Abstract
In continuation of structure activity relationship studies, a panel of fluorine containing sydnones with styryl ketone group 4-[1-oxo-3-(substituted aryl)-2-propenyl]-3-(3-chloro-4-fluorophenyl)sydnones 2a–i, was synthesized as better analgesic and anti-inflammatory agents. The title compounds were formed by condensing 4-acetyl-3-(3-chloro-4-fluorophenyl)sydnone with various substituted aryl aldehydes, characterized by spectral studies and evaluated at 100 mg\kg b.w., p.o. for analgesic, anti-inflammatory and ulcerogenic activities. Compounds 2c and 2e showed good analgesic effect in acetic acid-induced writhing while none showed significant activity in hot plate assay in mice. In carrageenan-induced rat paw oedema test, compound 2c and 2f exhibited good anti-inflammatory effect at 3rd h, whereas compounds 2c, 2e, 2d, 2g and 2h showed activity in croton oil induced ear oedema assay in mice. Compounds 2c and 2e were less ulcerogenic than ibuprofen in rats, when tested by ulcer index method. Compounds with electron attracting substituents such as 2c and 2e were found to be promising in terms of the ratio of efficacy and adverse effect. These compounds generally exhibited better activity than those of earlier series signifying fluorine substitution.
Introduction
Structure activity relationship (SAR) is one of the tools in drug design and development with which a medicinal chemist can explore the biological activity better by effecting structural variation in a panel of compounds. We undertook SAR studies of 4-[1-oxo-3-(substituted aryl)-2-propenyl]-3-(3-different substituted phenyl)sydnones as analgesic and anti-inflammatory agents on the basis that molecules containing both the structural features of sydnone and styryl ketone would be better acting, because these compounds have been reported separately in literature to exhibit analgesic and anti-inflammatory activitiesCitation1–6. As a part of this, we already reported 3-(4-chlorophenyl) and 3-(4-methylphenyl) substituted compounds of the said series for analgesic and anti-inflammatory activitiesCitation7,Citation8. In continuation with it, we synthesized 3-(3-chloro-4-fluorophenyl) substituted compounds of the above series i.e., 4-[1-oxo-3-(substituted aryl)-2-propenyl]-3-(3-chloro-4-fluorophenyl)sydnones 2a–i, and screened them for analgesic and anti-inflammatory activities. The findings of the study are presented in this paper.
Sydnones containing fluorine were planned to synthesize in an anticipation of good pharmacological profile because fluorine, when present, can alter the biological properties of a molecule. It affects lipophilicity thereby enhancing absorption, transport, recognition and interaction of the molecule with the biological target in vivo, thus increasing intrinsic activity, chemical and metabolic stabilityCitation9. In testimony of this, over 150 fluorinated drugs are there in the market today making up to ~20% of all pharmaceuticals. Some of the fluorinated molecules which made their way to clinic include the antidepressant fluoxetine, the cholesterol-lowering atorvastatin, the anticancer agent 5-fluorouracil, the general anaesthetic halothane, the anti-inflammatory drug flumethasone and the antibacterial ciprofloxacinCitation10,Citation11. Therefore, recently there is an increased interest in the synthesis of fluorinated compounds as pharmaceuticals.
Methods
The analytical reagent grade chemicals were used without further purification. Carrageenan was obtained from Sigma (St. Louis, MO), croton oil from Paras perfumers (Delhi, India) and pentazocine from Pharma Impex Laboratories (P) Ltd., (Kolkata, India). Embiotic Laboratories (P) Ltd., (Bengaluru, India) gifted acetyl salicylic acid (ASA), ibuprofen and indomethacin. The completion of reaction and purity of products were monitored by thin layer chromatography using silica gel 60 F254 pre-coated aluminium sheets (Merck, Darmstadt, Germany) and benzene-ethyl acetate 8.5:1.5 by volume as mobile phase. The spots were visualized by iodine vapour.
Instrumentation
Melting points were determined in Dolphin open capillary tube apparatus (Mumbai, India) and are uncorrected. Ultra Violet-Visible (UV-Vis) spectra were taken on Systronics V530 (Ahmedabad, India), Infrared (IR) spectra on ThermoNicolet 200 FT-IR (Madison, WI) by potassium bromide pellet technique and mass spectra (MS) on Finnegan-Mat1020 (electron impact, 70 ev) (Bremen, Germany). Proton nuclear magnetic resonance (1H-NMR) spectra in deuterochloroform were recorded on BrukerAC200F (200 MHz) (Rheinstetten, Germany), whereas 13C-NMR spectra were taken at 50 MHz. Chemical shifts were measured on δ scale in parts per million downfield to tetramethylsilane. Peak multiplicities are indicated as s (singlet), d (doublet), m (multiplet) and br (broad); coupling constant (J) values are given in Hz. Eddy’s Hot Plate (Kshitij International, Ambala, India) and digital micrometer (Digitrik mark II, NSK, Japan) were used in biological activities.
Animals
Adult healthy Albino mice (20–25 g b.w.) and Sprague-Dawley rats (100–150 g b.w.) of either sex, bred at the animal house facility of HSK College of Pharmacy, Bagalkote, were used. The animals were maintained on standard pellet diet and free access to water and housed in polypropylene cage (4 per cage) on paddy husk bedding under laboratory conditions at 22 ± 3°C temperature, 60 ± 10% humidity and 12-h light/dark cycle. They were acclimatized for 7 days. The food was withdrawn 18 h before the experiment, but free access to water was allowed. The experiments were performed on randomly formed groups during the light phase of the cycle and the animals were used for once experiment only. The test compounds and standard drugs were administered p.o., as aqueous suspension in 0.5% sodium caboxymethyl cellulose (Sod CMC). The animals in the vehicle control group were administered 0.5% Sod CMC 10 mL/kg b.w. p.o. All efforts were made to minimize animal suffering and to reduce the number of animals used. The guidelines of institutional animal ethics committee constituted as per committee for the purpose of control and supervision of experiments on animals, Ministry of Environment and Forestry, Government of India, were followed.
Statistics
The data of animal experiments were expressed as mean ± standard deviation (SD) and analyzed by Graph-pad Prism (San Diego, CA). Statistical differences between the treatments and the control were tested by analysis of variance, followed by Dunnett’s multiple comparison test. Data with p value <0.05 was considered significant.
Synthesis of 2a–g: 4-[1-oxo-3- (4-N, N-dimethylamino phenyl)-2-propenyl]-3-(3-chloro-4-fluoro phenyl)sydnone 2f
A mixture of 4-acetyl-3-(3-chloro-4-fluorophenyl)sydnone 1 (2.6 g, 0.01 mol), sodium hydroxide aqueous solution (0.6 g, 0.015 mol, 3 mL) and ethanol (95%, 20 mL) was cooled (5–10°C) and to this was added 4-N,N-dimethylaminobenzaldehyde (2 g, 0.012 mol) while being stirred. The reaction mixture was stirred further for 1 h. The precipitate obtained was filtered washed with cold water and re-crystallised from ethanol (95%) and ethyl acetate (1:1) to give the title compound (1.67 g, 0.0043 mol, 43%): mp 109–110°C; IR cm−1 1755 (C=O, sydnone), 1660 (C=O, styryl ketone); 1H-NMR δ 3.08 (s, 6H, N(CH3)2), 6.74–6.77 (m, 3H, ArH and olefinic αH), 7.36–7.49 (m, 2H, ArH), 7.78–7.83 (m, 4H, ArH and olefinic βH); 13C-NMR δ 40.06, 106.03, 111.01, 118.2, 121.48, 124.2, 125.11, 131.97, 133.26, 134.20, 136.50, 154.39, 157.66, 162.77, 168.61, 190.31; MS m/z 387.59 (M+).
Remaining compounds were prepared similarly using respective aryl aldehydes.
4-[1-oxo-3-(phenyl)-2-propenyl]-3-(3-chloro-4-fluorophenyl)sydnone 2a
IR cm−1 1753 (C=O, sydnone), 1662 (C=O, styryl ketone); 1H-NMR δ 6.73 (d, 1H, J = 10.00, olefinic αH), 7.2–7.61 (m, 5H, ArH), 7.64–7.72 (m, 4H, ArH and olefinic βH); 13C-NMR δ 106.31, 117.12, 120.83, 121.45, 125.16, 128.22, 128.63, 131.37, 135.48, 135.68, 145.36, 158.96, 167.35, 187.53; MS m/z 344.63 (M+).
4-[1-oxo-3-(2-furyl)-2-propenyl]-3-(3-chloro-4-fluorophenyl)sydnone 2b
IR cm−1 1765 (C=O, sydnone), 1676 (C=O, styryl ketone); 1H-NMR δ 6.92 (d, 1H, J = 11.76, olefinic αH), 7.03 (t, 1H, J = 11.76, furyl-4H), 7.26–7.42 (m, 2H, ArH), 7.73–7.96 (m, 3H, ArH, furyl-3H, and olefinic βH), 8.25 (d, 1H, J = 7.05, furyl-5H); 13C-NMR δ 105.18, 112.68, 113.93, 117.26, 120.88, 121.25, 125.37, 127.48, 131.24, 135.68, 143.78, 152.26, 159.15, 166.83, 187.28; MS m/z 334.46 (M+).
4-[1-oxo-3-(4-cholrophenyl)-2-propenyl]-3-(3-chloro-4-fluorophenyl)sydnone 2c
IR cm−1 1763 (C=O, sydnone), 1671(C=O, styryl ketone); 1H-NMR δ 6.81 (d, 1H, J = 10.52, olefinic αH), 7.28–7.43 (m, 2H, ArH), 7.6 (d, 2H, J = 5.26, ArH), 7.73–7.82 (m, 4H, ArH and olefinic βH); 13C-NMR δ 105.37, 117.32, 120.93, 121.35, 124.98, 128.98, 129.17, 131.48, 133.52, 135.73, 145.68, 159.47, 167.28, 186.79; MS m/z 379.18 (M+).
4-[1-oxo-3-(3,4,5-trimethoxyphenyl)-2-propenyl]-3-(3-chloro-4-fluorophenyl)sydnone 2d
IR cm−1 1752 (C=O, sydnone), 1657 (C=O, styryl ketone); 1H-NMR δ 3.93 (s, 9H, OCH3), 6.82 (d, 1H, J = 10.52, olefinic αH), 6.87 (s, 2H, ArH), 7.21–7.38 (m, 2H, ArH), 7.81 (t, 2H, J = 10.52, ArH and olefinic βH); 13C-NMR δ 55.97, 61.00, 104.16, 105.48, 117.32, 120.86, 121.74, 125.53, 126.84, 132.10, 136.21, 138.63, 153.46, 159.28, 166.58, 187.33; MS m/z 434.53 (M+).
4-[1-oxo-3-(4-nitrophenyl)-2-propenyl]-3-(3-chloro-4-fluorophenyl)sydnone 2e
IR cm−1 1755 (C=O, sydnone), 1667 (C=O, styryl ketone); 1H-NMR δ 7.38 (d, 1H, J = 11.56, olefinic αH), 7.42–7.56 (m, 2H, ArH), 7.76 (m, 1H, ArH), 7.93 (d, 1H, J = 11.56, olefinic βH), 8.69 (d, 2H, J = 5.78, ArH), 8.93 (d, 2H, J = 5.78, ArH); 13C-NMR δ 106.15, 117.28, 120.92, 121.36, 124.18, 125.38, 129.13, 130.96, 135.57, 141.46, 145.37, 147.14, 158.84, 167.35, 187.46; MS m/z 389.49 (M+).
4-[1-oxo-3-(2-nitrophenyl)-2-propenyl]-3-(3-chloro-4-fluorophenyl)sydnone 2 g
IR cm−1 1759 (C=O, sydnone), 1662 (C=O, styryl ketone); 1H-NMR δ 6.98 (d, 1H, J = 11.56, olefinic αH), 7.32–7.48 (m, 2H, ArH), 7.78–8.21 (m, 4H, ArH), 8.30–8.33 (m, 2H, ArH and olefinic βH); 13C-NMR δ 105.82, 117.30, 121.18, 121.63, 123.97, 125.46, 127.48, 129.00, 131.35, 134.68, 135.74, 145.56, 147.98, 159.12, 165.94, 188.20; MS m/z 389.52 (M+).
Synthesis of 2h and 2i: 4-[1-oxo-3-(4-hydroxy-3-methoxy phenyl)-2- propenyl]-3-(3-chloro-4-fluorophenyl)sydnone 2 h
To the suspension of compound 1 (2.6 g, 0.01 mol) and vanillin (1.8 g, 0.012 mol) in 20 mL ethanol (95%) was passed dry hydrogen chloride gas for 0.5 h under cooling (5°C). The reaction mixture was left overnight at room temperature and poured into cold water. The separated precipitate was filtered, washed, dried in air and re-crystallised from ethanol (95%) to give the title compound (2.18 g, 0.0056 mol, 56%): mp 158–160°C; IR cm−1 1758 (C=O, sydnone), 1660 (C=O, styryl ketone); 1H-NMR δ 3.97 (s, 3H, OCH3), 5.98 (s, br, 1H, OH), 6.79–6.88 (m, 2H, ArH and olefinic αH), 7.32–7.68 (m, 4H, Ar-H), 7.76–7.78 (m, 2H, ArH and olefinic βH); 13C-NMR δ 55.93, 105.73, 112.08, 117.32, 120.96, 121.48, 123.31, 125.83, 128.36, 131.89, 136.15, 146.10, 148.63, 149.76, 159.38, 166.48, 188.56; MS m/z 390.50 (M+).
Compound 2i was prepared similarly using 2-hydroxyquinolin-3-carboxaldehyde.
4-[1-oxo-3-(2-hydroxy-3-quinolinyl)-2-propenyl]-3-(3-chloro-4-fluorophenyl)sydnone 2i
IR cm−1 1757 (C=O, sydnone), 1672 (C=O, styryl ketone); 1H-NMR δ 6.76 (d, 1H, J = 12.30, olefinic αH) 7.27–7.39 (m, 2H, ArH), 7.57–7.8 (m, 2H, ArH and olefinic βH), 7.81–8.14 (m, 4H ArH), 8.42 (s, 1H, ArH), 11.47 (s, br, 1H, OH); 13C-NMR δ 106.18, 117.61, 121.13, 122.24, 124.30, 125.26, 125.60, 127.31, 127.86, 128.63, 130.75, 131.89, 136.12, 136.65, 145.74, 146.58, 159.38, 166.37, 174.92, 187.64; MS m/z 411.48 (M+).
Acute toxicity
Mice and rats were divided into groups of four each and the test compounds were administered p.o. to different groups in increasing dose levels of 250, 500, 750 and 1000 mg/kg b.w. They were observed continuously for 3 h, for neurological (ptosis, drowsiness, gait, tremors and convulsions), autonomic (salivation, lacrimation, perspiration, piloerection, urinary incontinence and defecation) and general behavioural profiles and then every 30 min for next 3 h and finally for lethality after 24 h.Citation12
Analgesic activity
It was assessed in mice by chemically as well as thermally induced pain using acetic acid-induced writhingCitation13 and hot plate assayCitation14, respectively.
Acetic acid-induced writhing
A sensitivity test was carried out a day prior to drug administration wherein the mice were injected 0.2–0.25 mL of 0.6% acetic acid i.p. Mice showing writhing within 30 min were chosen for study. Groups of six mice each were dosed with the test compounds or with ASA at a dose of 100 mg/kg b.w. p.o., 1 h before the i.p. injection of 0.6% acetic acid (10 mL/kg b.w.). After 5 min of acetic acid injection, they were observed for 20 min and the total number of writhes was recorded. The percent reduction of the number of writhes was calculated.
Hot plate assay
Groups of six mice each were administered with the test compounds at a dose of 100 mg/kg b.w. p.o. or with pentazocine (5 mg/kg b.w. p.o.) 1 h before the test. The animals were placed on hot plate maintained at 55 ± 1°C and the basal reaction time taken to cause a discomfort (licking of paw or jumping response whichever appeared first) was recorded at zero min. Cut-off period of 15 s was established to prevent damage to the paws. The reaction time in seconds was re-investigated at 30, 60, and 120 min. The activity was expressed as percent protection.
Anti-inflammatory activity
Acute systemic and local anti-inflammatory activities were carried out by carrageenan-induced paw oedema in ratsCitation15 and croton oil induced ear oedema in miceCitation16, respectively.
Carrageenan-induced paw oedema
Groups of six rats were dosed at 100 mg/kg b.w. p.o. with the test compounds or ibuprofen 1 h before 0.05 mL of a 1% suspension of carrageenan in saline was injected into the sub plantar region of the right hind paw. An equal volume of saline was injected into the other hind paw and served as control. Paw volumes were measured by plethismograph immediately after carrageenan injection and again after 1, 2, 3 and 5 h later. Data were reported as percent oedema inhibition.
Topical ear oedema
Groups of six mice received topical application (5 mg/ear) of test compounds or indomethacin dissolved in dimethyl sulfoxide (DMSO) on the anterior surface of the right ear while 0.05 mL croton oil (4 parts of croton oil and 96 parts of DMSO) was instantly applied on the posterior surface of the same ear. Control animals received an equivalent volume of DMSO. The ear thickness was measured by digital micrometer 4 h after challenge of croton oil to assess an increase in oedema. The % inhibition of oedema was calculated using the following relation.
Ulcerogenic assay
Groups of six rats were administered test compounds or ibuprofen at a dose of 100 mg/kg b.w. p.o. After 4 h, the rats were sacrificed, the stomachs removed, opened along the greater curvature and observed for gastric lesions on the mucosa. The lesion index for each group was determined by counting the number of lesions (x) in each of five size classes (y) which were defined as y = 1 (pinpoint lesion), y = 2 (lesions < 1 mm diameter), y = 3 (lesions 1–2 mm diameter), y = 4 (lesions 2–4 mm diameter) and y = 5 (lesions >4 mm diameter).Citation17
Results and discussion
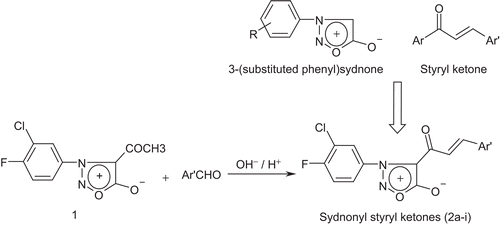
The starting material, 4-acetyl-3-(3-chloro-4-fluorophenyl)sydnone 1, was prepared by the action of glacial acetic acid on 3-(3-chloro-4-fluorophenyl)sydnone in presence of phosphorous pentoxideCitation18; the 3-(3-chloro-4-fluorophenyl)sydnone itself was synthesized by literature protocol from 3-chloro-4-fluoroanilineCitation19. The IR spectrum of compound 1 showed sydnone C=O and acetyl C=O stretching at 1772 and 1671 cm−1, respectively, and in 1H-NMR spectrum, it showed a singlet at δ 2.54 corresponding to three hydrogens of COCH3 and a multiplet at 7.61–7.89 accounting three aromatic protons (data not shown). Claisen-Schmidt reaction of compound 1 with different aryl aldehydes in presence of either alkali or acid afforded the title compounds 2a–i (). The UV-Vis spectra of compounds 2a–i exhibited considerable bathochromic shifts due to π-π* (343–601 nm) in comparison to 321 nm for compound 1 indicating the extension of conjugation due to formation of α,β-unsaturated ketone. In their IR spectra, compounds 2a–i showed sydnone C=O and styryl ketone stretching at 1753–1765 cm−1 and 1660–1676 cm−1, respectively. Compounds 2a–i in their 1H-NMR spectra displayed, apart from other hydrogens, doublets due to α protons of α,β-unsaturated ketone moiety at δ 6.73–6.98, whereas β protons were seen merged with aromatic protons at δ 7.64–8.33 in most instances. The 13C-NMR spectra of compounds 2a–i exhibited α-carbons of α,β-unsaturated ketone at δ 121.36–127.86, β-carbons at δ 146.10–159.47, carbons of styryl ketone at δ 186.79–190.31, the sydnone C=O at δ 165.94–168.61 and sydnone C-4 at δ 105.18–106.31 amidst other carbons. The mass spectra of compounds 2a–i showed the M+ ion peak at their respective m/z values which are consistent with their molecular weight. The physical data of compounds 2a–i are presented in .
Table 1. Physical data and yields of compounds 2a–i.
After 24 h of administration up to 1000 mg/kg b.w., compounds 2a–i produced no mortality in both rats and mice in acute toxicity testing. But few changes in the behavioural response like alertness, touch and restlessness were noticed. There were no significant changes in neurological and autonomic profiles. Hence, 1/10th of the maximum tolerated dose i.e., 100 mg/kg b.w. was chosen for the pharmacological studies.
Compound 2b, 2e and 2c showed good activity with 46, 44 and 40% reduction in writhes, respectively, in writhing test (). The presence of chloro and nitro groups at para position enhanced the activity. The furyl analogue was equiactive to that of phenyl. The presence of trimethoxy group on the phenyl ring sustained activity, whereas N, N-dimethylamino group at para position did not. It is noteworthy that compounds 2a–i were found to be more active than the corresponding 4-methyl analogues reported by us earlierCitation8. It is reported that intraperitoneal administration of an agent that irritates the serous membrane such as acetic acid results in the release of prostaglandins like PGE2 and PGF2α and sympathomimetic system mediators in the peritoneal fluidCitation20. It is speculated that the activity of the test compounds could be due to inhibition of synthesis of prostaglandins and or sympathomimetic system mediators. In hot plate assay, centrally acting analgesics increase the reaction time in laboratory animalsCitation21. Since none of the tested compounds significantly increase the reaction time in animals in hot plate test (), it can therefore be concluded that compounds 2a–i do not possess centrally mediated analgesic effect.
Table 2. Analgesic activity of compounds 2a–i.
The hind paw inflammation oedema produced by carrageenan is a biphasic event and the agents which act on the first stage inhibit the chemical mediators such as histamine, serotonin, and bradykinin, while the second stage of the oedema is related to the arachidonic acid metabolites since it is inhibited by aspirin, indomethacin, and other COX inhibitorsCitation22. In paw oedema test, compounds 2a–i displayed very weak anti-inflammatory activity at 1 and 2 h than the corresponding 4-chloro analogues reported by usCitation7 indicating none of the compounds 2a–i is inhibiting the synthesis or action of chemical mediators. Nitric oxide (NO) is known to inhibit the adhesion of leucocytes to the endothelium, preventing adhesion cascade and reduce inflammation in initial stagesCitation23. The weak and slow release of NO by sydnonesCitation24 may be responsible for the weak activity shown by these compounds in the early stage of inflammation. At 3 h, compounds 2a–i showed significant activity with 2c and 2f showing highest activities with 56 and 55% oedema inhibition followed by 2e, 2h and 2d with 49, 45 and 43% oedema inhibition, respectively (). These compounds showed more activity than the 4-chloro counterpartsCitation7 highlighting the effect of fluorine substitution. The good activity shown by these compounds in the second phase of inflammation may be attributed to more availability of these compounds at the site of action due to increased lipophilicity by fluorine substitution. They may act by inhibiting either COX and or lipoxygenase enzyme thereby preventing the formation of inflammatory prostaglandins and or leukotrienes from the arachidonic acid cascade. At 5th h, only compound 2c and 2f showed significant activity with 20 and 18% oedema inhibition, respectively. The chloro and N,N-dimethylamino substitution at para position seems to favour the activity.
Table 3. Anti-inflammatory activity of compounds 2a–i.
The good systemic anti-inflammatory activity displayed by title compounds and the paucity of data on local anti-inflammatory activity of sydnone derivatives prompted us to screen compounds 2a–i for topical anti-inflammatory activity. It is known that the oedematous inflammation induced by croton oil treated ears is related to the activation of phopholipaseA2, which releases arachidonic acid from the cell membrane that in turn, is metabolized to proinflammatory prostaglandins and leukotrienesCitation25. Reduction in the thickness of the croton oil induced ear oedema by any agent is an index of its topical anti-inflammatory activity. Compound 2c, 2e, 2d, 2g and 2h exhibited significant topical anti-inflammatory activity with 27, 25, 24, 23 and 22% oedema inhibition, whereas standard drug indomethacin showed 69% oedema inhibition (). Here also, the chloro derivative displayed good activity. However, the topical anti-inflammatory activity of these compounds was not pronounced compared to systemic activity. Again, their topical anti-inflammatory activity could be due to inhibition of COX and or lipoxygenase enzymes.
The major drawback of non-steroidal anti-inflammatory drugs is their propensity to cause gastric ulcerationCitation26, and the three active compounds in the series 2c, 2e and 2f were tested for this effect. The compounds with electron attracting substituents such as 2c and 2e showed ulcerogenic index of 11.12 and 14.38, respectively, that was less than that of ibuprofen (). From this study, compounds 2c and 2e have emerged to be the most promising in terms of the ratio of efficacy and adverse effect; 2c being the best. This could be due to selective inhibition of COX-2 by them.
Table 4. Ulcerogenicity of selected compounds.
Conclusion
The 4-[1-oxo-3-(substituted aryl)-2-propenyl]-3-(3-chloro-4-fluoro phenyl)sydnones prepared as a part of our ongoing SAR study showed good analgesic activity in acetic acid-induced writhing but failed to show appreciable activity in hot plate test suggesting their action is through peripheral mechanism. These compounds also exhibited systemic as well as topical anti-inflammatory activities. In general, the title compounds showed more activity than earlier series of compounds prepared by us suggesting the favourable effect of fluorine on activity. The mechanism of the effects exerted by the compounds covered in the study is under progress that probably would direct future course of action.
Acknowledgments
The authors thank Mr. Ramakrishna Anegundi, Research Fellow, Organic Chemical Synthesis Division, National Chemical Laboratory, Pune, and Prof. S.S. Karki for the help in obtaining the spectra. They also express thanks to Embiotic Laboratories (P) Ltd., Bengaluru, for providing drugs and Prof. AHMV Swamy for extending expertise in pharmacological studies.
Declaration of interest
The authors report no conflicts of interest.
References
- Satyanarayana K, Rao MNA. Synthesis and antiinflammatory, analgesic, and antipyretic testing of 4-[1-oxo-(3-substituted aryl)-2-propenyl]-3-phenylsydnones and of 3-[4-[3-(substituted aryl)-1-oxo-2-propenyl]phenyl]sydnones. J Pharm Sci 1995;84:263–266.
- Kalluraya B, Rahiman MA. Sydnone derivatives: Part X- synthesis and pharmacological activity of some novel cyclohexenones and indazolines. Indian J Chem 2003;42B:1141–1148.
- Satyanarayana K, Rao MNA. Synthesis of 3-[4-[2,3-dihydro-2-(substituted aryl)-1,5-benzothiazepin-4-yl]phenyl]sydnones as potential anti-inflammatory agents. Indian J Pharm Sci 1993;55:230–233.
- Kalluraya B, Rahiman MA, Banji D. Sydnone derivatives: Part V-synthesis and pharmacological properties of some novel triazolothiadiazepines. Indian J Chem 2002;41B:1712–1717
- Singh GB, Leach GD, Atal CK. Antiinflammatory actions of methyl- and phenyl-3-methoxy-4-hydroxy styryl ketones. Arzneimittelforschung 1987;37:435–440.
- Rao MNA, Naidoo L, Ramanan PN. Antiinflammatory activity of phenyl styryl ketones. Pharmazie 1991;46:542–543.
- Deshpande SR, Pai KV. Synthesis and biological activities of certain mesoionic sydnone compounds containing chalcone moiety. J Basic Clin Pharm 2010;1:147–152.
- Deshpande SR, Pai KV. Synthesis, antibacterial and analgesic activities of 4-[1-oxo-3-(substituted aryl)-2-propenyl]-3-(4-methylphenyl)sydnones. E-J Chem 2010;7:59–64.
- Chambers RD. Fluorine in organic chemistry. Oxford: Blackwell, 2004.
- Müller K, Faeh C, Diederich F. Fluorine in pharmaceuticals: Looking beyond intuition. Science 2007;317:1881–1886.
- Sandford G. Engineering fluorination. Nature Chemical Biol 2009;5:6–7.
- Dua PR. Testing of natural products for acute toxicity and CNS activity. Lucknow: Central Drug Research Institute 1992:15–17.
- Koster R, Anderson M, de Beer EJ. Acetic acid for analgesic screening. Fed Proc 1959;18:412–414.
- Eddy NB, Leimbach D. Synthetic analgesics. II. Dithienylbutenyl- and dithienylbutylamines. J Pharmacol Exp Ther 1953;107:385–393.
- Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med 1962;111:544–547.
- Tubaro A, Dri P, Delbello G, Zilli C, Della Loggia R. The croton oil ear test revisited. Agents Actions 1986;17:347–349.
- Rainsford KD. Electronmicroscopic observations on the effects of orally administered aspirin and aspirin-bicarbonate mixtures on the development of gastric mucosal damage in the rat. Gut 1975;16:514–527.
- Greco CV, Tobias J, Kier LB. Acylation of 3-phenylsydnone with carboxylic acids and phosphorus pentoxide. J Heterocycl Chem 1964;4:160–162.
- Earl JC, Mackney AW. The action of acetic anhydride on N-nitrosophenylglycine and some of its derivatives. J Chem Soc 1935;899–903.
- Deraedt R, Jouquey S, Delevallée F, Flahaut M. Release of prostaglandins E and F in an algogenic reaction and its inhibition. Eur J Pharmacol 1980;61:17–24.
- García MD, Fernández MA, Alvarez A, Saenz MT. Antinociceptive and anti-inflammatory effect of the aqueous extract from leaves of Pimenta racemosa var. ozua (Mirtaceae). J Ethnopharmacol 2004;91:69–73.
- Vinegar R, Schreiber W, Hugo R. Biphasic development of carrageenin edema in rats. J Pharmacol Exp Ther 1969;166:96–103.
- Panés J, Perry M, Granger DN. Leukocyte-endothelial cell adhesion: Avenues for therapeutic intervention. Br J Pharmacol 1999;126:537–550.
- Satyanarayana K, Deshpande SR, Subbarao B, Rao MNA. Synthesis and nitric oxide donor activity of phenylsydnones. Indian Drugs 2002;39:578–582.
- Bermejo Benito P, Abad Martínez MJ, Silván Sen AM, Sanz Gómez A, Fernández Matellano L, Sánchez Contreras S et al. In vivo and in vitro antiinflammatory activity of saikosaponins. Life Sci 1998;63:1147–1156.
- Roberts LJ II, Morrow JD. Analgesic-antipyretic and anti-inflammatory agents and drugs employed in the treatment of gout. In: Hardman JG, Limbird LE, eds. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. New York: McGraw-Hill Medical Publishing; 2001, pp. 694–695.