Abstract
The inhibitory effect of four structurally related flavonoids, apigenin, baicalein, luteolin and quercetin on the matrix metalloproteinase (MMP)-9 and -13 expressions in osteoblasts was investigated. Treatment with IL-1β induced both MMP-9 and -13 mRNA expressions as measured by quantitative real-time PCR. Luteolin and apigenin decreased IL-1β-induced MMP-9 and -13 mRNA expressions, whereas baicalein and quercetin showed little effects. Related to signalling, treatment with IL-1β induced ERK phosphorylation as measured by Western blotting. Further studies showed that transfection with a constitutively active form of the Ras protein (RasV12) induced stronger ERK phosphorylation and upregulated MMP-9 and -13 mRNA expressions. However, transfection with a dominant-negative form of the Ras protein (RasN17) inhibited the ERK activation and MMP-9 and -13 mRNA expressions induced by IL-1β, which supported the involvement of ERK signalling in IL-1β-induced MMP-9 and -13 expressions. Treatment with luteolin effectively inhibited the IL-1β-induced ERK activation in dose-dependent manner. Taken together, luteolin might inhibit IL-1β-induced MMP-9 and -13 expressions, in part, via inhibition of ERK signalling.
Introduction
Matrix metalloproteinases (MMPs) are a family of proteinase that comprises major components of the enzyme cascade responsible for degradation of extracellular matrix (ECM1). In bone, osteoblasts secrete several kinds of MMPs such as gelatinase A (MMP-2), stromelysin-1 (MMP-3), gelatinase B (MMP-9) and interstitial collagenase (MMP-13), which have different substrate specificity and expression patternCitation2. MMPs are expressed in low level at physiological condition and involved in development, differentiation and bone remodelling by the regulation of bone matrix degradationCitation3. Therefore, the activity of MMPs is tightly regulated and tissue inhibitors of metalloproteinases (TIMP) are their major intrinsic inhibitors of the MMPs. Imbalance between excessive levels of MMPs coupled with inadequate TIMP levels facilitates the joint destructive process. Therefore, high level of MMPs expression due to aberrant regulation of MMPs has been implicated in numerous pathologic processes, including osteoarthritis, rheumatoid arthritis and osteoporosisCitation4,Citation5. MMPs expression is upregulated in response to various bone-resorptive stimuliCitation2,Citation6. The proinflammatory cytokines such as interleukin-1β (IL-1β) and tumour necrosis factor-α (TNF-α), produced in inflammatory condition such as arthritis joint by activated synovial cells and infiltrating macrophages, are considered to be one of the most potent catabolic factors in boneCitation7. These cytokines induce the enhanced production of MMPs by osteoblasts, which can cause irreversible matrix degradation and subsequent joint destruction. Therefore, the inhibitors of MMPs expression might are considered as a therapeutic target for the treatment of inflammation-related bone diseasesCitation8,Citation9.
Many studies have focused on the development of MMPs inhibitors from natural productsCitation7,Citation10. Flavonoids are polyphenolic compounds present in a variety of fruits, vegetables and seeds, which show diverse biological and pharmacological activities. Related to bone, flavonoids have been suggested to have beneficial effects on bone health. Recently, several studies have been performed about their inhibitory effects on MMPs expression in osteoblastsCitation11,Citation12. In the present study, effects of four structurally related flavonoids, apigenin, baicalein, luteolin and quercetin () on IL-1β-induced MMPs expression in primary cultures of mouse osteoblasts were investigated. Related to signalling, the role of extracellular signal-regulated kinase (ERK) signalling pathway in IL-1β-induced MMPs expression was evaluated by using mutant Ras vectors in a mouse osteoblastic cell line, MC3T3-E1 cells. The effect of flavonoids on IL-1β-activated ERK signalling was also investigated.
Methods and materials
Materials
Apigenin, baicalein, luteolin and quercetin were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO) MC3T3-E1, a mouse osteoblastic cell line, was obtained from the American Type Culture Collection. Fetal bovine serum (FBS), Dulbecco’s modified Eagle’s medium (DMEM) and other culture supplements were obtained from Invitrogen (Carlsbad, CA). Antibodies against phospho-specific and phosphor-independent ERK were obtained from Santa Cruz Biotechnology. A constitutively active form of the Ras protein (RasV12) and a dominant-negative form of the Ras protein (RasN17) were obtained from Clontech (Mountain View, CA).
Cell culture
Murine calvarial osteoblasts were obtained from the calvariae of neonatal mice 1–2 days after birth by sequential collagenase digestion method. The osteoblasts were characterised by alkaline phosphatase staining and cultured in DMEM supplemented with 10% FBS, 100 IU/mL penicillin and 100 μg/mL streptomycinCitation13. MC3T3-E1, a mouse osteoblastic cell line, was grown in α-MEM supplemented with 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin. Test compounds were dissolved in dimethyl sulfoxide (DMSO) and our preliminary study showed that DMSO at a final concentration of 0.1% in media showed little effect. The cultures were pre-treated with test compounds 1 h before IL-1β exposure and incubated for further 30 min for ERK signalling or for 24 h for the evaluation of MMPs expression.
TaqMan quantitative real-time PCR analysis
TaqMan quantitative real-time PCR analysis was performed as we reported previouslyCitation13. Forward and reverse primer sequences for MMP-9 are 5′-GGA CGA CGT GGG CTA CGT-3′ and 5′-ACT GAA CTT GAC CGT ACA CAC GGT TGA AGC AAA GAA GGA-3′, respectively. Forward and reverse primer sequences for MMP-13 are 5′-TGG AAG GTT ATC CCA GAA AAA TAT CT -3′ and 5′-ACT GAA CTT GAC CGT ACA TCC CCG TGT TCT CAA AGT GAA-3′, respectively. The temperature cycling program was set at 2 min initial incubation at 50°C followed by 10 min at 95°C, and 40 cycles with 15 s at 95°C and 1 min at 60°C. Plasmids containing the cDNA were used as a template to generate a standard curve. The expression of each gene was normalised with GAPDH expression.
Western blot analysis
Western blotting was performed using phospho-specific ERK antibodies and developed using horseradish peroxidase-conjugated secondary antibodies and ECL, as we previously reportedCitation14. Equal loading of samples was checked by Ponceau staining and probing with phospho-independent ERK antibodies.
Transient transfection
MC3T3-E1 cells were seeded the day before transfection at a concentration of 1 × 104 cells/cm2. Cells were transfected with constructs of a constitutively active form of the Ras protein (RasV12) or a dominant negative form of the Ras protein (RasN17) using FuGene transfection reagent according to manufacturer’s protocol. One day after transfection, IL-1β was treated for 30 min for ERK signalling or treated for 24 h for MMP-9 and -13 mRNA expressions.
Statistical analysis
The evaluation of statistical significance was determined by the Student’s t-test with a value of p < 0.05 or less considered to be statistically significant.
Results
Effects of flavonoids on the IL-1β-induced MMP-9 and -13 expressions in primary osteoblast cultures
The MMPs expression is known to be increased in pathological conditions. IL-1β, one of the most potent proinflammatory cytokines, is known to activate MMPs in various cell typesCitation15,Citation16. Therefore, the effect of IL-1β on MMP-9 and -13 expressions was first examined in our assay system. Primary cultures of mouse osteoblasts expressed both MMP-9 and -13 mRNA in control cells and the basal level of MMP-13 was higher than that of MMP-9. In addition, treatment of primary cultures of mouse osteoblasts with 10 ng/mL IL-1β for 24 h induced both MMP-9 and -13 mRNA expressions up to 7.6 and 12.6 times, respectively, as measured by quantitative real-time PCR ().
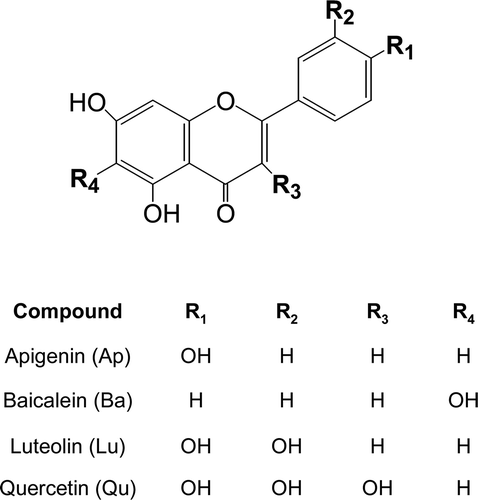
Figure 2. Effects of flavonoids on the mRNA expression of MMP-9 (A) and MMP-13 (B) in primary cultures of mouse osteoblasts. Cells were treated with 25 μM of each flavonoid, apigenin (Ap), bacalein (Ba), luteolin (Lu) and quercetin (Qu), for 1 h before IL-1β exposure. After further 24-h incubation, MMP-9 and -13 mRNA expressions were measured. *p < 0.05; **p < 0.01; ***p < 0.001 compared with IL-1β-treated control.
Next, the inhibitory effects of four structurally related flavonoids, apigenin, baicalein, luteolin and quercetin on IL-1β-induced MMP-9 and -13 expressions were investigated. At the concentration of 25 µM, luteolin decreased IL-1β-induced MMP-9 and -13 mRNA expressions almost up to control levels (). Treatment with 25 µM apigenin showed inhibitory effect on the MMP-9 mRNA expression induced by IL-1β, but less inhibitory effect on MMP-13 mRNA expression. Baicalein and quercetin, however, showed little effects on IL-1β-induced MMP-9 and -13 mRNA expressions.
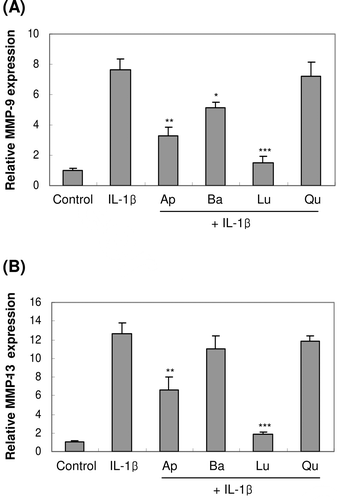
We further investigated the dose-dependent effects of luteolin on MMP-9 and -13 expressions. Treatment with luteolin concentrations ranging from 1 to 25 µM exerted dose-dependent inhibition on both MMP-9 and -13 mRNA expressions induced by IL-1β. MMP-13 expression was inhibited at concentrations ranging from 1 to 25 µM, whereas MMP-9 expression was inhibited at the concentrations ranging from 10 to 25 µM (). Taken together, these results suggested that luteolin inhibited IL-1β-induced MMP-9 and -13 mRNA expressions with more potent effect on MMP-13 expression.
Figure 3. Concentration-dependent effect of luteolin on the expression of MMP-9 and -13 in primary cultures of osteoblasts. Cells were treated with luteolin at concentrations from 1.0 to 25 μM for 1 h before IL-1β exposure. After further 24-h incubation, MMP-9 and -13 mRNA expressions were measured. *p < 0.05; **p < 0.01; ***p < 0.001 compared with IL-1β-treated control.
Role of ERK signalling pathway in the IL-1β-induced MMP-9 and -13 expressions in MC3T3-E1 osteoblasts
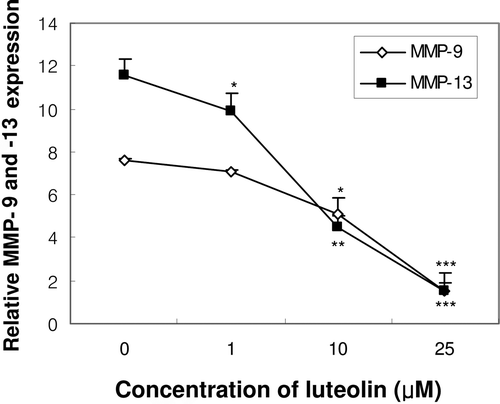
ERK signalling pathway is suggested to be involved in the IL-1β signallingCitation15. Treatment with 10 ng/mL IL-1β-induced ERK phosphorylation within 5 min and continued until 120 min in primary cultures of mouse osteoblasts (data not shown). The role of ERK signalling in IL-1β-induced MMP-9 and -13 expressions was further investigated by using mutant Ras vectors. Ras is known to activate ERK pathways, which in turn regulates gene transcriptionsCitation17,Citation18. Transfection with a constitutively active form of Ras protein (RasV12) induced ERK phosphorylation. In addition, exposure of RasV12-transfected cells to IL-1β exerted stronger ERK activation than that was produced by IL-1β alone. However, transfection with a dominant-negative form of the Ras protein (RasN17) prevented IL-1β-induced ERK activation ().
Figure 4. Effect of constitutively active form of the Ras protein (RasV12) and dominant-negative form of the Ras protein (RasN17) on ERK activation and the expression of MMP-9 and -13 mRNA in MC3T3-E1 cells. One day after the transfection with RasV12 or RasN17, cells were treated with IL-1β for 30 min for ERK phosphorylation (A) or treated for 24 h for the mRNA expression of MMP-9 (B) and MMP-13 (C). (CMV: empty vector, V: RasV12, N: RasN17). ##p < 0.01; ###p < 0.001 compared with control. **p < 0.01; ***p < 0.001 compared with IL-1β-treated control.
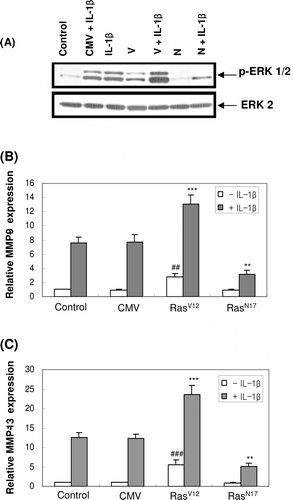
Next, we measured IL-1β-induced MMP-9 and -13 expressions in RasV12 or RasN17-transfected cells. Transfection with RasV12 significantly induced both MMP-9 and -13 mRNA expressions even though in the absence of IL-1β. Moreover, exposure of RasV12-transfected cells to IL-1β exerted much higher MMP-9 and -13 mRNA expressions than in control cells. On the contrary, inhibition of ERK phosphorylation by the transfection with RasN17 abolished IL-1β-induced MMP-9 and -13 mRNA expressions ( and ). These results suggested that ERK signalling is involved in IL-1β-induced MMP-9 and -13 expressions in osteoblasts.
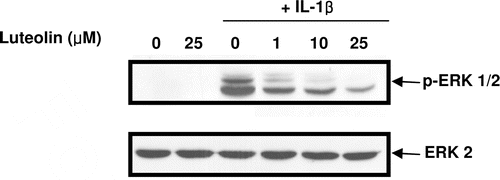
Effect of luteolin on IL-1β-induced ERK signalling in primary osteoblast cultures
The present study clearly showed the involvement of ERK signalling in the IL-1β-induced MMP-9 and -13 expressions in osteoblasts. Therefore, the effect of luteolin, which showed the inhibitory effect on IL-1β-induced MMP-9 and -13 expressions, on the IL-1β-induced ERK signalling was investigated. As shown in , luteolin inhibited IL-1β-induced ERK activation in dose-dependent manner. These results suggested that luteolin downregulates IL-1β-induced MMP-9 and -13 expressions by inhibiting ERK activation.
Figure 5. Effect of luteolin on IL-1β-induced ERK phosphorylation in primary cultures of mouse osteoblasts. Cells were pre-treated with luteolin at concentrations of 1, 10, 25 μM for 1 h before IL-1β exposure. After further 30-min incubation, ERK phosphorylation was measured using phosphor-specific ERK antibody.
Discussion
MMPs are major components of the enzyme cascade responsible for degradation of the extracelullar matrix in many organs including bone, brain and liverCitation7,Citation19,Citation20. The activities and expressions of MMPs are tightly regulated by various factors and aberrant regulations of MMPs are contributed to the pathogenesis of diverse diseases. MMPs expression can be induced in various pathological conditions including inflammation. Many studies reported the increased MMPs expression by the proinflammatory cytokines such as IL-1β and TNF-α in many cell types such as chondrocytes, synoviocytes, macrophages and astrocytesCitation7,Citation16,Citation20. In addition, IL-1 has been reported to induce MMPs expression in osteoblastic cell lines and primary cultured osteoblastsCitation21. Consistent with previous studies, the mRNA expression of MMP-9 and -13 were induced by the treatment with IL-1β in our assay system. Our present study also showed that IL-1β induced higher MMP-13 mRNA expression than that of MMP-9 in primary cultures of osteoblasts.
Since increased expression of MMPs are considered to be involved in various pathogenesis, the inhibitors of MMPs expressions might be considered as a therapeutic target for the treatment of inflammation-related bone diseasesCitation8,Citation9. Despite its therapeutic importance, however, few compounds were reported to inhibit MMPs expression in pathological condition. In the present study, the inhibitory effects of four related flavonoids such as apigenin, baicalein, luteolin and quercetin on IL-1β-induced MMP-9 and -13 expressions was tested. Our study showed that, of the four flavonoids tested, luteolin showed most potent inhibitory effect on IL-1β-induced MMP-9 and -13 mRNA expression in osteoblasts. MMPs were also produced from other types of cells such as chondrocyte and synoviocyte in bone systemCitation8. Therefore, the effect of luteolin on MMPs produced in other cell type needs to be clarified in further study.
Flavonoids are widely distributed in higher plants and exhibit diverse biological activities. Flavonoids share similar structural skeleton; however, they can be divided into several subfamilies. As shown in , all the four flavonoids tested in our study have a similar structure with a double bond between C2 and C3 but differ in their hydroxyl substituents (R1–R4). Therefore, structure-activity relationship of flavonoids in the inhibitory effects on IL-1β-induced MMP-9 and -13 expressions was investigated. Luteolin and quercetin have an identical structure except a hydroxyl group at C-3 (R3) but only luteolin showed potent inhibitory effect on IL-1β-induced MMP-9 and -13 expressions, which suggests that the introduction of a hydroxyl group at C-3 (R3) reduced the inhibitory effect on IL-1β-induced MMP-9 and -13 expressions. Luteolin, which has an identical structure to apigenin except hydroxyl group at C-4′ (R2), showed more potent inhibition than apigenin, which suggests the importance of hydroxyl group at C-4′ (R2) in inhibition on MMPs expression. Taken together, it has been postulated that the absence of hydroxyl group in C-3 and a 3′, 4′-dihydroxyl groups are important determinants for their inhibitory effects of flavonoids on IL-1β-induced MMPs expression. Consistent with this postulation, luteolin which fulfills these structural requirements showed the most potent inhibition in this study.
IL-1β appears to activate multiple signalling pathways including Akt, NF-κB and protein kinase C (PKC) as well as three mitogen activated kinases (MAPKs), ERK, p38 and JNKCitation22,Citation23. Moreover, MMPs expression is known to be regulated by multiple signalling such as MAPK, NF-κB, AP-1 and Akt in a variety of cell typesCitation21,Citation23. In the present study, treatment of IL-1β induced rapid activation (within 5 min) of ERK in osteoblast culture, which suggested that ERK activation is the early event during the MMPs induction by IL-1β. In addition, the inhibition of IL-1β-induced ERK activation by dominant-negative Ras mutant failed to induce MMPs expression, suggesting the importance of ERK activation in IL-1β-induced MMPs expression. Luteolin, which showed potent inhibitory effect on IL-1β-induced MMPs expression inhibited ERK activation induced by IL-1β. These results suggested that the inhibitory effect of luteolin was achieved via the inhibition of IL-1β-induced ERK activation, an important signalling in MMPs expression. Several reports also showed the inhibitory effect of luteolin on AP-1 and NF-κB, which is known to be involved in MMPs expressionCitation24,Citation25. Therefore, inhibition of MMPs expression by luteolin might be also exerted by its ability to inhibit other signalling pathway involved in IL-1β-induced MMPs expression. More detailed studies will be required to investigate the role of luteolin in IL-1β-induced signalling in MMPs expression.
In conclusion, the present study showed that luteolin inhibited IL-1β-induced MMP-9 and -13 mRNA expressions in primary cultures of mouse osteoblasts by inhibition of ERK activation. Because excessive levels of MMPs promote joint destructive process in both OA and RA, luteolin might be beneficial to retard the progress joint damage in degenerative bone disease, especially in inflammatory condition. In addition, proinflammatory cytokines including In-1β are increased in collagen-induced arthritis, a widely used pre-clinical animal model. Therefore, the inhibitory effect of luteolin on IL-1β-induced MMPs might be applied to animal model of arthritis and will provide further insight into the design of new approaches to anti-osteoporosis and anti-inflammatory drugs.
Acknowledgment
This work was supported by the Medical Research Center program through the National Research Foundation of Korea (2010-0029480).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Krane SM. Clinical importance of metalloproteinases and their inhibitors. Ann N Y Acad Sci 1994;732:1–10.
- Kusano K, Miyaura C, Inada M, Tamura T, Ito A, Nagase H et al. Regulation of matrix metalloproteinases (MMP-2, -3, -9, and -13) by interleukin-1 and interleukin-6 in mouse calvaria: Association of MMP induction with bone resorption. Endocrinology 1998;139:1338–1345.
- Nagase H, Woessner JF Jr. Matrix metalloproteinases. J Biol Chem 1999;274:21491–21494.
- Shlopov BV, Lie WR, Mainardi CL, Cole AA, Chubinskaya S, Hasty KA. Osteoarthritic lesions: Involvement of three different collagenases. Arthritis Rheum 1997;40:2065–2074.
- Teitelbaum SL. Bone resorption by osteoclasts. Science 2000;289:1504–1508.
- Uchida M, Shima M, Shimoaka T, Fujieda A, Obara K, Suzuki H et al. Regulation of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) by bone resorptive factors in osteoblastic cells. J Cell Physiol 2000;185:207–214.
- Ahmed S, Wang N, Lalonde M, Goldberg VM, Haqqi TM. Green tea polyphenol epigallocatechin-3-gallate (EGCG) differentially inhibits interleukin-1β-induced expression of matrix metalloproteinase-1 and -13 in human chondrocytes. J Pharmacol Exp Ther 2004;308:767–773.
- Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science 2000;289:1508–1514.
- Mengshol JA, Mix KS, Brinckerhoff CE. Matrix metalloproteinases as therapeutic targets in arthritic diseases: Bull’s-eye or missing the mark? Arthritis Rheum 2002;46:13–20.
- Mix KS, Mengshol JA, Benbow U, Vincenti MP, Sporn MB, Brinckerhoff CE. A synthetic triterpenoid selectively inhibits the induction of matrix metalloproteinases 1 and 13 by inflammatory cytokines. Arthritis Rheum 2001;44:1096–1104.
- Lee SO, Jeong YJ, Yu MH, Lee JW, Hwangbo MH, Kim CH, Lee IS. Suppression of PMA-induced tumor cell invasion by capillarisin via the inhibition of NF-κB-dependent MMP-9 expression. Biochem Biophys Res Commun 2006;351:118–125.
- Lim H, Kim HP. Inhibition of mammalian collagenase, matrix metalloproteinase-1, by naturally-occurring flavonoids. Planta Med 2007;73:1267–1274.
- Lee MK, Choi H, Gil M, Nikodem VM. Regulation of osteoblast differentiation by Nurr1 in MC3T3-E1 cell line and mouse calvarial osteoblasts. J Cell Biochem 2006;99:986–994.
- Lee MK, Nikodem VM. Differential role of ERK in cAMP-induced Nurr1 expression in N2A and C6 cells. Neuroreport 2004;15:99–102.
- Amin R, Senga T, Oo ML, Thant AA, Hamaguchi M. Secretion of matrix metalloproteinase-9 by the proinflammatory cytokine, IL-1β: A role for the dual signalling pathways, Akt and Erk. Gene cells 2003;8:515–523.
- Wu CY, Hsieh HL, Jou MJ, Yang CM. Involvement of p42/p44 MAPK, p38 MAPK, JNK and nuclear factor-κB in interleukin-1β-induced matrix metalloproteinase-9 expression in rat brain astrocytes. J Neurochem 2004;90:1477–1488.
- Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: Conserved structure and molecular mechanism. Nature 1991;349:117–127.
- Campbell SL, Khosravi-Far R, Rossman KL, Clark GJ, Der CJ. Increasing complexity of Ras signaling. Oncogene 1998;17:1395–1413.
- Wu J, Zern MA. Hepatic stellate cells: A target for the treatment of liver fibrosis. J Gastroenterol 2000;35:665–672.
- Lin N, Sato T, Takayama Y, Mimaki Y, Sashida Y, Yano M et al. Novel anti-inflammatory actions of nobiletin, a citrus polymethoxy flavonoid, on human synovial fibroblasts and mouse macrophages. Biochem Pharmacol 2003;65:2065–2071.
- de Bart AC, Quax PH, Löwik CW, Verheijen JH. Regulation of plasminogen activation, matrix metalloproteinases and urokinase-type plasminogen activator-mediated extracellular matrix degradation in human osteosarcoma cell line MG63 by interleukin-1α. J Bone Miner Res 1995;10:1374–1384.
- Chen CC, Chen JJ, Chou CY. Protein kinase c-α but not p44/42 mitogen-activated protein kinase, p38, or c-Jun NH(2)-terminal kinase is required for intercellular adhesion molecule-1 expression mediated by interleukin-1β: Involvement of sequential activation of tyrosine kinase, nuclear factor-κB-inducing kinase, and IκB kinase 2. Mol Pharmacol 2000;58:1479–1489.
- Liacini A, Sylvester J, Li WQ, Zafarullah M. Inhibition of interleukin-1-stimulated MAP kinases, activating protein-1 (AP-1) and nuclear factor κB (NF-κB) transcription factors down-regulates matrix metalloproteinase gene expression in articular chondrocytes. Matrix Biol 2002;21:251–262.
- Chen CC, Chow MP, Huang WC, Lin YC, Chang YJ. Flavonoids inhibit tumor necrosis factor-α-induced up-regulation of intercellular adhesion molecule-1 (ICAM-1) in respiratory epithelial cells through activator protein-1 and nuclear factor-κB: Structure-activity relationships. Mol Pharmacol 2004;66:683–693.
- Kim JS, Jobin C. The flavonoid luteolin prevents lipopolysaccharide-induced NF-κB signalling and gene expression by blocking IκB kinase activity in intestinal epithelial cells and bone-marrow derived dendritic cells. Immunology 2005;115:375–387.