Abstract
Glucosyltransferase (GTF) plays an important role in the development of dental caries. This study was carried out to compare the efficiency of green mate (GM) and roasted mate (RM) water extracts, drinks rich in polyphenolic compounds consumed in the subtropical region of South America, on the extracellular GTF activity from Streptococcus mutans. The RM extract exhibited a greater inhibitory effect (IC50 of 10 mg/mL) despite presenting lower polyphenolic content. The kinetic analysis showed that there were significant differences (P < 0.05) between the extracts with respect to the values for Km and Ki, whereas the values for Vmax were the same, implying the competitive nature of GTF inhibition. GTF activity was also measured using selected polyphenols as inhibitors, and the most effective inhibitors were rutin and caffeoylshikimic acid. The characterization of the extracts by ESI-MS and UPLC-MS showed that the compounds formed during roasting, possibly shikimic acid derivatives and other unindentified compounds formed by the Maillard reaction, appeared to contribute to the inhibition of GTF activity.
Introduction
The glucosyltransferase (GTF; EC 2.4.1.5) enzymes produced by Streptococcus mutans have been recognized as virulence factors in the pathogenesis of dental caries.Citation1,Citation2 They use sucrose as their substrate yielding fructose and glucans with predominantly α(1→3) and α(1→6) bonds as the reaction products.Citation3 S. mutans produces at least three GTFs: GTF B, which synthesizes a polymer of mostly insoluble α 1,3-linked glucan; GTF C, which synthesizes a mixture of insoluble α 1,3-linked glucan and soluble α 1,6-linked glucan; and GTF D, which synthesizes α 1,6-linked soluble glucan.Citation4 Glucans promote the adherence and accumulation of cariogenic streptococci on the tooth surface and play an essential role in the development of the pathogenic dental plaque related to carie-forming activity.Citation2,Citation5,Citation6 The inhibition of GTFs both in solution and adsorbed to the pellicle of the tooth surface is one of the strategies used to prevent dental caries and other plaque-related diseases. Some studies have demonstrated an inhibitory activity of natural herbs such as oolong tea,Citation7 Chinese black teaCitation8 and rosemary extractCitation9 on GTF.
Yerba mate (Ilex paraguariensis) is a plant native to the subtropical region of South America. It is found in the South of Brazil, North of Argentina, Paraguay and Uruguay and contains many bioactive compounds. In addition to substantial amounts of purine alkaloids and phenolic acids (mainly caffeoylquinic acid derivatives), the yerba mate leaves also contain flavonoids (quercetin and rutin) and triterpenoid saponins.Citation10,Citation11 The dried and minced leaves and branches of I. paraguariensis are called yerba mate when not roasted and mate tea when roasted, as determined by Brazilian and Argentinean legislation. Several beverages are produced with yerba mate leaves: chimarrão and tererê are prepared with yerba mate and hot and cold water, respectively; mate tea can be prepared as a typical hot tea or in the form of a refreshing ready-to-drink iced mate tea. Roasted mate (RM) tea is a beverage with a mild, pleasant aroma, which is consumed in southern and southeastern Brazil and in Argentina. The use of leaves from different origins as well as different forms of preparation result in extracts with varied chemical compositions.Citation11,Citation12 Previous studies showed that I. paraguariensis extracts exhibited strong antioxidant activity.Citation13,Citation14 The present authors recently reported that mate tea was able to protect unsaturated fatty acids from oxidation and decrease DNA damage in the liver of mice.Citation15,Citation16 It also presents a hypocholesterolemic effect, which is associated with the inhibition of pancreatic lipase activity.Citation17 Other biological effects such as chemopreventive activity,Citation18,Citation19 choleretic effect,Citation20 vasodilatation effect,Citation21 inhibition of glycation,Citation22 and anti-atherosclerotic propertiesCitation23 were also reported.
Although the effect of some polyphenol-containing herbs on the inhibition of GTF has been investigated,Citation8 no reports were found on the inhibitory activity of I. paraguariensis extracts on GTF. Thus the aim of the present study was to quantify and compare the in vitro inhibition of GTF by green mate (GM) and RM water extracts, to determine the kinetic parameters of the inhibition reaction and to evaluate the effects of several pure compounds identified in mate tea on the inhibition of the enzyme. Furthermore, the effect of both extracts on the growth of S. mutans was compared. The profile of the main bioactive compounds present in both the GM and RM water extracts was determined by electrospray ionization mass spectrometry (ESI-MS), and these compounds were quantified in both extracts by ultraperformance liquid chromatography coupled to mass spectrometry (UPLC-MS).
Materials and methods
Materials
Brain heart infusion (BHI) broth, mitis salivarius (MSA) and phenol red broth were purchased from Difco Laboratories (Detroit, MI). The Folin-Ciocalteu reagent, glucose, dimethyl sulfoxide (DMSO), phenol, catechin, caffeine, rutin and caffeic, chlorogenic, 5-O-caffeoylshikimic and quinic acids were obtained from Sigma (St. Louis. MO). S. mutans ATCC 25175 was purchased from the American Type Culture Collection (ATCC; Rockville, MD). Purified water was obtained from a Milli-Q purifier (Millipore, MA) and HPLC grade methanol and acetonitrile were purchased from Tedia (Fairfield, OH). All other chemicals were of analytical grade.
Plant material and extraction
The extracts were prepared from commercially available dried GM leaves and RM leaves of I. paraguariensis purchased from Leão Jr. S/A (Curitiba, Brazil). The minced leaves were soaked in boiling deionized water (5% w/v) for 15 min. The water infusion was cooled to room temperature, filtered under vacuum, centrifuged (12,000 g for 10 min) and freeze-dried. The freeze-dried water extract was kept at 20°C until analyzed. The extraction yields of solids were 17.4 and 19.7 g per 100 g of dried GM and RM leaves, respectively.
Preparation of extracellular GTFs
S. mutans was cultivated in a shaker (100 rpm) for 18 h at 37°C in 1.2 L of BHI broth (Difco, Detroit, MI). After incubation under anaerobic conditions, the bacteria were removed by refrigerated centrifugation at 8000 g for 30 min at 4°C. The pH of the culture supernatant was adjusted to 6.8 by the addition of 2 M NaOH. The supernatant fluid was treated with ammonium sulfate at 50% saturation and then centrifuged. The precipitate was dialyzed against 10 mM sodium phosphate buffer, pH 6.8, containing 1 mM phenylmethylsulfonyl fluoride (PMSF) as a protease inhibitor. The extract was then freeze-dried for 12 h and named crude GTF, which was used in the subsequent studies. The total protein content of the crude GTF was estimated according to the method of Lowry et al.,Citation24 using bovine serum albumin (Sigma) as the standard.
Determination of GTF activity and inhibition of water-insoluble glucan synthesis
The determination of the GTF activity was based on the amount of water-insoluble glucans formed, expressed as the glucose content. Quantification of the glucan was carried out according to the method described by Ooshima et al.Citation25 with some modifications. The reaction mixtures contained 400 µL of sterile sucrose (150 mM) as the substrate and 50 µL of crude GTF (1 mg/mL) buffered in PBS, pH 6.0. Aliquots of 50 µL of the final concentrations of each extract (5–35 mg/mL) were tested to see if they inhibited the synthesis of water-insoluble glucans. The mixture was then incubated for 1 h at 37°C, and the reaction terminated by placing the tube in a boiling water bath for 5 min. The mixture was then centrifuged at 2000 g for 10 min and the supernatant discarded. The precipitate was washed three times with 5 mL of distilled water, suspended by gentle agitation in 1 mL of distilled water and used for the determination of glucose by the phenol–sulfuric acid methodCitation26 with glucose as the standard. The enzyme activity was calculated and expressed as μmol glucose in the glucan produced per minute (U), and the specific activity was expressed as U/mg protein. The results showed that the GTF activity of the crude enzyme preparation was 1.33 U/mg protein.
The effect of exposure time of each extract on GTF activity was also examined. The GTF was incubated with 10 mg/mL of GM and RM extracts for 10, 20 and 30 min, and for 1, 5, 10 and 15 h at 37°C, prior to the addition of sucrose. Inhibition of the synthesis of water-insoluble glucans was then determined as described above. Three replicates were made for each concentration of the test extracts. The test for inhibition was also carried out using standard solutions of polyphenols obtained commercially (concentrations ranging from 125 to 500 µM). The compounds were dissolved in DMSO–ethanol (1:4 v/v) or ethanol (99.9%; high-performance liquid chromatographic grade).
Measurement of the kinetic constants
In order to determine the Michaelis–Menten constant, Km, the inhibition constant, Ki and Vmax, a series of substrate concentrations (5–200 mM) were tested in the assay system. Each analysis was performed with and without the extract as inhibitor. Lineweaver–Burk plots were fitted to determine the mechanism of the effect of the extracts on the GTF activity using the GraphPad Prism enzyme kinetic program (GraphPad Software, Inc., San Diego, CA). The inhibition constant, Ki, was calculated from the following equation:
where Km,app and Km represent the Km with or without the extracts and [I] represents the concentration of the test extracts.
Effect on the growth of S. mutans
The anti-streptococcal activities of the GM and RM extracts were examined by determining the minimal inhibitory concentration (MIC) values using the macrodilution in broth technique. In brief, the extracts, dissolved in 1% DMSO, were added to the first tube containing 1 mL BHI broth and serially diluted with broth to give concentrations of 1–20 mg/mL of the GM and RM extracts. A S. mutans suspension (0.1 mL) containing 106 colony-forming units (CFU)/mL was added to each tube and incubated for 24 and 48 h at 37°C. After incubation under anaerobic conditions, the growth of S. mutans was estimated spectrophotometrically at 630 nm. The MIC was defined as the minimum concentration of the test compound limiting turbidity to <0.05 absorbance units. The MIC was recorded as the lowest concentration that completely inhibited growth. The experiments were performed in triplicate.
Determination of total phenolic and flavonoid contents
The total phenolic content was determined using the Folin-Ciocalteu reagent as described by Tsai et al.Citation9 and the results were expressed as chlorogenic acid equivalents (CAE). The total flavonoid contents of the extracts were determined using a colorimetric assay. In brief, 0.25 mL of each extract was added to a tube containing 1 mL of distilled water and 0.75 mL of 5% NaNO2, 0.075 mL of 10% AlCl3 and 0.5 mL of 1 M NaOH sequentially added. The volume of the reaction solution was then adjusted to 2.5 mL with distilled water and the absorbance was read at 510 nm. The flavonoid content of each extract was calculated using a standard curve prepared with catechin and expressed as milligrams of catechin equivalents (CE) per gram of solid extract.
ESI-MS fingerprinting
The extracts were directly infused into the source by means of a syringe pump (Harvard apparatus) at a flow rate of 10 μL/min. ESI-MS fingerprints of the extracts were acquired in the negative ion mode using a hybrid high-resolution and high-accuracy (5 ppm) Micromass-Waters Q-TOF mass spectrometer (Manchester, UK). The capillary and cone voltages were set at −3000 and −40 V, respectively, with a desolvation temperature of 100°C. The extracts were diluted in a solution containing 70% (v/v) chromatographic grade methanol, 30% (v/v) purified water and 0.5% of ammonium hydroxide.
UPLC-MS quantification
The chromatographic separation was achieved using an Acquity UPLC system (Waters, Milford, MA) equipped with a Waters UPLC BEH column (2.1 × 50 mm, 1.7 µm particle size) at a temperature of 25°C, injecting 5 μL of each extract. A gradient was applied using 2 mobile phases—(A) purified water with 1% formic acid and (B) acetonitrile with 1% formic acid—starting with 2% B, ramping to 35% B in 6 min, and then to 100% B from 6.10 to 6.50 min, and finally returning to the initial conditions. Detection was carried out in both the positive and negative ion modes using an Acquity TQD mass spectrometer with an ESI source (Micromass Waters, Milford, MA) under the following conditions: capillary ± 3000 V, cone ± 30 V, temperature 150°C, ranging between 110 and 700 m/z. The compounds were quantified by an external standard method based on calibration curves prepared with chlorogenic and quinic acids.
Statistical analysis
The data were expressed as the mean ± SEM, and the groups of data were compared using a one-way ANOVA followed by the Dunnett’s multiple comparisons test. Statistical significance for the expression analysis was also assessed by ANOVA, and the differences identified were pinpointed by an unpaired Student’s t-test. An associated probability (P-value) of <5% was considered significant.
Results
shows the inhibitory activity of different concentrations (4–20 mg/mL) of GM and RM extracts against the GTF from S. mutans. Both extracts inhibited the synthesis of glucan by crude GTF in a dose-dependent manner. It is noteworthy that the RM extract was a much more effective inhibitor of the GTF activity than the GM extract. At a concentration of 4 mg/mL, the percent inhibition by the RM extract was 32.8 ± 2.7% whereas by GM it was 6.4 ± 1.5% (P < 0.05). Under the assay conditions used, the values for IC50 (the concentration required to inhibit enzymatic activity by 50%) of the RM and GM extracts were 10 and 18 mg/mL, respectively. The inhibitory activity of the RM extract reached a maximum at 16 mg/mL, corresponding to 35 mg of extract per gram of substrate (77.8 ± 3.6%). A comparison (t-test) between the inhibitory effects of the RM and GM extracts at all the concentrations tested showed that their effects were significantly different (P < 0.05). The inhibitory effect of the GM extract at 4, 6, 8 and 10 mg/mL was not significantly different from that of the blank control (ANOVA).
Figure 1. Effect of roast and green mate extracts on the activity of the glucosyltransferase (GTF) of S. mutans. The GTF activity was calculated by considering the control as having maximum GTF activity (100%). The results are expressed as the mean ± standard deviation (SD) of three determinations. *Significant difference at the level of P < 0.05 as compared with the control.
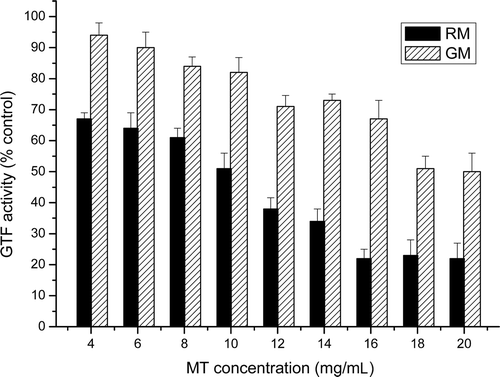
shows the reduction in GTF activity as a function of the time of incubation of the enzyme with the inhibitory extracts. An incubation time of 30 min with 10 mg/L of the RM extract was sufficient to cause a significant inhibition of the GTF activity (47.6 ± 2.7%). However, an incubation time of >5 h was required to cause the maximum inhibition of glucan synthesis with 10 mg/L of GM extract (34.8 ± 3.1%).
Figure 2. Effect of the time of incubation of the crude glucosyltransferase (GTF) extract with the roast (•) and green (○) mate extracts (10 mg/mL) on the activity of the GTF of S. mutans. The results are expressed as the mean ± standard deviation (SD) of three determinations.
GTF activity in the absence and presence of the extracts (8 and 16 mg/mL) at various sucrose concentrations (5–200 mM) was also examined. The Lineweaver–Burk analysis was carried out to characterize the mechanism of the inhibitory effect of the extracts on the GTF activity. summarizes the results obtained for the kinetic parameters. The value for Km without any extract was 20.5 mM. The addition of 8.0 and 16.0 mg/mL of RM extracts significantly changed the apparent Km values to 31.3 and 43.03 mM (P < 0.05), respectively, whereas the Vmax remained constant (25.8 and 23 μmol glucose per minute). The Ki values were 15.1 and 14.5 mM for 8 and 16 mg/mL of the RM extracts, respectively. The same kinetic profile was observed for the GM extract, but with lower Km values (25.7 and 29.8 mM at 8 and 16 mg/mL, respectively) and higher Ki values (34.5 and 35.1 mM, at 8 and 16 mg/mL, respectively), demonstrating that this extract was less efficient in inhibiting GTF activity than the RM extract. Of the two extracts tested, only the RM extracts exhibited an inhibitory effect against the growth of S. mutans, with a MIC value of 16 mg/mL.
Table 1. Kinetic parameters of the inhibitory effect of green and roast mate extracts on the activity of glucosyltransferase (GTF) and growth of S. mutans.
The value for the y-intercept in the equation (1/V = Vmax) for each curve remained at a fixed point with variation in the RM extract concentration, indicating that the inhibition of glucan synthesis by these extracts was of a competitive nature ( and Supplementary material).
Figure 3. Lineweaver–Burk double reciprocal plot of the glucosyltransferase (GTF) activity. V is the initial velocity and [S] is the concentration of sucrose. The values are shown for the absence (▪), 8 mg/mL (•) and 16 mg/mL (▴) of roast mate extract. The values are means of triplicate determinations and the error bars indicate SD (n = 3).
![Figure 3. Lineweaver–Burk double reciprocal plot of the glucosyltransferase (GTF) activity. V is the initial velocity and [S] is the concentration of sucrose. The values are shown for the absence (▪), 8 mg/mL (•) and 16 mg/mL (▴) of roast mate extract. The values are means of triplicate determinations and the error bars indicate SD (n = 3).](/cms/asset/b8b5b7ac-7ead-4af3-b9cd-400c90bae05b/ienz_a_585986_f0003_b.gif)
The phenolic compounds in the GM and RM water extracts were characterized by negative ion mode ESI-MS fingerprints ( and Supplementary material) and quantified by UPLC-MS (). The following compounds were identified in the extracts as their deprotonated molecules [M - H]¯ of m/z 179 (caffeic acid), m/z 191 (quinic acid), m/z 335 (caffeoylshikimic acid) m/z 341 (caffeoyl glucose), m/z 353 (caffeoylquinic acid), m/z 367 (feruloylquinic acid), m/z 497 (dicaffeoylshikimic acid), m/z 515 (dicaffeoylquinic acid) and m/z 609 (rutin). The similarity between the sets of ions in the ESI-MS fingerprints of GM and RM indicated that both extracts were qualitatively similar, but the substantial differences in the abundances indicated important quantitative differences, and therefore the compounds were quantified. Since all isomers of caffeoylquinic acid, feruloylquinic acid, dicaffeoylshikimic acid and dicaffeoylquinic acid are common components of green yerba mate and have antioxidant activity,Citation27 they were quantified as a group.
Table 2. Phenolic compounds identified and quantified by UPLC-MS in green and roast mate.a
Figure 4. ESI-MS fingerprints of water extracts of: (A) green mate and (B) roasted mate. For the compounds identified, see .
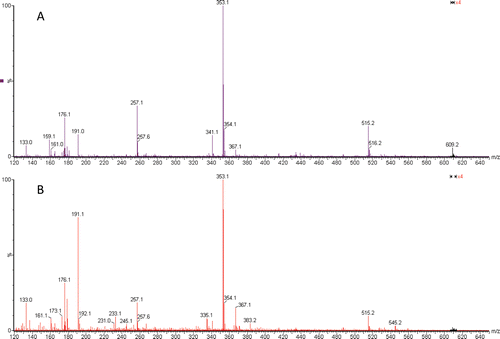
The following phenolic compounds decreased in concentration during the roasting process: caffeoyl glucose, caffeoylquinic acid, feruloylquinic acid, dicaffeoylquinic acid and rutin; whereas caffeic acid, quinic acid, caffeoylshikimic acid and dicaffeoylshikimic acid increased in concentration, as indicated by the ESI-MS of . The reduction in concentration of the phenolic compounds was detected by both analytical methods employed ( and ), but the profiles changed to a great extent, increasing the relative concentrations of some specific molecules. The presence of caffeine and theobromine were determined by UPLC-MS in the positive ion mode, and the areas of their peaks in the two extracts were only slightly different.
Table 3. Total phenolic and flavonoid contents of the aqueous extracts of green and roasted mate.
shows that the total phenolic and flavonoid contents of the water extracts of GM were significantly higher than those of the roasted extracts.
The effects of several selected polyphenols on GTF activity are shown in . Caffeic acid, caffeine and quinic acid showed negligible effects on GTF activity (<10% inhibition). Chlorogenic acid and 5-O-caffeoylshikimic acid showed moderate inhibitory effects (35–40% inhibition) at a concentration of 500 µM. Rutin (quercetin 3-O-rutinoside) displayed the most potent inhibition of GTF activity (50% to 70% inhibition) with an IC50 of 154 µM.
Table 4. Effects of several selected polyphenols on the glucosyltransferase (GTF) activity of S. mutans.
Discussion
The important role of tea polyphenols against S. mutans was confirmed by studies on oolong tea polyphenols, which reported their effects on adherence and GTF activity.Citation7,Citation28 Many substances found in green tea (Camellia sinensis) exhibit antibacterial activity against S. mutans, including several polar polyphenolic compoundsCitation29 and non-polar volatile flavour compounds.Citation30 Some polyphenolic compounds identified in green tea, such as (−)-epicatechin and (−)-epigallocatechin, showed no antibacterial activity against S. mutans up to 500 µg/mL,Citation31 but chlorogenic and caffeic acids have been reported to inhibit the growth of enterobacteria and S. mutans.Citation32
There are few reports available on the effect of mate extracts (I. paraguariensis) on the growth of S. mutans. It was recently observed that the methanolic extract of GM possessed antimicrobial activity against S. sanguinis (MIC 4 mg/mL) but no inhibitory effect on the growth of S. mutans.Citation33 According to the present study, the RM water extract inhibited the growth of S. mutans with a MIC value of 16 mg/mL, which was not observed for the GM extract. Similar results were reported for water extracts of rosemary (Rosmarinus officinalis L.) against S. sobrinus (MIC of 16 mg/mL).Citation9
In the present study, it was demonstrated that the RM extract, as compared with the GM extract, exhibited a greater inhibitory effect on GTF activity, despite having lower phenolic and flavonoid contents. The kinetic results indicated that the RM extract competitively inhibited GTF activity in a concentration-dependent manner, with an IC50 value of 10 mg/mL, whereas a drastic decrease in GTF activity (~80% that of the control) was observed in the presence of 16 mg/mL. The estimated concentration of ‘‘a cup” of mate tea (5 g of leaves/200 mL of boiling water) was ~4.92 mg of water-soluble extract per ml of infusion, which is practically half the IC50 value of the GTase-inhibitory dose.
The kinetic analysis showed significant differences in the values for Km (43.0 ± 1.3 vs. 29.8 ± 0.5 mM, P < 0.05) and Ki (14.5 vs. 35.1 mM P < 0.05) between the RM and GM extracts at 16 mg/mL, as determined by the Lineweaver–Burk plot. These results showed that the GTF was strongly inhibited by the RM extract, since the Ki value was less than half that obtained with the GM extract. The magnitude of Ki indicates the level of dissociation of each inhibitor in the presence of enzyme and substrate. The smaller the value for Ki the less dissociation occurs, indicating it to be a better inhibitor.
Km values for other GTF from S. mutans have been reported. S. mutans Ingbritt (serotype c) was found to secrete basic GTF with Km values of 4.3 mM for sucrose,Citation34 and the GTF produced by the mutant of S. mutans 6715 had a Km value of 2.4 mM.Citation35 It is difficult to establish comparisons between the results reported by the above authors, since they were determined with different substrates as well as under different conditions of temperature and pH.
Phenolic acids and rutin were the main phenolic compounds observed in the water mate extracts. Ten different phenolic constituents, including the three natural isomers of 5-CQA (neo-chlorogenic acid, chlorogenic acid and crypto-chlorogenic acid), as well as three isomeric dicaffeoylquinic acids, rutin (quercitin-3 rutinoside) and two isomeric caffeoyl glucosides had been previously identified in a GM water infusion.Citation27 Roasting clearly changes the bioactive compound content of the water extract and increases the overall efficiency. The majority of the components in GM and RM leaves were extractable with hot water, and the extracts obtained presented different profiles. The compounds that increased in concentration during the roasting process could have been formed by the thermal decomposition of some caffeoylquinic acids (caffeic and quinic acid) or by dehydration (caffeoylshikimic acid and dicaffeoylshikimic acid) as shown in . These compounds contain one less water molecule than the analogous caffeoyl- and dicaffeoylquinic acids, and they have previously been found in RM extracts.Citation12
The results for the inhibitory capacity of the isolated compounds () showed that at least three compounds appeared to contribute to this effect: rutin, 5-O-caffeoylshikimic acid and chlorogenic acid. Despite having a smaller total phenolic compound content, especially with respect to the chlorogenic and dicaffeoylquinic acids and total flavonoids (rutin), the RM showed greater GTF inhibitory activity than the GM extract. The results suggested that new compounds formed during roasting, possibly caffeoylshikimic acid and others formed by the Maillard reaction, acted in a synergistic manner and hence contributed to the inhibition of the GTF activity from S. mutans. Further research is required to elucidate the mechanistic details of GTF inhibition by these compounds, as also the characterization of the as yet unidentified compounds formed during the processing of mate.
Supplementary Material
Download PDF (549.4 KB)Acknowledgements
The authors would like to thank Dr. Paulo Mazzafera for the use of the UPLC-MS (BIOEN-FAPESP grant no. 2008/58035-6). This work was partially supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 2009/10548-8). A.C.H.F.S. would like to thank CAPES for the post-doctoral fellowship.
Declaration of interest
The authors declare no conflicts of interest.
References
- Tanzer JM, Freedman ML, Fitzgerald RJ. Virulence of mutants defective in glucosyltransferase, dextran-mediated aggregation, or dextranase activity. In: Mergenhagen SE, Rosan B, editors. Molecular Basis of Oral Microbial Adhesion. American Society for Microbiology, Washington, DC, 1985, pp. 204–211.
- Yamashita Y, Bowen WH, Burne RA, Kuramitsu HK. Role of the Streptococcus mutans gtf genes in caries induction in the specific-pathogen-free rat model. Infect Immun 1993;61:3811–3817.
- Kawabata S, Hamada S. Studying biofilm formation of mutans streptococci. Meth Enzymol 1999;310:513–523.
- Hanada N, Kuramitsu HK. Isolation and characterization of the Streptococcus mutans gtfC gene, coding for synthesis of both soluble and insoluble glucans. Infect Immun 1988;56:1999–2005.
- Ando T, Tsumori H, Shimamura A, Sato Y, Mukasa H. Classification of oral streptococci by two-dimensional gel electrophoresis with direct activity stain for glycosyltransferases. Oral Microbiol Immunol 2003;18:171–175.
- Konishi N, Torii Y, Yamamoto T, Miyagi A, Ohta H, Fukui K et al. Structure and enzymatic properties of genetically truncated forms of the water-insoluble glucan-synthesizing glucosyltransferase from Streptococcus sobrinus. J Biochem 1999;126:287–295.
- Matsumoto M, Minami T, Sasaki H, Sobue S, Hamada S, Ooshima T. Inhibitory effects of oolong tea extract on caries-inducing properties of mutans streptococci. Caries Res 1999;33:441–445.
- Limsong J, Benjavongkulchai E, Kuvatanasuchati J. Inhibitory effect of some herbal extracts on adherence of Streptococcus mutans. J Ethnopharmacol 2004;92:281–289.
- Tsai PJ, Tsai TH, Ho SC. In vitro inhibitory effects of rosemary extracts on growth and glucosyltransferase activity of Streptococcus sobrinus. Food Chem 2007;105:311–316.
- Gosmann G, Guillaume D, Taketa AT, Schenkel EP. Triterpenoid saponins from Ilex paraguariensis. J Nat Prod 1995;58:438–441.
- Bastos DHM, Oliveira DM, Matsumoto RLT, Carvalho PO, Ribeiro ML. Yerba mate: pharmacological properties, research and biotechnology. Med Aromat Plant Sci Biotechnol 2007;1:37–46.
- Bastos DH, Saldanha LA, Catharino RR, Sawaya AC, Cunha IB, Carvalho PO et al. Phenolic antioxidants identified by ESI-MS from Yerba maté (Ilex paraguariensis) and green tea (Camelia sinensis) extracts. Molecules 2007;12:423–432.
- Gugliucci A. Antioxidant effects of Ilex paraguariensis: induction of decreased oxidability of human LDL in vivo. Biochem Biophys Res Commun 1996;224:338–344.
- Gugliucci A, Menini T. The botanical extracts of Achyrocline satureoides and Ilex paraguariensis prevent methylglyoxal-induced inhibition of plasminogen and antithrombin III. Life Sci 2002;72:279–292.
- Martins F, Suzan AJ, Cerutti SM, Arçari DP, Ribeiro ML, Bastos DH et al. Consumption of mate tea (Ilex paraguariensis) decreases the oxidation of unsaturated fatty acids in mouse liver. Br J Nutr 2009;19:1–6.
- Miranda DD, Arçari DP, Pedrazzoli J Jr, Carvalho PO, Cerutti SM, Bastos DH et al. Protective effects of mate tea (Ilex paraguariensis) on H2O2-induced DNA damage and DNA repair in mice. Mutagenesis 2008;24:375–381.
- Martins F, Noso TM, Porto VB, Curiel A, Gambero A, Bastos DH et al. Maté tea inhibits in vitro pancreatic lipase activity and has hypolipidemic effect on high-fat diet-induced obese mice. Obesity (Silver Spring) 2010;18:42–47.
- Ramirez-Mares MV, Chandra S, de Mejia EG. In vitro chemopreventive activity of Camellia sinensis, Ilex paraguariensis and Ardisia compressa tea extracts and selected polyphenols. Mutat Res 2004;554:53–65.
- Filip R, Sebastian T, Ferraro G, Anesini C. Effect of Ilex extracts and isolated compounds on peroxidase secretion of rat submandibulary glands. Food Chem Toxicol 2007;45:649–655.
- Gorzalczany S, Filip R, Alonso MR, Miño J, Ferraro GE, Acevedo C. Choleretic effect and intestinal propulsion of ‘mate’ (Ilex paraguariensis) and its substitutes or adulterants. J Ethnopharmacol 2001;75:291–294.
- Baisch ALM, Johnston FL, Stein P. Endothelium-dependent vasorelaxing activity of water extracts of Ilex paraguariensis on mesenteric arterial bed of rats. J Ethnopharmacol 1998;60:133–139.
- Lunceford N, Gugliucci A. Ilex paraguariensis extracts inhibit AGE formation more efficiently than green tea. Fitoterapia 2005;76:419–427.
- de Morais EC, Stefanuto A, Klein GA, Boaventura BC, de Andrade F, Wazlawik E et al. Consumption of yerba mate (Ilex paraguariensis) improves serum lipid parameters in healthy dyslipidemic subjects and provides an additional LDL-cholesterol reduction in individuals on statin therapy. J Agric Food Chem 2009;57:8316–8324.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265–275.
- Ooshima T, Osaka Y, Sasaki H, Osawa K, Yasuda H, Matsumura M et al. Caries inhibitory activity of cacao bean husk extract in in-vitro and animal experiments. Arch Oral Biol 2000;45:639–645.
- Dubois M. Colorimetric method for determinations of sugars and related compounds. Anal Chem 1956;28:350–356.
- Carini M, Facino RM, Aldini G, Calloni M, Colombo L. Characterization of phenolic antioxidants from mate (Ilex paraguariensis) by liquid chromatography/mass spectrometry and liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 1998;12:1813–1819.
- Ooshima T, Minami T, Aono W, Izumitani A, Sobue S, Fujiwara T et al. Oolong tea polyphenols inhibit experimental dental caries in SPF rats infected with mutans streptococci. Caries Res 1993;27:124–129.
- Sakanaka S, Kim M, Taniguchi M, Yamamoto T. Antibacterial substances in Japanese tea extract against Streptococcus mutans, a cariogenic bacterium. Agric Biol Chem 1989;53:2307–2311.
- Kubo I, Muroi H, Himejima M. Antimicrobial activity of green tea flavor components and their combination effects. J Agric Food Chem 1992;40:245–248.
- Muroi H, Kubo I. Combination effects of antibacterial compounds in green tea flavor against Streptococcus mutans. J Agric Food Chem 1993;41:1102–1105.
- Almeida AA, Farah A, Silva DA, Nunan EA, Glória MB. Antibacterial activity of coffee extracts and selected coffee chemical compounds against enterobacteria. J Agric Food Chem 2006;54:8738–8743.
- Tsai TH, Chien YC, Lee CW, Tsai PJ. In vitro antimicrobial activities against cariogenic streptococci and their antioxidant capacities: a comparative study of green tea versus different herbs. Food Chem 2008;110:859–864.
- Mukasa H, Shimamura A, Tsumori H. Purification and characterization of basic glucosyltransferase from Streptococcus mutans serotype c. Biochim Biophys Acta 1982;719:81–89.
- Shimamura A, Tsumori H, Mukasa H. Purification and properties of Streptococcus mutans extracellular glucosyltransferase. Biochim Biophys Acta 1982;702:72–80.