Abstract
An economical and efficient one-pot synthesis of a series of novel 5-aryl-6-cinnamoyl-7-methyl-flavanones has been developed by simple refluxing of cinnamoyl chalcones with NaOAc in aqueous ethanol in quantitative yields. These flavanones were screened for their in vitro antioxidant and in vivo antidyslipidemic activities. Among 24 compounds screened, four compounds 28, 29, 30, and 48 showed significant antidyslipidemic activities. However, out of all the compounds, only compound 28 exhibited significant antioxidant activity and other compounds showed moderate antioxidant activities.
Introduction
Oxidative stress is intricately involved in the pathogenesis and development of several diseases and more particularly atherosclerosisCitation1. It is one of the important factors for the development and progression of CHD (coronary heart diseaseCitation2). On the other hand, dyslipidemia is also a threat to serious cardiovascular problems, including atherosclerosis, stroke, and cardiac arrest. The most common dyslipidemia treatment, however, is a carefully regulated regimen of diet and exercise. More serious conditions may require a combination of exercise, medication, and surgery to prevent life-threatening complicationsCitation3. Therefore, antioxidants and lipid lowering agents play a major role in prevention of CHD. Flavanones, the cyclic isomer of chalcones, are naturally occurring antioxidants and have been investigated in great detail for their antioxidant, hypolipidemic and free radical scavenging activitiesCitation4. They are also known to play an important role in defence mechanism of plants against several toxicants, microbes and parasitesCitation5. They are associated with antibacterial, antifungalCitation6, estrogen receptor modulatoryCitation7,Citation8, TNF-α inhibitory and hormone-dependent anticancer activitiesCitation9–11. Several naturally occurring and synthetic chalcones also possess most of the pharmacological activities of the flavanonesCitation12–20. Therefore, it was envisaged to synthesize hybrid molecules where both the chalcone and flavanone skeletons are in the same molecule and screen them for their antioxidant antidyslipidemic activities (). There are several methods to prepare flavanones involving the oxidative cyclization of 2′-hydroxychalcones using different reagentsCitation21–25.
Herein, we have reported one-pot, economical and eco-friendly syntheses of 6-cinnamoyl flavanones by reacting preformed cinnamoyl chalconesCitation26 with NaOAc in refluxing aqueous ethanol in quantitative yields. The method of synthesis is quite simple as it does not involve any sophisticated chemical or apparatus. The purification of the compounds is either by crystallization or by simple filtration of compounds on a short column of silica gel. The compounds synthesized were screened for their antioxidant and antihyperlipidemic activities.
Results and discussion
Chemistry
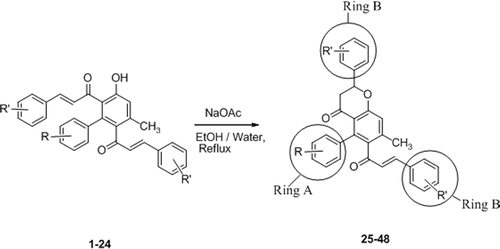
The starting chalcones (1–24) were prepared in a straightforward manner by reaction of diacetyl benzenesCitation27 and different aromatic aldehydes as reported earlier by us26. The compounds were identical in all respects to those reported earlier. The reaction of the above chalcones (1–24) separately with sodium acetate in ethanol and water (1:1) at 70–80°C for different time intervals gave the hybrid molecules of 5-aryl-6-cinnamoyl-7-methyl-flavanones (25–48) in almost quantitative yields ( and ).
Table 1. Synthesis of 5-aryl-6-cinnamoyl-7-methyl-flavanone derivatives (25–48) by cyclization of different cinnamoyl chalcones.
Structures of these compounds were established on the basis of their spectroscopic data and microanalyses. The IR spectra of all the flavanones exhibited the absorption bands around 1670 and 3060 cm−1 for their carbonyl group and alkene CH stretching vibrations. The ESMS (mass spectra) of the compounds showed [M+H]+ peaks corresponding to their molecular formulae. The NMR spectra (1H and 13C) were consistent with the proposed structures.
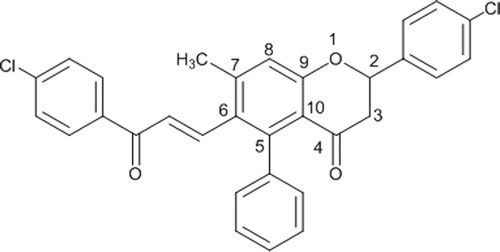
As a prototype, the detailed NMR spectra of (E)-2-(4-chlorophenyl)-6-(3-(4-chlorophenyl) acryloyl)-7-methyl-5-phenylchroman-4-one (25, ) has been described herein.
In the 1H NMR spectrum of compound 25, the aromatic protons and one of the olefinic protons of the cinnamoyl moiety, adjacent to aromatic ring were observed as m in the range of δ 7.42–6.95. The other olefinic proton of the cinnamoyl group appeared as a d at δ 6.36 (J = 16.1 Hz). The benzylic proton (H-2) was visible as dd at δ 5.56 with J1 = 12.8 Hz and J2 = 3.1 Hz, whereas the two methylene protons (H-3) were observed as dd at two different field strengths at δ 3.07 (J1 = 16.5 Hz and J2 = 12.8 Hz), and at δ 2.81 (J1 = 16.5 Hz and J2 = 3.1 Hz), respectively. The C-7 methyl was visible as singlet at δ 2.30.
In the 13C NMR spectrum, the two carbonyl carbons appeared at δ 197.1 and 190.0, whereas the quaternary aromatic carbon C-9 was visible at δ 162.5 ppm and C-7 at δ 143.9 ppm. The other aromatic quaternary carbons (ArC) were observed at their usual chemical shifts of δ 141.5, 138.6, 137.4, 136.9, 136.3, 135.1, 133.2, 129.7, and 116.9 ppm. The C-2 carbon appeared at δ 78.8 ppm, whereas the other tertiary aromatic carbons (ArCH) appeared at δ 143.2, 129.6, 129.5, 129.4, 129.3, 128.5, 128.2, 128.0, 127.8, and 119.6 ppm. The C-3 carbon was visible at δ 46.1 ppm, whereas the methyl carbon was visible at δ 20.8 ppm. Almost similar patterns were observed in 1H NMR and 13C NMR spectra of other compounds 26–48 of the series.
Biology
Antioxidant activities of 5-aryl-6-cinnamoyl-7-methyl-flavanones
The antioxidant activities of compounds 25–48 were evaluated by generating free radicals (superoxide ions (O2−), hydroxyl radicals (OH·), microsomal lipid peroxidation) in vitro in the presence of 200 µg/mL compounds dissolved in DMSO and compared with the control where no compound was added. Superoxide anions were generated enzymaticallyCitation28 from xanthine (160 mM) using xanthine oxidase (0.04 U) and nitroblue tetrazolium (320 μM). Hydroxyl radicals (OH·) were generated in a nonenzymatic system comprising deoxyribose (2.8 mM), FeSO4.7H2O (2.0 mM), sodium ascorbate (2.0 mM) and H2O2 (2.8 mM) in 50 mM KH2 PO4 buffer (pH 7.4) to a final volume of 2.5 mL. The test compounds were also studied for their inhibitory action against microsomal lipid peroxidation in vitro by nonenzymatic inducer. The scavenging potential of the compounds for O2−, OH·, and microsomal lipid peroxidation is depicted in . Alloprinol, mannitol and α-tocopherol were used as standard scavengers for the superoxide ions (O2−), hydroxyl free radicals (OH·) and microsomal lipid peroxidation, respectively.
Figure 3. Showing the effect of compounds 25–48 at 200 µg/mL on superoxide ion (n mole formazone formed/min), hydroxyl radicals (n mole MDA formed/h) and lipid peroxidation in microsomes (n mole MDA formed/mg protein).
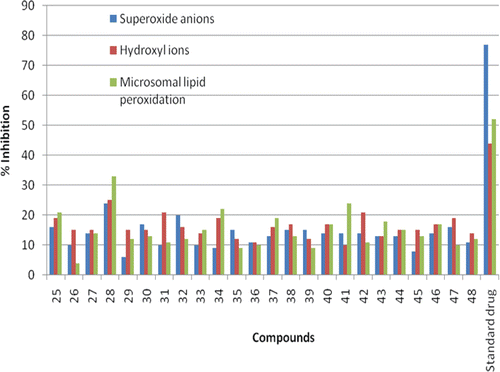
Alloprinol showed 77% superoxide ions scavenging activity at 200 µg/mL, whereas mannitol showed 44% hydroxyl radical scavenging activity at the same concentration. α-tocopherol showed 52% inhibition of lipid peroxidation at 200 µg/mL concentrations. The compounds of the series showed moderate to significant antioxidant activities. Compounds 25–48 inhibit superoxide ions by 7, 4, 8, 13, 3, 11, 5, 14, 5, 5, 6, 5, 6, 6, 13, 7, 7, 8, 11, 8, 4, 10, 7, and 7%, respectively. They inhibit hydroxyl radicals by 13, 8, 10, 18, 9, 7, 11, 8, 8, 11, 6, 7, 9, 9, 10, 11, 5, 12, 8, 7, 11, 10, 10, and 8%, respectively, whereas inhibition of microsomal lipid peroxidation is 6, 1, 8, 17, 6, 7, 5, 9, 13, 13, 4, 9, 12, 6, 9, 8, 16, 5, 11, 7, 7, 13, 6, and 6%, respectively. The compound 28 having Br group in ring B was found to be the most active compound of the series. It showed significant inhibition of superoxide anions (24%), hydroxyl radicals (19%) and microsomal lipid peroxidation (21%), respectively. The properties of these cinnamoyl flavanones as antioxidant and free radical scavenger appear to be due to extended conjugation in the molecule which facilitates the electron transfer and the resonance stabilization through keto-enol tautomerism of propenone moiety. The latter has the ability to delocalize unpaired electrons of free radicalsCitation29–36. No definite structure activity relationship could be established in the series for antioxidant activity.
Effect of 5-aryl-6-cinnamoyl-7-methyl-flavanones on hyperlipidemia
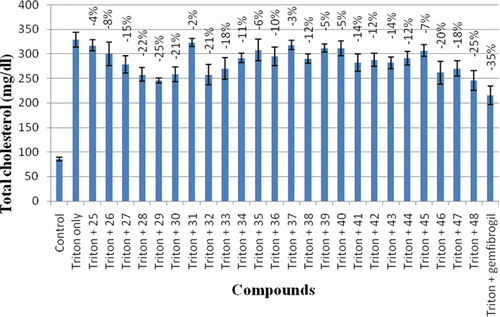
The total cholesterol (TC) of control groups was estimated to be 85.40 ± 4.00 mg/dl (). Administration of triton WR-1339 in rats induced marked hyperlipidemia as evidenced by 3.87-fold increase in the plasma levels of TC (329.04 ± 15.00). The standard drug gemfibrozil decreased the TC level by 35% as compared to triton only group. The compounds 25–48 exhibited their TC lowering activity in 2–25% range. Five compounds of the series, compounds 28, 29, 30, 32, and 48, were found to have significant TC inhibitory activities as they decreased the TC level by 22, 25, 21, 21 and 25%, respectively.
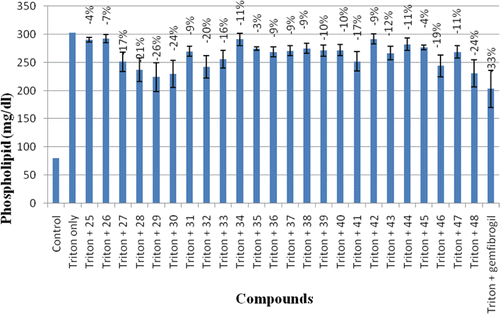
Treatment of rats with triton WR-1339 increased their plasma phospholipids (PL) by 3.75-folds (). The animal group treated with triton only were compared with the animal group treated with triton and compounds both. The compounds 25–28 showed their PL-lowering activities in a range of 4–26%. Five compounds of the series 28, 29, 30, 32, and 48 decreased the PL level by 21, 26, 24, 20 and 24%, respectively. Thus compound 29 with 26% PL-lowering activity was the most active compound of the series as compared to the standard drug gemfibrozil, which displayed 33% PL-lowering activity.
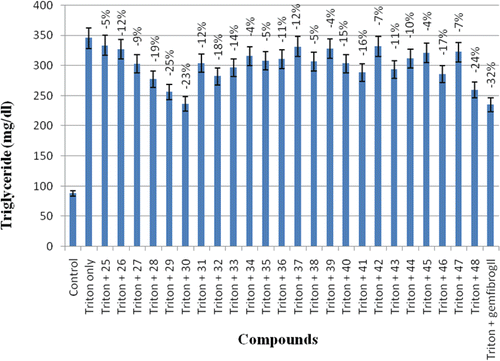
Treatment of rats with triton WR-1339 increased their triglyceride (Tg) level by 3.92-folds (). The Tg levels in the two animal groups, one group treated with triton only and the other group with triton and compounds both were compared. The compounds showed their Tg lowering activities in a range of 4–24%. Five compounds 28, 29, 30, 32, and 48 of the series were found to decrease the Tg level by 19, 25, 23, 18 and 24%, respectively. Thus compound 29 with 25% Tg lowering was the most active compound of the series as compared to the drug gemfibrozil with 32% Tg lowering activity.
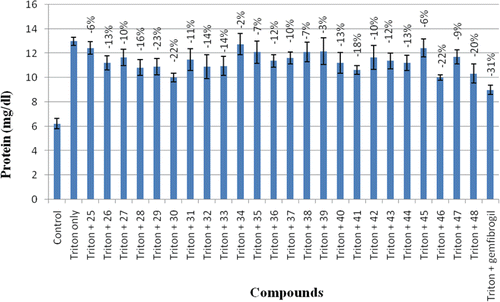
Administration of triton in rats elevated their protein levels by 2.08-fold (). Triton WR-1339 acts as surfactant, suppresses the action of lipase and blocks the uptake of lipoproteins from the circulation of extra hepatic tissues resulting in an increased level of circulatory lipidsCitation37,Citation38. Treatment of hyperlipidemic rats with compounds 25–48 reversed the plasma level of protein with varying extents. Compounds 28, 29, 30, 46 and 48 exhibited 16, 23, 22, 22 and 20% protein lowering activity, respectively, whereas other compounds exhibited mild lowering of protein levels as compared to triton. These data were compared with gemfibrozil, which showed a decrease of 36% in protein levels.
With this sizable number of compounds, although no definite SAR could be established on protein lowering activity, yet a closure look into the structure activity relationship revealed that in general, compounds (32) B ring as naphthyl substituent are more active than those with phenyl ring substituent. The effect of halo substituents in the aryl ring B, in general, results in the increased activity with Br > F > Cl trend with the exception of compound 35. Further, positional substitution of halo substituents follows the pattern m > p > o. It is also observed that substitution of phenyl ring of the cinnamoyl group with 4-OCH2Ph results in better activity as compared to 4-OCH3 or halogen. In general, the compounds having phenyl or chlorophenyl as ring A are more active than compounds with benzyloxy or bromophenyl substituents.
Conclusion
In conclusions, a series of novel 5-aryl-6-cinnamoyl-7-methyl-flavanones has been synthesized from (2E,2′E)-1,1′-(3-hydroxy-5-methylbiphenyl-2,6-diyl)bis(3-phenylprop-2-en-1-one) derivatives in very good yields. The compounds were evaluated for their antidyslipidemic and antioxidant activities. A number of compounds showed significant to moderate activities. Further work with these molecules is underway to prepare more potent compounds having drug like properties.
Experimental
Chemistry
Commercially available reagent grade chemicals were used as received. All reactions were followed by TLC on E. Merck Kieselgel 60 F254, with detection by UV light, spraying a 20% KMnO4 aq solution. Column chromatography was performed on silica gel (100–200 mesh E. Merck). IR spectra were recorded as thin films or in KBr solution with a Perkin-Elmer Spectrum RX-1 (4000–450 cm−1) spectrophotometer. 1H and 13C NMR spectra were recorded on a Brucker DRX-200 in CDCl3 and CDCl3+CCl4. Chemical shift values are reported in ppm relative to TMS (tetramethylsilane) as internal reference, unless otherwise stated; s (singlet), d (doublet), dd (doublet of doublet), m (multiplet); J in hertz. ESI mass spectra were performed using Quattro II (Micromass). Elemental analyses were performed on a Perkin-Elmer 2400 II elemental analyzer.
General procedure for the synthesis of cinnamoyl flavanones
To a stirring solution of (2E,2′E)-1,1′-(3-hydroxy-5-methylbiphenyl-2,6-diyl)-bis(3-pheylprop-2-ene-1-ones derivatives (1 equiv.) in minimum amount of a mixture of EtOH: H2O (1:1) and NaOAc (4 equiv.) was added. The reaction mixture was heated to reflux till the disappearance of the starting materials. After completion of reaction (TLC), the reaction mixture was allowed to cool to room temperature. The mixture was then diluted with H2O and extracted with Et2O. The combined organic phases were washed with brine, dried over anhydrous sodium sulphate, and concentrated under reduced pressure. The crude product was purified either by crystallization or by filtration through a short column of SiO2 (60–120 mesh) using appropriate eluent to give the desired flavanone.
(E)-2-(4-chlorophenyl)-6-[3-(4-chlorophenyl)acryloyl]-7-methyl-5-phenylchroman-4-one (25)
It was obtained as light yellow solid, mp 190–192°C, in 94% yield; Rf = 0.6 (8:2 hexane:ethylacetate); IR (KBr): υmax in cm−1 3360, 3060, 1691, 1670, 1597, 1489, 1425, 1319, 1159, 1088, 818, 700; 1H NMR (200 MHz, CDCl3+CCl4): δ = 7.42 (s, 4H, 3 × ArH & = CH), 7.31–7.14 (m, 8H, ArH), 7.06–6.95 (m, 3H, ArH), 6.36 (d, J = 16.1 Hz, 1H, = CH), 5.56 (dd, J1 = 12.8 Hz, J2 = 3.1 Hz, 1H, CH), 3.07 (dd, J1 = 16.5 Hz, J2 = 12.8 Hz, 1H, Ha, CH2), 2.81 (dd, J1 = 16.5 Hz, J2 = 3.1 Hz, 1H, Hb, CH2), 2.30 (s, 3H, CH3); 13C NMR (50 MHz, CDCl3+CCl4): δ = 197.1, 190.0 (2 × CO), 162.5, 143.9, 141.5, 138.6, 137.4, 136.9, 135.1, 133.2, 129.7, 129.6, 129.5, 129.4, 129.3, 128.5, 128.2, 128.0, 127.8, 119.6, 116.9, 78.8, 46.1, 20.8; MS (ESI+): m/z: 513[M+H]+. Elemental analysis for C31H22O3Cl2: Calcd. C, 72.52; H, 4.32. Found: C, 72.50; H, 4.28.
6-cinnamoyl-7-methyl-2,5-diphenylchroman-4-one (26)
It was obtained as light yellow solid, mp 148–150°C, in 93% yield; Rf = 0.6 (8:2 hexane:ethylacetate); IR (KBr): υmax in cm−1 3410, 3021, 2359, 1636, 1596, 1217, 769; 1H NMR (200 MHz, CDCl3+CCl4): δ = 7.47–7.41 (m, 5H, 4 × ArH & = CH), 7.32–7.24 (m, 9H, ArH), 7.11–7.02 (m, 3H, ArH), 6.44 (d, J = 16.1 Hz, 1H, = CH), 5.59 (d, J = 12.6 Hz, 1H, CH), 3.13 (dd, J1 = 16.3 Hz, J2 = 13.3 Hz, 1H, Ha, CH2), 2.82 (d, J = 16.3 Hz, 1H, Hb, CH2), 2.31 (s, 3H, CH3); 13C NMR (50 MHz, CDCl3+CCl4): δ = 197.6, 190.5 (2 × CO), 162.7, 145.1, 143.6, 141.5, 139.0, 138.7, 136.2, 134.8, 130.8, 129.8, 128.6, 128.4, 128.1, 127.8, 126.4, 119.6, 117.0, 79.6, 46.3, 20.8; MS (ESI+): m/z: 445[M+H]+. Elemental analysis for C31H24O3: Calcd. C, 83.76; H, 5.44, Found: C, 83.69; H, 5.40.
(E)-2-(4-fluorophenyl)-6-[3-(4-fluorophenyl)acryloyl]-7-methyl-5-phenylchroman-4-one (27)
It was obtained as yellow solid, mp 152–154°C, in 93% yield; Rf = 0.6 (8:2 hexane:ethylacetate); IR (KBr): υmax in cm−1 3862, 3429, 3021, 2359, 1639, 1515, 1216, 1043, 766, 671; 1H NMR (200 MHz, CDCl3+CCl4): δ = 7.49–7.00 (m, 15H, 14 ArH & = CH), 6.34 (d, J = 16.1 Hz, 1H, = CH), 5.55 (d, J = 12.6 Hz, 1H, CH), 3.09 (dd, J1 = 16.2 Hz, J2 = 13.3 Hz, 1H, Ha, CH2), 2.80 (d, J = 16.3 Hz, 1H, Hb, CH2), 2.30 (s, 3H, CH3); 13C NMR (50 MHz, CDCl3+CCl4): δ = 197.3, 190.2 (2 × CO), 162.6, 143.8, 143.6, 141.5, 138.6, 136.3, 134.8, 134.7, 131.0 130.9, 130.5, 130.3, 129.7, 129.3, 128.4, 128.2, 128.1, 128.0, 127.9, 119.6, 116.9, 116.6, 116.4, 116.2, 116.0, 78.9, 46.2, 20.8; MS (ESI+): m/z: 481[M+H]+. Elemental analysis for C31H22O3F2: Calcd. C, 77.49; H, 4.61. Found: C, 77.38; H, 4.57.
(E)-2-(4-bromophenyl)-6-[3-(4-bromophenyl)acryloyl]-7-methyl-5-phenylchroman-4-one (28)
It was obtained as light yellow solid, mp 188–190°C, in 93% yield; Rf = 0.6 (8:2 hexane:ethylacetate); IR (KBr): υmax in cm−1 3779, 3459, 2366, 1670, 1596, 1318, 1159, 1008, 815; 1H NMR (200 MHz, CDCl3+CCl4): δ = 7.59–6.93 (m, 15H, 14 ArH & = CH), 6.37 (d, J = 16.0 Hz, 1H, = CH), 5.55 (dd, J1 = 12.7 Hz, J2 = 2.8 Hz, 1H, CH), 3.06 (dd, J1 = 16.5 Hz, J2 = 13.1 Hz, 1H, Ha, CH2), 2.81 (dd, J1 = 16.5 Hz, J2 = 2.9 Hz, 1H, Hb, CH2), 2.30 (s, 3H, CH3); 13C NMR (50 MHz, CDCl3+CCl4): δ = 197.2, 189.9 (2 × CO), 162.5, 143.9, 143.3, 138.5, 137.9, 133.6, 132.5, 132.4, 129.8, 129.7, 129.3, 128.6, 128.2, 128.1, 125.3, 123.2, 119.6, 116.9, 78.8, 46.1, 20.8; MS (ESI+): m/z: 601[M+H]+. Elemental analysis for C31H22O3Br2: Calcd. C, 61.82; H, 3.68. Found: C, 61.81; H, 3.65.
(E)-2-[4-(benzyloxy)phenyl]-6-[3-{4-(benzyloxy)phenyl}acryloyl]-7-methyl-5-phenylchroman-4-one (29)
It was obtained as light yellow solid, mp 163–165°C, in 94% yield; Rf = 0.4 (8:2 hexane:ethylacetate); IR (KBr): υmax in cm−1 3449, 3055, 2368, 1692, 1597, 1510, 1240, 1170, 1002, 743, 697; 1H NMR (200 MHz, CDCl3+CCl4): δ = 7.41–7.23 (m, 18H, 17 ArH & = CH), 7.04–6.88 (m, 7H, ArH), 6.33 (d, J = 16.4 Hz, 1H, = CH), 5.51 (d, J = 12.5 Hz, 1H, CH), 5.10 (s, 2H, OCH2), 5.07 (s, 2H, OCH2), 3.13 (dd, J1 = 15.5 Hz, J2 = 13.8 Hz, 1H, Ha, CH2), 2.78 (d, J = 16.1 Hz, 1H, Hb, CH2), 2.29 (s, 3H, CH3); 13C NMR (50 MHz, CDCl3+CCl4): δ = 197.3, 190.2 (2 × CO), 162.6, 143.8, 143.6, 141.4, 138.6, 136.3, 134.8, 134.7, 131.0, 130.9, 130.5, 130.3, 129.7, 129.3, 128.4, 128.2, 128.1, 127.9, 119.6, 116.9, 116.6, 116.4, 116.2, 116.0, 78.9, 70.4, 46.2, 20.8; MS (ESI+): m/z: 657[M+H]+. Elemental analysis for C45H36O5: Calcd. C, 82.29; H, 5.52. Found: C, 82.10; H, 5.50.
(E)-2-(3-chlorophenyl)-6-[3-(3-chlorophenyl)acryloyl]-7-methyl-5-phenylchroman-4-one (30)
It was obtained as light yellow solid, mp 190–192°C, in 94% yield; Rf = 0.6 (8:2 hexane:eyhylacetate); IR (KBr): υmax in cm−1 3434, 3020, 2365, 1690, 1595, 1218, 768; 1H NMR (200 MHz, CDCl3+CCl4): δ = 7.51 (s, 1H, ArH), 7.39–7.15 (m, 11H, 10 ArH & = CH), 7.07–7.02 (m, 2H, ArH), 6.94 (s, 1H, ArH), 6.39 (d, J = 16.0 Hz, 1H, = CH), 5.56 (dd, J1 = 12.9 Hz, J2 = 3.1 Hz, 1H, CH), 3.08 (dd, J1 = 16.5 Hz, J2 = 12.9 Hz, 1H, Ha, CH2), 2.83 (dd, J1 = 16.5 Hz, J2 = 3.2 Hz, 1H, Hb, CH2), 2.31 (s, 3H, CH3); 13C NMR (50 MHz, CDCl3+CCl4): δ = 197.1, 189.8 (2 × CO), 162.5, 143.9, 143.0, 141.6, 141.0, 138.5, 136.6, 136.2, 135.3, 130.6, 130.5, 130.4, 129.8, 129.4, 129.3, 128.4, 128.2, 128.0, 126.7, 126.5, 124.4, 119.6, 116.9, 78.8, 46.2, 20.8; MS (ESI+): m/z: 513[M+H]+. Elemental analysis for C31H22O3Cl2: Calcd. C, 72.52; H, 4.32. Found: C, 72.50; H, 4.28.
(E)-2-(3,4-dimethoxyphenyl)-6-[3-(3,4-dimethoxyphenyl)acryloyl]-7-methyl-5-phenylchroman-4-one (31)
It was obtained as light yellow solid, mp 90–92°C, in 92% yield; Rf = 0.4 (7:3 hexane:ethylacetate); IR (KBr): υmax in cm−1 3445, 2960, 2370, 1691, 1637, 1596, 1514, 1428, 1261, 1024, 859, 555; 1H NMR (200 MHz, CDCl3+CCl4): δ = 7.26–7.13 (m, 4H, 3 ArH & = CH), 7.04–6.75 (m, 9H, ArH), 6.31 (d, J = 16.0 Hz, 1H, = CH), 5.50 (dd, J1 = 2.6 Hz and J2 = 12.9 Hz, 1H, CH), 3.92–3.84 (m, 12H, 4 × OCH3), 3.12 (dd, J1 = 13.1 Hz and J2 = 16.4 Hz, 1H, Ha, CH2), 2.78 (dd, J1 = 16.5 Hz, J2 = 2.8 Hz, 1H, Hb, CH2), 2.29 (s, 3H, CH3); 13C NMR (50 MHz, CDCl3+CCl4): δ = 197.6, 190.7 (2 × CO), 162.6, 151.9, 149.9, 149.8, 149.6, 145.6, 143.6, 141.3, 138.8, 136.3, 131.4, 129.7, 129.3, 128.0, 127.8, 127.7, 126.6, 123.4, 119.6, 119.2, 117.1, 111.6, 111.4, 110.1, 109.9, 79.5, 56.3, 56.2, 56.1, 46.2, 20.8; MS (ESI+): m/z: 565[M+H]+. Elemental analysis for C35H32O7: Calcd. C, 74.45; H, 5.71. Found: C, 74.39; H, 5.67.
(E)-7-Methyl-2-(naphthalen-1-yl)-6-[3-(naphthalen-1-yl)acryloyl]-5-phenylchroman-4-one (32)
It was obtained as light yellow solid, mp 180–181°C, in 92% yield; Rf = 0.4 (7:3 hexane:ethylacetate); IR (KBr): υmax in cm−1 3428, 3056, 2367, 1695, 1635, 1596, 1342, 1178, 980, 775, 698; 1H NMR (200 MHz, CDCl3+CCl4): δ = 8.10 (d, J = 7.5 Hz, 1H, ArH), 7.99–7.77 (m, 7H, 6 ArH & = CH), 7.55–7.09 (m, 12H, ArH), 6.54 (d, J = 15.8 Hz, 1H, = CH), 6.35 (d, J = 12.7 Hz, 1H, CH), 3.31 (dd, J1 = 16.4 Hz, J2 = 13.3 Hz, 1H, Ha, CH2), 3.05 (d, J = 16.2 Hz, 1H, Hb, CH2), 2.40 (s, 3H, CH3); 13C NMR (50 MHz, CDCl3+CCl4): δ = 197.5, 190.8 (2 × CO), 163.0, 143.8, 141.8, 141.6, 138.8, 136.6, 134.4, 134.3, 134.0, 132.3, 131.8, 131.0, 130.6, 130.1, 129.8, 129.6, 129.5, 129.1, 128.3, 128.0, 127.2, 127.1, 126.6, 126.3, 125.7, 124.1, 123.7, 123.2, 119.8, 117.1, 78.0, 45.6, 20.9; MS (ESI+): m/z: 545[M+H]+. Elemental analysis for C39H28O3: Calcd. C, 86.01; H, 5.18. Found: C, 85.91; H, 5.15.
(E)-7-methyl-2-(naphthalen-2-yl)-6-[3-(naphthalen-2-yl)acryloyl]-5-phenylchroman-4-one (33)
It was obtained as light yellow solid, mp 184–185°C, in 94% yield; Rf = 0.4 (8:2 hexane:ethylacetate); IR (KBr): υmax in cm−1 3465, 3050, 2369, 1690, 1597, 1429, 1263, 1187, 984, 747, 473; 1H NMR (200 MHz, CDCl3+CCl4): δ = 7.95–7.73 (m, 8H, 7 ArH & = CH), 7.62–7.40 (m, 7H, ArH), 7.27–7.19 (m, 4H, ArH), 7.12–7.07 (m, 2H, ArH), 6.55 (d, J = 16.0 Hz, 1H, = CH), 5.77 (dd, J1 = 13.6 Hz, J2 = 2.9 Hz, 1H, CH), 3.24 (dd, J1 = 16.5 Hz, J2 = 13.0 Hz, 1H, Ha, CH2), 2.93 (dd, J1 = 16.5 Hz, J2 = 3.0 Hz, 1H, Hb, CH2), 2.35 (s, 3H, CH3); 13C NMR (50 MHz, CDCl3+CCl4): δ = 197.7, 190.5 (2 × CO), 162.7, 145.3, 143.8, 141.5, 138.8, 136.3, 134.7, 133.8, 133.6, 132.3, 130.7, 129.8, 129.4, 129.2, 129.0, 128.9, 128.5, 128.2, 127.9, 127.8, 127.7, 127.1, 126.9, 125.7, 123.9, 123.8, 119.7, 117.1, 79.7, 46.3, 20.8; MS (ESI+): m/z: 545[M+H]+. Elemental analysis for C39H28O3: Calcd. C, 86.01; H, 5.18. Found: C, 85.89; H, 5.15.
5-(4-bromophenyl)-6-cinnamoyl-7-methyl-2-phenylchroman-4-one (34)
It was obtained as light yellow solid, mp 168–170°C in 92% yield; Rf = 0.6 (8:2 hexane:ethylacetate); IR (KBr): υmax in cm−1 3434, 3022, 2364, 1639, 1216, 767, 671; 1H NMR (200 MHz, CDCl3+CCl4): δ = 7.46–7.26 (m, 12H, 11 ArH & = CH), 7.03–6.91 (m, 4H, ArH), 6.49 (d, J = 16.1 Hz, 1H, = CH), 5.58 (d, J = 12.1 Hz, 1H, CH), 3.13 (dd, J1 = 16.3 Hz, J2 = 13.7 Hz, 1H, Ha, CH2), 2.82 (d, J = 16.1 Hz, 1H, Hb, CH2), 2.30 (s, 3H, CH3); 13C NMR (50 MHz, CDCl3+CCl4): δ = 197.7, 190.6 (2 × CO), 162.7, 146.1, 143.7, 140.0, 138.8, 137.6, 136.0, 134.6, 131.4, 131.3, 131.1, 130.9, 129.3, 129.2, 128.7, 128.6, 126.4, 122.1, 119.9, 116.9, 79.6, 46.2, 20.7; MS (ESI+): m/z: 523[M+H]+. Elemental analysis for C31H23BrO3: Calcd. C, 71.13; H, 4.43. Found: C, 71.06; H, 4.40.
(E)-2,5-bis(4-bromophenyl)-6-[3-(4-bromophenyl)acryloyl]-7-methylchroman-4-one (35)
It was obtained as yellow solid, mp 221–223°C in 94% yield; Rf = 0.5 (8:2 hexane:ethylacetate); IR (KBr): υmax in cm−1 3426, 3021, 2363, 1633, 1584, 1216, 761, 671; 1H NMR (200 MHz, CDCl3+DMSO): δ = 7.59–7.30 (m, 10H, 9 ArH & = CH), 7.05–6.89 (m, 4H, ArH), 6.54 (d, J = 16.0 Hz, 1H, = CH), 5.67 (d, J = 11.9 Hz, 1H, CH), 3.08 (dd, J1 = 16.4 Hz, J2 = 13.0 Hz, 1H, Ha, CH2), 2.79 (d, J = 16.3 Hz, 1H, Hb, CH2), 2.23 (s, 3H, CH3); 13C NMR (50 MHz, CDCl3+CCl4): δ = 197.4, 190.2 (2 × CO), 162.5, 144.9, 143.3, 139.6, 138.6, 138.4, 135.9, 133.8, 132.6, 132.3, 132.1, 131.5, 130.9, 130.7, 129.1, 128.9, 125.3, 122.8, 121.5, 120.1, 117.2, 78.7, 45.9, 20.7; MS (ESI+): m/z: 679[M+H]+. Elemental analysis for C31H21Br3O3: Calcd. C, 54.66; H, 3.11. Found: C, 54.50; H, 3.09.
(E)-5-(4-bromophenyl)-2-(4-fluorophenyl)-6-[3-(4-fluorophenyl)acryloyl]-7-methylchroman-4-one (36)
It was obtained as light yellow solid, mp 168–170°C in 92% yield; Rf = 0.5 (8:2 hexane:ethylacetate); IR (KBr): υmax in cm−1 3426, 2927, 2365, 1636, 1595, 1509, 1230, 1160, 836, 755, 514; 1H NMR (200 MHz, CDCl3+CCl4): δ = 7.49–7.26 (m, 6H, 5 ArH & = CH), 7.18–6.80 (m, 8H, ArH), 6.38 (d, J = 16.1 Hz, 1H, = CH), 5.55 (dd, J1 = 13.0 Hz, J2 = 2.9 Hz, 1H, CH), 3.09 (dd, J1 = 16.5 Hz, J2 = 13.0 Hz, 1H, Ha, CH2), 2.80 (dd, J1 = 16.5 Hz, J2 = 3.0 Hz, 1H, Hb, CH2), 2.29 (s, 3H, CH3); 13C NMR (50 MHz, CDCl3+CCl4): δ = 197.1, 190.1 (2 × CO), 162.5, 144.3, 143.8, 140.0, 137.5, 136.1, 134.6, 131.4, 131.3, 131.1, 130.9, 130.7, 130.6, 130.4, 128.4, 128.2, 119.9, 116.8, 116.4, 116.3, 116.0, 78.9, 46.1, 20.7; MS (ESI+): m/z: 559[M+H]+. Elemental analysis for C31H21BrF2O3: Calcd. C, 66.56; H, 3.78. Found: C, 66.50; H, 3.71.
(E)-5-(4-bromophenyl)-2-(4-chlorophenyl)-6-[3-(4-chlorophenyl)acryloyl]-7-methylchroman-4-one (37)
It was obtained as light yellow solid, mp 174–176°C in 94% yield; Rf = 0.5 (8:2 hexane:ethylacetate); IR (KBr): υmax in cm−1 3458, 3020, 2365, 1691, 1594, 1216, 1089, 765, 501; 1H NMR (200 MHz, CDCl3+CCl4): δ = 7.41–7.23 (m, 10H, 9 ArH & = CH), 7.01–6.88 (m, 4H, ArH), 6.41 (d, J = 16.1 Hz, 1H, = CH), 5.55 (d, J = 11.0 Hz, 1H, CH), 3.07 (dd, J1 = 16.3 Hz, J2 = 12.9 Hz, 1H, Ha, CH2), 2.80 (d, J = 15.7 Hz, 1H, Hb, CH2), 2.29 (s, 3H, CH3); 13C NMR (50 MHz, CDCl3+CCl4): δ = 197.1, 190.0 (2 × CO), 162.5, 144.1, 143.8, 140.1, 137.4, 137.2, 136.1, 135.2, 133.0, 131.3, 131.1, 131.0, 129.7, 129.6, 129.5, 128.7, 127.7, 122.3, 119.9, 116.8, 78.8, 46.0, 20.7; MS (ESI+): m/z: 591[M+H]+. Elemental analysis for C31H21BrCl2O3: Calcd. C, 62.86; H, 3.57. Found: C, 62.80; H, 3.43.
(E)-2-(4-(benzyloxy)phenyl)-6-[3-{4-(benzyloxy)phenyl}acryloyl]-5-(4-bromophenyl)-7-methylchroman-4-one (38)
It was obtained as light yellow solid, mp 192–195°C in 92% yield; Rf = 0.5 (7:3 hexane:ethylacetate); IR (KBr): υmax in cm−1 3752, 3454, 3021, 2364, 1563, 1216, 1017, 761; 1H NMR (200 MHz, CDCl3+CCl4): δ = 7.43–7.26 (m, 16H, 15 ArH & = CH), 7.06–6.92 (m, 8H, ArH), 6.42 (d, J = 16.1 Hz, 1H, = CH), 5.53 (dd, J1 = 13.1 Hz, J2 = 2.6 Hz, 1H, CH), 5.11–5.09 (m, 4H, 2 × OCH2), 3.15 (dd, J1 = 16.6 Hz, J2 = 13.2 Hz, 1H, Ha, CH2), 2.79 (dd, J1 = 16.6 Hz, J2 = 2.8 Hz, 1H, Hb, CH2), 2.27 (s, 3H, CH3); 13C NMR (50 MHz, CDCl3+CCl4): δ = 198.2, 190.4 (2 × CO), 162.7, 161.5, 159.6, 146.6, 143.7, 139.8, 137.8, 137.1, 136.7, 136.0, 131.3, 131.2, 131.1, 130.8, 130.6, 129.1, 128.6, 128.5, 128.1, 127.8, 127.4, 126.6, 122.0, 119.9, 116.9, 115.7, 115.6, 79.4, 70.5, 45.9, 20.7; MS (ESI+): m/z: 735[M+H]+. Elemental analysis for C45H35O5Br: Calcd. C, 73.47; H, 4.80. Found: C, 73.41; H, 4.75.
(E)-5-(4-bromophenyl)-2-(4-methoxyphenyl)-6-[3-(4-methoxyphenyl)acryloyl]-7-methylchroman-4-one (39)
It was obtained as light yellow solid, mp 178–180°C in 91% yield; Rf = 0.4 (7:3 hexane:ethylacetate); IR (KBr): υmax in cm−1 3756, 3019, 2364, 1598, 1216, 1168, 1030, 763; 1H NMR (200 MHz, CDCl3+CCl4): δ = 7.41–7.26 (m, 6H, 5 ArH & = CH), 7.04–6.83 (m, 8H, ArH), 6.38 (d, J = 16.1 Hz, 1H, = CH), 5.52 (dd, J1 = 13.1 Hz, J2 = 2.7 Hz, 1H, CH), 3.84–3.80 (m, 6H, 2 × OCH3), 3.13 (dd, J1 = 16.5 Hz, J2 = 13.2 Hz, 1H, Ha, CH2), 2.78 (dd, J1 = 16.5 Hz, J2 = 2.8 Hz, 1H, Hb, CH2), 2.28 (s, 3H, CH3); 13C NMR (50 MHz, CDCl3+CCl4): δ = 197.7, 190.9 (2 × CO), 162.7, 162.3, 160.4, 146.2, 143.6, 139.9, 137.8, 136.1, 131.4, 131.2, 131.0, 130.8, 130.5, 128.0, 127.2, 126.5, 122.0, 119.8, 116.8, 114.8, 114.6, 79.3, 55.7, 55.6, 46.0, 20.7; MS (ESI+): m/z: 583[M+H]+. Elemental analysis for C33H27O5Br: Calcd. C, 67.93; H, 4.66. Found: C, 67.80; H, 4.55.
5-(4-(benzyloxy)phenyl)-6-cinnamoyl-7-methyl-2-phenylchroman-4-one (40)
It was obtained as light yellow solid, mp 168–169°C in 90% yield; Rf = 0.4 (7:3 hexane:ethylacetate); IR (KBr): υmax in cm−1 3779, 3380, 3021, 2359, 1596, 1216, 762, 671; 1H NMR (200 MHz, CDCl3+CCl4): δ = 7.47–7.26 (m, 15H, 14 ArH & = CH), 7.14–7.11 (m, 2H, ArH), 7.03–6.89 (m, 2H, ArH), 6.88–6.84 (m, 2H, ArH), 6.44 (d, J = 16.1 Hz, 1H, = CH), 5.59 (dd, J1 = 13.1 Hz, J2 = 3.0 Hz, 1H, CH), 4.95 (s, 2H, OCH2), 3.13 (dd, J1 = 16.5 Hz, J2 = 13.1 Hz, 1H, Ha, CH2), 2.84 (dd, J1 = 16.5 Hz, J2 = 3.0 Hz, 1H, Hb, CH2), 2.31 (s, 3H, CH3); 13C NMR (50 MHz, CDCl3+CCl4): δ = 197.8, 190.6 (2 × CO), 162.8, 158.7, 144.7, 143.7, 141.3, 139.0, 137.3, 136.5, 134.9, 131.1, 130.8, 130.7, 129.2, 128.8, 128.6, 128.4, 128.2, 127.8, 126.4, 119.5, 114.3, 79.5, 70.2, 46.4, 20.8; MS (ESI+): m/z: 551[M+H]+. Elemental analysis for C38H30O4: Calcd. C, 82.89; H, 5.49. Found: C, 82.70; H, 5.45.
(E)-5-(4-(benzyloxy)phenyl)-2-(4-bromophenyl)-6-[3-(4-bromophenyl)acryloyl]-7-methylchroman-4-one (41)
It was obtained as light yellow solid, mp 170–171°C in 93% yield; Rf = 0.4 (7:3 hexane:ethylacetate); IR (KBr): υmax in cm−1 3762, 3427, 3022, 2368, 1635, 1217, 767, 671; 1H NMR (200 MHz, CDCl3+CCl4): δ = 7.59–7.26 (m, 11H, 10 ArH & = CH), 7.17–7.07 (m, 3H, ArH), 7.01–6.83 (m, 5H, ArH), 6.37 (d, J = 16.0 Hz, 1H, = CH), 5.54 (d, J = 12.6 Hz, 1H, CH), 4.95 (m, 2H, OCH2), 3.06 (dd, J1 = 16.1 Hz, J2 = 12.9 Hz, 1H, Ha, CH2), 2.81 (d, J = 16.4 Hz, 1H, Hb, CH2), 2.30 (s, 3H, CH3); 13C NMR (50 MHz, CDCl3+CCl4): δ = 197.8, 191.0 (2 × CO), 162.7, 158.6, 151.9, 149.9, 149.6, 145.3, 143.7, 137.3, 131.4, 130.6, 128.8, 128.2, 127.8, 126.6, 123.4, 119.2, 109.9, 70.5, 20.9; MS (ESI+): m/z: 707[M+H]+. Elemental analysis for C38H28O4Br2: Calcd. C, 64.42; H, 3.98. Found: C, 64.39; H, 3.88.
(E)-5-(4-(benzyloxy)phenyl)-2-(4-chlorophenyl)-6-[3-(4-chlorophenyl)acryloyl]-7-methylchroman-4-one (42)
It was obtained as light yellow solid, mp 164–165°C in 93% yield; Rf = 0.4 (7:3 hexane:ethylacetate); IR (KBr): υmax in cm−1 3420, 2368, 1687, 1598, 1511, 1244, 1173, 1029, 838, 760, 522; 1H NMR (200 MHz, CDCl3+CCl4): δ = 7.42–7.24 (m, 13H, 12 ArH & = CH), 7.20–6.95 (m, 4H, ArH), 6.86–6.83 (m, 2H, ArH), 6.36 (d, J = 16.0 Hz, 1H, = CH), 5.56 (dd, J1 = 12.7 Hz, J2 = 3.2 Hz, 1H, CH), 4.95 (s, 2H, OCH2), 3.07 (dd, J1 = 16.5 Hz, J2 = 12.7 Hz, 1H, Ha, CH2), 2.82 (dd, J1 = 16.5 Hz, J2 = 3.2 Hz, 1H, Hb, CH2), 2.30 (s, 3H, CH3); 13C NMR (50 MHz, CDCl3+CCl4): δ = 197.1, 190.0 (2 × CO), 162.6, 158.8, 143.9, 142.6, 141.4, 137.4, 137.2, 136.8, 136.6, 135.1, 133.4, 130.9, 129.6, 129.5, 129.4, 128.8, 128.6, 128.2, 127.7, 119.5, 117.0, 78.7, 70.2, 46.2, 20.8; MS (ESI+): m/z: 619[M+H]+. Elemental analysis for C38H28O4Cl2: Calcd. C, 73.67; H, 4.56. Found: C, 73.60; H, 4.52.
(E)-2,5-bis(4-(benzyloxy)phenyl)-6-[3-{4-(benzyloxy)phenyl}acryloyl]-7-methylchroman-4-one (43)
It was obtained as light yellow solid, mp 64–65°C in 92% yield; Rf = 0.4 (7:3 hexane:ethylacetate); IR (KBr): υmax in cm− 3781, 3324, 3036, 2362, 1602, 1505, 1247, 1016, 829, 731, 607; 1H NMR (200 MHz, CDCl3+CCl4): 7.43–7.26 (m, 19H, 18 ArH & = CH), 7.19–6.30 (m, 10H, ArH), 6.38 (d, J = 16.0 Hz, 1H, = CH), 5.53 (dd, J1 = 13.0 Hz, J2 = 2.4 Hz, 1H, CH), 5.11–4.96 (m, 6H, 3 × OCH2), 3.15 (dd, J1 = 16.5 Hz, J2 = 13.4 Hz, 1H, Ha, CH2), 2.80 (dd, J1 = 16.5 Hz, J2 = 2.7 Hz, 1H, Hb, CH2), 2.28 (s, 3H, CH3); 13C NMR (50 MHz, CDCl3+CCl4): δ = 198.5, 191.4 (2 × CO), 162.8, 161.2, 159.6, 158.6, 145.4, 143.7, 137.4, 137.1, 136.7, 131.4, 131.2, 131.0, 130.5, 129.1, 128.9, 128.6, 128.5, 128.3, 128.1, 128.0, 127.8, 127.7, 126.5, 119.5, 115.6, 115.5, 114.6, 114.2, 79.3, 70.2, 46.1, 20.8; MS (ESI+): m/z: 763[M+H]+. Elemental analysis for C52H42O6: Calcd. C, 81.87; H, 5.55. Found: C, 81.80; H, 5.53.
(E)-5-(4-(benzyloxy)phenyl)-2-(4-methoxyphenyl)-6-[3-(4-methoxyphenyl)acryloyl]-7-methylchroman-4-one (44)
It was obtained as yellow solid, mp 69–70°C in 91% yield; Rf = 0.4 (7:3 hexane:ethylacetate); IR (KBr): υmax in cm−1 3783, 2838, 2362, 1628, 1604, 1509, 1248, 1169, 1027, 830, 728, 561; 1H NMR (200 MHz, CDCl3+CCl4): 7.42–7.20 (m, 9H, 8 ArH & = CH), 7.12–6.80 (m, 10H, ArH), 6.34 (d, J = 16.0 Hz, 1H, = CH), 5.52 (dd, J1 = 13.1 Hz, J2 = 2.7 Hz, 1H, CH), 4.95 (m, 2H, OCH2), 3.84–3.81 (m, 6H, 2 × OCH3), 3.13 (dd, J1 = 16.5 Hz, J2 = 13.2 Hz, 1H, Ha, CH2), 2.79 (dd, J1 = 16.5 Hz, J2 = 2.8 Hz, 1H, Hb, CH2), 2.29 (s, 3H, CH3); 13C NMR (50 MHz, CDCl3+CCl4): δ = 197.7, 190.9 (2 × CO), 162.8, 162.0, 160.4, 158.6, 144.8, 143.5, 141.2, 137.4, 136.6, 131.3, 131.1, 130.6, 130.3, 128.8, 128.1, 128.0, 127.8, 127.5, 126.4, 119.4, 117.1, 114.7, 114.2, 79.2, 70.1, 55.6, 46.2, 20.8; MS (ESI+): m/z: 611[M+H]+. Elemental analysis for C40H34O6: Calcd. C, 78.67; H, 5.61. Found: C, 78.65; H, 5.58.
(E)-5-(4-(benzyloxy)phenyl)-2-(3,4-dimethoxyphenyl)-6-[3-(3,4-dimethoxyphenyl)acryloyl]-7-methylchroman-4-one (45)
It was obtained as light yellow solid, mp 138–140°C in 93% yield; Rf = 0.4 (7:3 hexane:ethylacetate); IR (KBr): υmax in cm−1 3767, 3427, 2932, 2363, 1594, 1510, 1261, 1150, 1021, 770; 1H NMR (200 MHz, CDCl3+CCl4): δ = 7.32–7.26 (m, 5H, 4 ArH & = CH), 7.11–6.75 (m, 12H, ArH), 6.32 (d, J = 16.0 Hz, 1H, = CH), 5.51 (dd, J1 = 12.8 Hz, J2 = 2.6 Hz, 1H, CH), 4.95 (m, 2H, OCH2), 3.93–3.85 (m, 12H, 4 × OCH3), 3.13 (dd, J1 = 16.5 Hz, J2 = 13.2 Hz, 1H, Ha, CH2), 2.80 (dd, J1 = 16.5 Hz, J2 = 2.8 Hz, 1H, Hb, CH2), 2.29 (s, 3H, CH3); 13C NMR (50 MHz, CDCl3+CCl4): δ = 197.8, 191.0 (2 × CO), 162.7, 158.6, 151.9, 149.9, 149.6, 145.3, 143.7, 137.3, 131.4, 130.6, 128.8, 128.2, 127.8, 126.6, 123.4, 119.2, 114.5, 111.6, 111.4, 110.1, 109.9, 79.5, 70.2, 56.3, 46.3, 20.8. MS (ESI+): m/z: 671[M+H]+. Elemental analysis for C42H38O8: Calcd. C, 75.21; H, 5.71. Found: C, 75.12; H, 5.69.
(E)-2-(2-chlorophenyl)-5-(4-chlorophenyl)-6-[3-(2-chlorophenyl)acryloyl]-7-methylchroman-4-one (46)
It was obtained as light yellow solid, mp 190–192°C in 91% yield; Rf = 0.4 (7:3 hexane:ethylacetate); IR (KBr): υmax in cm−1 3757, 3065, 2364, 1696, 1591, 1436, 1316, 1161, 1041, 861, 746; 1H NMR (200 MHz, CDCl3+CCl4): δ = 7.76–7.72 (m, 1H, ArH), 7.50–7.23 (m, 11H, 10 ArH & = CH), 7.19–6.98 (m, 2H, ArH), 6.42 (d, J = 16.2 Hz, 1H, = CH), 5.98 (dd, J1 = 11.7Hz, J2 = 4.4 Hz, 1H, CH), 2.91–2.84 (m, 2H, CH2), 2.32 (s, 3H, CH3); 13C NMR (50 MHz, CDCl3+CCl4): δ = 197.7, 190.0 (2 × CO), 162.8, 143.8, 141.9, 140.3, 136.9, 135.9, 135.3, 134.0, 133.0, 132.1, 131.7, 131.5, 130.8, 130.5, 130.1, 130.0, 128.3, 128.1, 127.8, 127.5, 119.9, 116.9, 76.5, 44.9, 20.8; MS (ESI+): m/z: 547[M+H]+. Elemental analysis for C31H21O3Cl3: Calcd. C, 67.94; H, 3.86. Found: C, 67.88; H, 3.85.
(E)-5-(4-chlorophenyl)-7-methyl-2-(naphthalen-1-yl)-6-[3-(naphthalen-1-yl)acryloyl]chroman-4-one (47)
It was obtained as light yellow solid, mp 184–185°C in 923% yield; Rf = 0.4 (7:3 hexane:ethylacetate); IR (KBr): υmax in cm−1 3778, 3362, 3060, 2920, 2365, 1691, 1639, 1594, 1340, 1180, 1085, 775, 528; 1H NMR (200 MHz, CDCl3+CCl4): δ = 8.10–7.76 (m, 8H, 7 ArH & = CH), 7.59–7.11 (m, 12H, ArH), 6.57 (d, J = 15.8 Hz, 1H, = CH), 6.35 (dd, J1 = 12.8 Hz, J2 = 2.7 Hz, 1H, CH), 3. 31 (dd, J1 = 16.7 Hz, J2 = 12.9 Hz, 1H, Ha, CH2), 3.06 (dd, J1 = 16.7 Hz, J2 = 2.9 Hz, 1H, Hb, CH2), 2.39 (s, 3H, CH3); 13C NMR (50 MHz, CDCl3+CCl4): δ = 197.4, 190.8 (2 × CO), 163.0, 143.8, 142.7, 140.2, 137.2, 136.5, 134.3, 134.2, 134.1, 132.1, 131.7, 131.4, 131.3, 131.1, 130.9, 130.6, 129.8, 129.5, 129.2, 128.5, 128.3, 127.4, 126.6, 126.4, 125.9, 125.8, 125.7, 124.1, 123.5, 123.1, 120.1, 117.0, 76.90, 45.51, 20.8; MS (ESI+): m/z: 579 [M+H]+. Elemental analysis for C39H27O3Cl: Calcd. C, 80.89; H, 4.70. Found: C, 80.81; H, 4.67.
(E)-5-(4-chlorophenyl)-7-methyl-2-(naphthalen-2-yl)-6-[3-(naphthalen-1-yl)acryloyl]chroman-4-one (48)
It was obtained as light yellow solid, mp 184–185°C in 923% yield; Rf = 0.4 (7:3 hexane:ethylacetate); IR (KBr): υmax in cm−1 3765, 3412, 2922, 2364, 1636, 1596, 1429, 1183, 1088, 814, 473; 1H NMR (200 MHz, CDCl3+CCl4): δ = 7.94–7.78 (m, 8H, 7 ArH & = CH), 7.62–7.45 (m, 6H, ArH), 7.26–7.01 (m, 6H, ArH), 6.60 (d, J = 16.0 Hz, 1H, = CH), 5.77 (dd, J1 = 12.9 Hz, J2 = 2.6 Hz, 1H, CH), 3. 24 (dd, J1 = 16.5 Hz, J2 = 13.0 Hz, 1H, Ha, CH2), 2.93 (dd, J1 = 16.5 Hz, J2 = 2.8 Hz, 1H, Hb, CH2), 2.34 (s, 3H, CH3); 13C NMR (50 MHz, CDCl3+CCl4): δ = 197.7, 190.5 (2 × CO), 162.7, 146.2, 143.7, 140.1, 137.1, 136.1, 134.9, 133.9, 133.8, 133.6, 132.1, 131.1, 130.9, 130.7, 129.2, 128.9, 128.7, 128.5, 128.3, 128.2, 127.9, 127.2, 127.0, 125.7, 123.9, 123.7, 120.0, 117.0, 79.75, 46.20, 30.1, 20.8; MS (ESI+): m/z: 579 [M+H]+. Elemental analysis for C39H27O3Cl: Calcd. C, 80.89; H, 4.70. Found: C, 80.81; H, 4.67.
Biology
Animal used
Rats (Charles Foster strain, male, adult, body weight 200–225 g) were kept in a room with controlled temperature (25–26°C), humidity (60–80%) and 12/12-h light/dark cycle (light on from 8.00 a.m. to 8.00 p.m.) under hygienic conditions. Animals, which were acclimatized for 1 week before starting the experiment, had free access to the normal diet and water.
Antidyslipidemic activity
The antidyslipidemic activities of all compounds were evaluated in a triton modelCitation39. Rats were divided in control, triton induced, triton plus compounds and gemfibrozil (standard drug, 100 mg/kg) treated groups containing six rats in each group. Hyperlipidemia was developed by administration of triton WR-1339 (sigma chemical company, St Louis, MO) at a dose of 400 mg/kg body wt. intraperitoneally to animals of all groups except the control. All the compounds were macerated with gum acacia suspension (vehicle). After 18 h of treatment 3.0 mL, blood was withdrawn from retro-orbital sinus using glass capillary in EDTA coated eppendorf tube (3.0 mg/mL) and plasma was separated. Plasma was diluted with normal saline (ratio 1:3) and used for the analysis of TC, PL, Tg, and protein by standard enzymatic procedures using spectrometer and standard kits purchased from Beckmann Coulter International (USA).
Antioxidant activity determination
Superoxides anions were generated enzymaticallyCitation28 from xanthine (160 mM) using xanthine oxidase (0.04 U) and nitro blue tetrazolium (320 μM) in the absence or presence of compounds 25–48 (200 μg/mL) in 100 mM phosphate buffer (pH-8.2). Fractions were sonicated well in phosphate buffer before use. The reaction mixtures were incubated at 37°C, and after 30 min, the reaction was stopped by adding 0.5 mL glacial acetic acid. The amount of formazone formed was calculated spectrophotometrically. In another set of experiment, an effect of compounds on generation of hydroxyl radicals (OH·) was also studied by nonenzymatic reactants. Briefly, hydroxyl radicals (OH·) were generated in a nonenzymatic system comprising deoxyribose (2.8 mM), FeSO4·7H2O (2.0 mM), sodium ascorbate (2.0 mM) and H2O2 (2.8 mM) in 50 mM KH2 PO4 buffer, pH 7.4 to a final volume of 2.5 mL. The above reaction mixtures in the absence or presence of test compounds (200 μg/mL) were incubated at 37°C for 90 min. The test compounds were also studied for their inhibitory action against microsomal lipid peroxidation in vitro by non enzymatic inducer. Reference samples and reagent blanks were also run simultaneously. Malondialdehyde content in both experimental and reference samples were estimated spectrophotometriclly by thiobarbituric acid method as mentioned earlierCitation40. Alloprinol, mannitol, and α-tocopherol were used as standard drugs for superoxide, hydroxylations and microsomal lipid peroxidations.
Biochemical analysis of plasma/serum
Serum lipids TCCitation41, PlCitation42, TgCitation43 and proteinCitation44 were estimated by the standard procedures reported in literature.
Statistical evaluation
Data were analysed using Student’s t-test. The hyperlipedemic groups were compared with control drug treated groups. Similarly, the generation of oxygen free radicals with different 6-cinnamoyl-7-methyl-5-phenyl-flavanones derivatives were compared with that of their formation without compounds. P < 0.05 was considered to be significant.
Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.3109/14756366.2011.585134.
Declaration of interest
This is CDRI Communication No 8052. The authors thank CSIR and DRDO New Delhi for the SRF and PA to Anindra, Namrata and Rahul, respectively. We are also thankful to the SAIF Division of C.D.R.I. for providing all the spectral data. We sincerely acknowledge the financial assistance from DRDO New Delhi.
Supplementary Material
Download PDF (1.4 MB)References
- Vassalle C, Pratali L, Boni C, Mercuri A, Ndreu R. An oxidative stress score as a combined measure of the pro-oxidant and anti-oxidant counterparts in patients with coronary artery disease. Clin Biochem 2008;41:1162–1167.
- Wahle KW. Atherosclerosis: Cell biology and lipoproteins. Curr Opin Lipidol 2002;13:347–349.
- Kreisberg RA, Oberman A. Medical management of hyperlipidemia/dyslipidemia. J Clin Endocrinol Metab 2003;88:2445–2461.
- Rajani GP, Ashok P. In vitro antioxidant and antihyperlipidemic activities of Bauhinia variegata Linn. Indian J Pharmacol 2009;41:227–232.
- Delriao JA, Goamez A, Baidez G, Arcas MC, Botia JM, Ortuno A. Changes in the levels of polymethoxyflavones and flavanones as part of the defense mechanism of Citrus sinensis (Cv. Valencia Late) fruits against Phytophthora citrophthora. J Agric Food Chem 2004;52:1913–1917.
- Wächter GA, Hoffmann JJ, Furbacher T, Blake ME, Timmermann BN. Antibacterial and antifungal flavanones from Eysenhardtia texana. Phytochemistry 1999;52:1469–1471.
- Chen HY, Dykstra KD, Birzin ET, Frisch K, Chan W, Yang YT et al. Estrogen receptor ligands. Part 1: The discovery of flavanoids with subtype selectivity. Bioorg Med Chem Lett 2004;14:1417–1421.
- Tan Q, Blizzard TA, Morgan JD 2nd, Birzin ET, Chan W, Yang YT et al. Estrogen receptor ligands. Part 10: Chromanes: old scaffolds for new SERAMs. Bioorg Med Chem Lett 2005;15:1675–1681.
- Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA. Flavonoids: A review of probable mechanisms of action and potential applications. Am J Clin Nutr 2001;74:418–425.
- Middleton E, Kandaswami C. Potential health promoting properties of citrus flavonoids. Food Technol 1994;48:115–119.
- Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J Nutr Biochem 2002;13:572–584.
- Harborne JB. The Flavonoids. Chapman & Hall: London, U.K. 1994, pp. 406–416.
- Harborne JB, Williams CA. Anthocyanins and other flavonoids. Nat Prod Rep 2001;18:310–333.
- Harbone JB, ed. The Flavonoids, Advances in Research since 1980. Chapman & Hall, New York. 1988, 303–328.
- Harborne JB, Williams CA. Anthocyanins and other flavonoids. Nat Prod Rep 1995;12:639–657.
- Chang LC, Kinghorn AD. In bioactive compounds from natural sources: Isolation characterisation and biological properties. Tringali, C., ed., Taylor & Francis Ltd., London, 2001: Chapter 5. pp 159–188.
- Anderson ØM, Markham, KR. eds., Flavonoids: Chemistry, Biochemistry and Applications. Taylor & Francis Ltd., London 2006, 749–856
- Avila HP, Smânia Ede F, Monache FD, Smânia A Jr. Structure-activity relationship of antibacterial chalcones. Bioorg Med Chem 2008;16:9790–9794.
- Cushnie TP, Lamb AJ. Antimicrobial activity of flavonoids. Int J Antimicrob Agents 2005;26:343–356.
- Rukachaisirikul T, Innok P, Aroonrerk N, Boonamnuaylap W, Limrangsun S, Boonyon C et al. Antibacterial pterocarpans from Erythrina subumbrans. J Ethnopharmacol 2007;110:171–175.
- Bohm BA. Introduction to Flavonoids. Harwood Academic Publishers: Amsterdam, Netherlands, 1998: p. 243.
- Harborne JB. The Flavonoids, Chapman & Hall: London, U.K. 1994, pp. 427–431.
- Kasahara A, Izumi T, Ooshima M. A new method of preparing flavones. Bull Chem Soc Jpn 1974;47:2526–2528.
- Maruyama K, Tamanaka K, Nishinaga A. Conversion of 2′-hydroxychalcones to flavanones catalyzed by cobalt Schiff base complex. Tetrahedron Lett 1989;30:4145–4148.
- Makrandi JK, Bala S. Potassium ferricyanide mediated cyclisation of 2′-hydroxychalcones to flavanones using phase transfer catalysis. Syn Comm 2000;30:3555–3558.
- Sharma A, Chakravarti B, Gupt MP, Siddiqui JA, Konwar R, Tripathi RP. Synthesis and anti breast cancer activity of biphenyl based chalcones. Bioorg Med Chem 2010;18:4711–4720.
- Sharma A, Pandey J, Tripathi RP. An efficient regioselective synthesis of functionalized biphenyls via sequential reactions of aromatic aldehydes and β-keto esters or ketones. Tetrahedron Lett 2009;50:1812–1816.
- Bindoli A, Valente M, Cavallini L. Inhibitory action of quercetin on xanthine oxidase and xanthine dehydrogenase activity. Pharmacol Res Commun 1985;17:831–839.
- Rohdewald P. A review of the French maritime pine bark extract (Pycnogenol), a herbal medication with a diverse clinical pharmacology. Int J Clin Pharmacol Ther 2002;40:158–168.
- Bors W, Michel C. Antioxidant capacity of flavanols and gallate esters: Pulse radiolysis studies. Free Radic Biol Med 1999;27:1413–1426.
- Bors W, Michel C, Stettmaier K. Electron paramagnetic resonance studies of radical species of proanthocyanidins and gallate esters. Arch Biochem Biophys 2000;374:347–355.
- Robak J, Gryglewski RJ. Flavonoids are scavengers of superoxide anions. Biochem Pharmacol 1988;37:837–841.
- Husain SR, Cillard J, Cillard P. Hydroxyl radical scavenging activity of flavonoids. Phytochemistry 1987;26:2489–2491.
- Wang W, Goodman MT. Antioxidant property of dietary phenolic agents in a human LDL-oxidation ex vivo model: Interaction of protein binding activity. Nutr Res 1999;19:191–202.
- Fuchs J, Huflejt ME, Rothfuss LM, Wilson DS, Carcamo G, Packer L. Impairment of enzymic and nonenzymic antioxidants in skin by UVB irradiation. J Invest Dermatol 1989;93:769–773.
- Lotito SB, Frei B. Relevance of apple polyphenols as antioxidants in human plasma: Contrasting in vitro and in vivo effects. Free Radic Biol Med 2004;36:201–211.
- Halliwell B, Gutteridge JM, Aruoma OI. The deoxyribose method: A simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem 1987;165:215–219.
- Schotz MC, Scanu A, Page IH. Effect of triton on lipoprotein lipase of rat plasma. Am J Physiol 1957;188:399–402.
- Schurr PE, Schultz JR, Parkinson TM. Triton-induced hyperlipidemia in rats as an animal model for screening hypolipidemic drugs. Lipids 1972;7:68–74.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95:351–358.
- Deeg R, Ziegenhorn J. Kinetic enzymic method for automated determination of total cholesterol in serum. Clin Chem 1983;29:1798–1802.
- Bucolo G, David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem 1973;19:476–482.
- Zilversmit DB, Davis AK. Microdetermination of plasma phospholipids by trichloroacetic acid precipitation. J Lab Clin Med 1950;35:155–160.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265–275.