parmar SS. Monoamine oxidase inhibitory and anticonvulsant properties of 1,2,4-trisubstituted 5-imidazolones. J Pharm Sci 1974;63:1740–1744.
- Naithani PK, Srivastava VK, Barthwal JP, Saxena AK, Gupta TK, Shanker K, Synthesis and antiparkinsonian activity of newer imidazolones. Indian J Chem 1989;28:990–992
- Harfenist M, Soroko FE, McKenzie GM. 2-(Alkoxyaryl)-2-imidazoline monoamine oxidase inhibitors with antidepressant activity. J Med Chem 1978;21:405–409.
- Magd-El-Din AA, Abd-El-All AS, Roaiah HMF, El-Baroudy MMS. New synthesis of furochromenyl imidazo [2a-1b] thiazole derivatives, studies on their antitumor activities. J Am Sci 2010;6:251–256.
- Desai NC, Bhavsar AM, Baldaniya BB. Synthesis and antimicrobial activity of 5-imidazolinone derivatives. Indian J Pharm Sci 2009;71:90–94.
- Joshi H, Upadhyay P, Karia D, Baxi AJ. Synthesis of some novel imidazolinones as potent anticonvulsant agents. Eur J Med Chem 2003;38:837–840.
- Siddiqui SA, Bhusare SR, Jorikot DV, Pawa RP, Vibhute YB. New novel synthesis and antibacterial activity of 1-(substituted phenyl)-2-phenyl-4-(3′-halo, 4′-hydroxy, 5′-methoxy benzylidene)-imidazole-5-ones. Bull Korean Chem Soc 2001;22:1033–1036.
- Patel HJ, Sarra J, Caruso F, Rossi M, Doshi U, Stephani RA. Synthesis and anticonvulsant activity of new N-1′,N-3′-disubstituted-2′H,3H,5′H-spiro-(2-benzofuran-1,4′-imidazolidine)-2′,3,5′-triones. Bioorg Med Chem Lett 2006;16:4644–4647.
- Librowski T, Kubacka M, Meusel M, Scolari S, Müller CE, Gütschow M. Evaluation of anticonvulsant and analgesic effects of benzyl- and benzhydryl ureides. Eur J Pharmacol 2007;559:138–149.
- Bhusare SR, Patil PS, Chavan VP, Pawar RP, Bhawal BM, Vibhute YB. Synthesis of 1-(substituted phenyl)-2-phenyl-4- (2′-hydroxy 3′-iodo-5′-choro benzylidene) -imidazole-5-ones. Mendeleev Commun 2002;12:94–95.
- Thiry A, Dogné JM, Supuran CT, Masereel B. Anticonvulsant sulfonamides/sulfamates/sulfamides with carbonic anhydrase inhibitory activity: drug design and mechanism of action. Curr Pharm Des 2008;14:661–671.
- Masereel B, Rolin S, Abbate F, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: anticonvulsant sulfonamides incorporating valproyl and other lipophilic moieties. J Med Chem 2002;45:312–320.
- Wilhelm H, Regina T, Michael W. The virtual laboratory approaches to pharmacokinetics: design principles and concepts. Drug Dis Today 2006;11:800–805.
- Dearden JC. In silico prediction of drug toxicity. J Comput Aided Mol Des 2003;17:119–127.
- Sean E, John R. In silico ADME/Tox: the state of the art. J Mol Graph Model 2002;20:305–309.
- Moorthy HN, Singh RJ, Singh HP, Gupta SD. Synthesis, biological evaluation and in silico metabolic and toxicity prediction of some flavanone derivatives. Chem Pharm Bull 2006;54:1384–1390.
- Moorthy HN, Karthikeyan C, Trivedi P. Design, synthesis, cytotoxic evaluation, and QSAR study of some 6H-indolo[2,3-b]quinoxaline derivatives. J Enzyme Inhib Med Chem 2010;25:394–405.
- Hari Narayana Moorthy HN, Karthikeyan C, Trivedi P. Synthesis, cytotoxic evaluation and in silico pharmacokinetic prediction of some benzo[a]phenazine-5-sulfonic acid derivatives. Med Chem 2009;5:549–557.
- Pallas 3.1.1.2., ADME-Tox software, CompDrug International Inc., USA; 2000.
- q-ADME, q-Tox and q-hERG software 2008, developed by Quantum Pharmaceuticals, Moscow, Russia; 2008. http://www.q-lead.com/.