Abstract
Claisen-Schmidt condensation of 3-(1,2,3,6-tetrahydro-1-methylpyridin-4-yl)-2,4,5- trimethoxybenzaldehyde 3 and various aromatic, heterocyclic and alicyclic amides of 3- aminoacetophenone 6(a–s) afforded novel curcumin mimics. All the synthesized compounds were characterized by IR, 1H NMR, Mass spectroscopy and evaluated for antioxidant, cytotoxicity and antimicrobial activity. Out of the 20 compounds screened, compounds 7i, 7l, 7q, and 7n have shown excellent radical scavenging activity, compounds 7o, 7t, 7f, and 7r have shown significant xanthine oxidase inhibition, and compounds 7a, 7k and 7l were found to be potent inhibitors of selected cancer cell lines. Compounds 7h, 7t, 7l, 7i, and 7e have shown good antibacterial activity, whereas compounds 7j, 7f, 7o, 7h, and 7t exhibited significant antifungal activity.
Introduction
Reactive oxygen species (ROS), including the hydroxyl radical, superoxide anion, hydrogen peroxide and peroxynitric species are continuously generated in the process of respiration as natural byproduct of oxygen metabolismCitation1. These species are sufficiently reactive to cause injury to cells through the destruction of components such as proteins, lipids, sugars and nucleotidesCitation2,Citation3. Under normal circumstances, cells are able to defend against ROS damage through the use of enzymes such as superoxide dismutase and catalaseCitation4–6. Small molecule antioxidants such as ascorbic acid, uric acid and glutathione also play an important role as cellular antioxidantsCitation7–9. Therefore, under normal condition, ROS are maintained at constant level in the body by the antioxidant defence mechanism. However, imbalances between the formation and detoxification of ROS can result in significant damage to cells, a situation known as oxidative stress. Oxidative stress leads to the initiation or progression of various diseases such as cancerCitation10, ischemia-reperfusion injuryCitation11, atherosclerosisCitation12, cardiovascularCitation13, inflammationCitation14, and neurodegenerative disorders such as Alzheimer’s and ParkinsonCitation15,Citation16.
Xanthine oxidase (XO) is a key enzyme that catalyzes the oxidation of xanthine and hypoxanthine into uric acid and plays a vital role in causing hyperuricemia and goutCitation17. It is considered to be a main source of oxidative stress and destructive free radicals in ischemia-reperfusion injury associated with heart attack, stroke and spinal cord injury, as well as being a destructive force myocardial or renal hypoxia and infarctionCitation18–20. Because of this, there is a growing interest in the discovery of natural and unnatural antioxidants that attenuate oxidative stress and as a result serve as protective agents against these diseasesCitation21.
Curcumin is a β-diketone constituent of the turmeric that is obtained from the powdered rhizome of Curcuma longa. Curcumin has a wide range of interesting biological activities such as anti-inflammatory, antioxidant, antiviral, cutaneous wound healing, hypocholesterolemic effects in diabetic patients, anti-angiogenic and stimulatory response to stress-induced biological activityCitation22,Citation23. Curcumin has been demonstrated to possess preventative activity against Aβ-aggregation in Alzheimer’s modelCitation24. Several studies have shown that curcumin manifests anti-proliferative activity against various cancers, including leukemia, colon, liver, breast and prostrate cancersCitation23,Citation25–27. Although curcumin is non-toxic and has promising biological activities, preclinical and clinical studies have indicated that poor bioavailability and pharmacokinetic profile are poor due to its instability under physiological condition which has limited its application in anticancer therapiesCitation28–30. Evidences from both in vitro and in vivo studies show that β-diketone moiety is responsible for instability and weak pharmacokinetic profiles of curcumin. During last decade, synthetic modifications of curcumin, which were aimed at enhancing its bioactivities, suggested that the stability and metabolic profile of curcumin could be enhanced by deleting β-diketone moiety. Recent studies from several independent groups demonstrated that curcumin analogues without β-diketone either retained or increased various biological activities such as anticancerCitation31,Citation32, antibacterialCitation33 and anti-inflammatoryCitation34. In continuation of our studies in synthesizing various biologically active compoundsCitation35, in the present study we have synthesized and characterized the novel curcumin mimics and evaluated these for antioxidant, cytotoxic and antimicrobial activity.
Chemistry
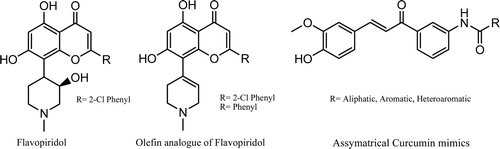
Although curcumin is non-toxic and has promising biological activities, clinical studies indicate its poor bioavailability and pharmacokinetic profile. To overcome these barriers several research groups have synthesized curcumin analogues. Robinson et alCitation31. have synthesized enone and dienone analogues, and Woo et alCitation36. have synthesized the curcumin mimics which were shown to possess increased anti-angiogenic activity. Flavopiridol (NSC 649890) [cis-5,7-dihydroxy-2-(2-chlorophenyl)-8-[4-(3-hydroxy-1-methyl)-piperidinyl]-1-benzopyran-4-one] is a synthetic flavone that has been shown to possess anti-tumour activity against various tumour cell lines such as human lung carcinoma and breast carcinomaCitation37. Olefin analogues of flavopiridol have been synthesized and shown to be potent inhibitor of CDKCitation38 and glycogen phosphorylaseCitation39 . We herein report on the synthesis of the novel curcumin mimic incorporating olefin as well as aromatic, alicyclic or heteroaromatic amide moities. The title compounds were prepared using straight forward chemistry (Scheme 1). Compound 2 (olefin) was prepared by reacting 1, 3, 5-trimethoxy benzene with 1-methyl-4-piperidone in presence of hydrogen chloride gas in glacial acetic acidCitation40. Compound 2 on Vilsmier-Hack formylation gave 3-(1,2,3,6-tetrahydro-1-methylpyridine-4-yl)-2,4,6-trimethoxybenzaldehyde 3. Compounds 6a–s were prepared by acylation of 3-aminoacetophenone with different acylchlorides in basic medium, except compound 6r and 6s which were prepared using an earlier reported methodCitation41. Compounds 6a–s on Claisen-Schmidt condensation with compound 3 under basic media afforded a residue, which on purification by column chromatography with 1% ammonia and 0.5–1% methanol in chloroform as eluting solvent furnished title compounds in good yields (). All the synthesized curcumin mimics were characterized by IR, 1H NMR and Mass spectral analysis. All the synthesized curcumin mimics 7(a–s) and synthetic intermediate 7t were evaluated for antioxidant, cytotoxicity and antimicrobial activity.
Table 1. Synthesis of curcumin mimics.
Results and discussion
Taking into the account of multifactorial character of oxidative stress which is involved in many pathological states, we have evaluated antioxidant activity of curcumin mimics against DPPH stable free radical. Free radical scavenging activity was measured in terms of % DPPH inhibition and results are presented in . All the synthesized compounds have shown excellent to moderate radical scavenging activity. Compounds 7i, 7l, 7q, 7f, 7r, 7j, 7e, 7o, 7t and 7c have shown excellent radical scavenging activity (90–80%) as compared to standard trolox (80%), compounds 7k, 7s have shown good radical scavenging activity (77–75%), whereas the rest of the compounds have shown moderate radical scavenging activity. Structure activity relationship (SAR) study of a curcumin mimics demonstrated that the presence of electron donating substituents on the amide ring increases antioxidant activity, whereas the presence of electron withdrawing substituents on the amide ring decreases the antioxidant activity except in the case of compound 7e and 7o. Butylated compounds are excellent inhibitors of reactive oxygen species (ROSCitation42); however, in the present case, compound 7m has shown moderate antioxidant activity. This may be ascribed to the absence of hydroxyl group ortho to butyl group on aromatic ring. Compound 7n containing cinnamide moiety shows good antioxidant activity which is as expected since cinnamic derivatives have been known to be excellent ROS scavengerCitation43. A comparison of the antioxidant activity of compounds 7a, 7k, 7l reveal that the replacement of aromatic amide moiety (7a) with heterocyclic amide moiety such as, thiphen (7k) or furan (7l) results in increased antioxidant activity, particularly in the latter case. Similarly, incorporation of alicyclic amide moiety (7r) also showed increased activity compared to (7a) which contains aromatic amide moiety.
Table 2. Antioxidant activity of curcumin mimics.
Xanthine Oxidase (XO) is a cytosolic enzyme that is an important source of oxygen free radicals. Results of XO inhibition by curcumin mimics are presented in . These results indicate that all the synthesized compounds have excellent to moderate XO inhibition. Among all synthesized compounds, 7o, 7t, 7f, 7r, 7l, 7i, 7k and 7q were excellent inhibitors of XO (94-90%) as compared to standard allopurinol (90%), compounds 7d, 7n, 7m, 7c, and 7h were good inhibitors of XO (89-80%), whereas the rest of the compounds showed moderate inhibition. SAR study revealed that compounds containing electron withdrawing substituents on amide ring are excellent inhibitors of XO compared to their counterparts containing electron donating groups with the exception 7i and 7q. Interestingly, compound 7t an intermediate of curcumin mimics, has shown excellent XO inhibition. Changing the position of fluorine on amide ring from para position (7f) to meta position (7d) showed a decrease in activity. There is further decrease in the activity as the position of fluorine is changed from meta (7d) to ortho (7b). This suggests that a substituent at para or meta position is favourable for enzyme inhibition. In addition it was found that heteroaromatic amides such as furan amide (7l), thiophen amide (7k) are better inhibitors than the aromatic amide (7a). Similarly, curcumin mimics containing alicyclic cyclopropyl amide moiety (7r) shows excellent XO inhibition as compared to one containing aromatic amide moiety (7a).
Results shown in indicate that all synthesized compounds have shown cytotoxicity against all selected cancer cell lines. Compounds 7a, 7k, and 7l completely inhibited selected ACHN (human renal carcinoma), Panc 1 (human pancreatic carcinoma), Calu 1 (human non-small cell lung carcinoma), H460 (human non cell lung carcinoma) and HCT 116 (human colon carcinoma) cancer cell lines (92–100%) at 10 μM as compared to standard flavopiridol (68–73%) and gemcitabine (78–84%) at 700 and 500 nM, respectively. Compounds 7e, 7g, and 7s have moderate cytotoxicity. Of the 20 compounds synthesized, compound 7a and 7k are cytotoxic to all selected five cancer cell lines studied. The cytotoxicity of these compounds are in compliance with the literatureCitation36. Compound 7l has preferential cytotoxicity against pancreas, lung and non-small cell lung carcinoma cancer cell lines. Similarly, compound 7g shows cytotoxicity against non-small cell lung carcinoma, human colon and renal cancer cell lines. In general, the following trend was observed: (i) electron withdrawing groups on the amide ring are more cytotoxic than their counterparts containing electron donating groups, (ii) curcumin mimics containing heteroaromatic amide moiety showed highest cytotoxicity, (iii) the order of cytotoxicity with respect to position of substituents on the amide moiety was found to be meta > para > ortho.
Table 3. Cytotoxicity profile of curcumin mimics against selected cancer cell lines.
All synthesized compounds were subjected to antimicrobial study by in vitro disk diffusion method against Salmonella typhi, Proteus vulgaris, Pseudomonas aeruginosa, Staphylococcus aureus, Bacilus subtilis, Penicillium chrysogenum, Trichoderma viridae, Candida albicans, Microsporum cannis and Fusarium moniliformae. Nystatin and tetracycline used as standards against fungi and bacteria, respectively. The results are reported as a mean of triplicate measurements. Antibacterial and antifungal activities of compounds are shown in . Results show that all the synthesized compounds possess good antimicrobial activity. Compounds 7h, 7t, 7l, 7i, 7e, 7j, 7o, 7c, and 7r show antibacterial activity more or less equal to that of standard at tested concentration; however, the rest of the compounds displayed weak antibacterial activity. It is observed that of the synthesized 20 compounds screened against three gram negative strains S. typhi, P. vulgaris, P. aeruginosa and two gram positive strain S. aureus, B. subtilis, almost all the compounds are active against gram positive strains, whereas some of the compounds are inactive against gram negative strain. In addition, it was observed that substituents on amide ring at meta and para positions showed higher antibacterial activity as compared to the respective ortho derivatives with the exception of 7h. Interestingly, compound 7t, an intermediate of curcumin mimic, has shown equal antibacterial activity as that of curcumin mimics. Curcumin mimics containing heterocyclic and alicyclic amides moieties have shown promising antibacterial activity. Compounds 7j, 7f, 7o, 7h, 7t, 7c, 7e, and 7r showed strong antifungal activity as compared to nystatin at tested concentration. Some of the compounds are inactive against fungal strains especially against C. albicans. Structure activity relationship study indicates that compounds containing electron withdrawing groups on the amide moiety have shown higher antifungal activity than their counterparts containing electron donating groups except 7j. Alicyclic cyclopropyl amide 7r showed higher antifungal activity than the aromatic amide 7a. Compounds having substituents at para and meta position of amide ring have higher antifungal activity than the ortho substituted counterparts.
Table 4. Antimicrobial activity of curcumin mimics (zone of inhibition in mm).
Conclusion
In this study, we have synthesized a series of novel curcumin mimics and evaluated for antioxidant, cytotoxicity and antimicrobial activity. Compounds 7i, 7l, 7q, and 7n showed excellent antioxidant activity. The compounds 7o, 7t, 7f, and 7r have shown promising xanthine oxidase inhibition. The presence of substituents at meta and para position of amide was found to be essential for antioxidant activity. Compounds 7a, 7k, and 7l have shown potent cytotoxicity against selected human cancer cell lines. The presence of substituents at meta position was found to be necessary for cytotoxicity. Compounds 7h, 7t, 7l, 7i, and 7e showed good antibacterial activity and compounds 7j, 7f, 7o, 7h, and 7t exhibited antifungal activity against all the strains tested. Overall compounds 7l, 7t, 7i, 7o, and 7r need to be screened further for other cancer cell lines and in vivo study and toxicology. These molecules can be considered as lead molecules for novel cytotoxic and antioxidant agent.
Experimental
Chemistry
All the chemicals were purchased from Aldrich Chemical Co. (Nanded, Maharashtra, India). Melting points were determined with digital thermometer and were uncorrected. IR spectra were recorded on FT-IR Shimadzu 8300 spectrophotometer and 1H NMR spectra were recorded on a Bruker 400 MHz spectrometer in CDCl3 using tetramethyl silane as an internal standard. Mass spectra were obtained with a Shimadzu LCMS-2010 EV. Chromatographic purification was performed with silica gel (100–200 Mesh). Thin layer chromatography was performed on pre-coated silica plates (Merks kiesegel 60F254, 0.2 mm thickness) sheet. The spots could be visualized easily under UV light.
Synthesis of 3-(1,2,3,6-tetrahydro-1-methylpyridin-4-yl)-2,4,5-trimethoxybenzaldehyde (3)
Olefin 2 (1.0 g, 4 mmol) was dissolved in dry DMF (13.3 mL) under anhydrous condition. It was cooled to 0°C, POCl3 (7.2 mL) was added drop wise for 30 min and stirring continued for 2 h at 25°C. After completion of reaction (TLC), reaction mass was poured over crushed ice (50 g) and basified with Na2CO3, extracted with chloroform, dried over anhydrous Na2SO4, purified through silica gel column using 0.5–1% methanol + 1% liquor ammonia in chloroform as eluting solvent afforded product 3 (0.6 g) in 51% yield.
General procedure for the synthesis of curcumin mimics (7a-s)
Amides 6a–s (1 mmol) were dissolved in ethanol (15 mL), NaOH (20%) was added to it and stirred for 5 min. 3-(1,2,3,6-tetrahydro-1-methylpyridine-4-yl)-2,4,6-trimethoxybenzaldehyde 3 (1 mmol) was added and stirring continued for 24 h at room temperature. After completion of reaction (TLC), reaction mixture was poured over crushed ice, extracted with chloroform, organic extract was washed with water, dried (anhydrous Na2SO4) and concentrated. Purification was carried using silica gel column using mixture of 0.5–1% methanol + 1% liquor ammonia in chloroform as an eluent to obtain the title compounds.
N-(3-((2E)-3-(1,2,3,6-tetrahydro-1-methylpyridin-4-yl)-2,4,6-trimethoxyphenyl)acryloyl)phenyl)benzamide (7a):
IR (KBr) cm−1: 3470, 3170, 2986, 2852, 1649, 1617, 1590, 1570, 1510, 1420, 1020, 945, 844, 763. 1H NMR (CDCl3, 400 MHz) δ: 10.50 (s, 1H, -NH), 8.48 (s, 1H), 8.08 (d, 1H, J = 8 Hz), 7.95 (m, 2H), 7.85 (d, 1H, J = 15.6 Hz), 7.72 (d, 1H, J = 7.6 Hz), 7.45 (m, 4H), 7.40 (m, 1H), 6.30 (s, 1H), 5.58 (1H), 3.98 (s, 3H), 3.86 (s, 3H), 3.77 (s, 3H), 3.12 (s, 2H), 2.64 (s, 2H), 2.42 (s, 3H), 2.35 (s, 2H). MS: m/z 513 [M+1].
2-fluoro-N-(3-((2E)-3-(3-(1,2,3,6-tetrahydro-1-methylpyridin-4-yl)-2,4,6-trimethoxyphenyl)acryloyl)phenyl)benzamide(7b):
IR (KBr) cm−1: 3410, 3115, 2984, 1660, 1603, 1429, 1139, 1016, 982, 828, 760. 1H NMR (CDCl3, 400 MHz) δ: 10.67 (s, 1H, -NH), 8.44 (s, 1H), 8.04 (m, 3H), 7.95 (d, 1H, J = 16 Hz), 7.79 (d, 1H, J = 7.08 Hz), 7.53 (m, 4H), 6.30 (s, 1H), 5.53 (s, 1H), 3.99 (s, 3H), 3.80 (s, 3H), 3.70 (s, 3H), 3.27 (s, 2H), 2.88 (s, 2H), 2.53 (s, 3H), 2.46 (s, 2H). MS: m/z 531 [M+1].
Biological activities
DPPH antioxidant assay
The DPPH radical scavenging activity of curcumin mimics were measured according to the procedure described by Bandgar et al. with minor modificationCitation44. A purple-coloured (DPPH˙) is a stable free radical, which is reduced to DPPH (yellow coloured) by reacting with an antioxidant. The reaction mixture consisted of 100 μM DPPH in absolute ethanol with different concentrations (0–1000 μM) of test compounds. A negative control with the same DPPH concentration in ethanol without sample was used. The mixture was shaken vigorously and allowed to stand in the dark at room temperature for 10 min. The decrease in absorbance of resulting solution was then measured spectrophotometrically at 517 nm against ethanol. All measurements were made in triplicate and averaged. Trolox was used as standard.
Xanthine oxidase inhibition
Xanthine oxidase inhibitory activity was assayed spectrophotometrically at 295 nm under aerobic conditionCitation45. The reaction mixture contained 200 mM sodium pyrophosphate buffer (pH 7.5), 100 μm xanthine, and 0.4 U of XO. The absorption rate at 295 nm indicate the formation of uric acid at 25°C. Samples were dissolved directly in the buffer and incorporated with enzyme assay to assess the inhibitory activity. The experiments were carried out with three sets of apparatus testing XO inhibitory activity. XO activity was expressed as % inhibition of XO, calculated as % inhibition = (1-B/A) × 100 where A is the change in absorbance of the assay without the curcumin mimics and B is the change in absorbance of the assay with curcumin mimics. The enzyme kinetics was similar to XO assay method.
Cytotoxicity assay
The selected cancer cell lines such as ACHN (human renal carcinoma), Panc 1 (human pancreatic carcinoma), Calu-1 (human non-small cell lung carcinoma), H460 (human non cell lung carcinoma) and HCT 116 (human colon carcinoma) were used for evaluation of cytotoxicity of test compounds. The propidium iodide florescence assay was performed for the demonstration of cytotoxic effect of test compound. Seed cells of 3000–7500 cells/well of individual cell line were placed in 2000 μL of tissue culture grade 96-well plates and allowed them to recover for 24 h in humidified 5% CO2 incubator at 37°C. After culturing for 24 h, individual test compounds (10 μM in 0.3% DMSO) were added onto triplicate wells. DMSO (0.3%) alone was used as control. After 48 h in humidified 5% CO2 incubator at 37°C condition, the medium was removed and treated with 25 μL of propidium iodide (50 μg/mL in water/medium) per well. The plates were frozen at −80°C for 24 h then thawed and allowed it to room temperature, and the plate absorbance was read on fluorometer (polar-star BMG Tech), using 530 nm excitation and 620 nm emission wavelengths. Percent cytotoxicity of the test compounds were calculated by using following formula.
T = OD of treated cells
C = OD of control
Flavopiridol (700 nM) and Gemcitabine (500 nM) were used as standard anticancer drugs for comparisonCitation46.
Antimicrobial activity
Antimicrobial activities of all the synthesized compounds were determined by agar diffusion methodCitation35. All the bacteria and fungal species used were procured from Institute of Microbial Type Culture Collection (IMTCC), Chandigarh, India, and National Collection of Industrial Microorganisms (NCIM), Pune, India, namely, P. vulgaris, P. aeruginosa, S. aureus, B. subtilis, P. chrysogenum, T. viridae, C. albicans, M. cannis and F. moniliformae. All the synthesized compounds were dissolved to prepare a stock solution of 1 mg/mL using DMSO. Stock solution was aseptically transferred and suitably diluted to have solutions of concentration ranging 50–100 μg. For antifungal activity, different fungal spore suspension in sterile distilled water was adjusted to give a final concentration of 106 cfu/mL. Inoculum of 0.1 mL spore suspension of each fungus was spread on Sabouraud’s Dextrose agar plates (HiMedia). For antibacterial activity, Muller Hinton agar was used (HiMedia) seeded with 0.1 mL of respective bacterial strains suspension prepared in sterile saline (0.85%) of 105 cfu/mL dilution. The wells of 6 mm diameter were filled with 0.1 mL each test compound separately for each fungi and bacterial strain. The DMSO alone was used as control. The antibiotics tetracycline (10 μg/mL) and nystatin (30 μg/mL) were used as reference for antibacterial and antifungal, respectively. Inoculated plates in duplicate were then incubated at 37 ± 0.5°C for antibacterial activity for 24 h and at 28 ± 0.2°C for antifungal activity for 48 h. After incubation, the antimicrobial activity was measured in terms of the zone of inhibition in mm.
Acknowledgments
HVC is thankful to the Council of Scientific and Industrial Research (CSIR), New Delhi, for the award of JRF, as well as to Mahesh Nambiar and Mrs Asha Almeida Piramal Life Sciences Ltd., Mumbai, for anticancer activity.
Declaration of interest
The authors report no conflicts of interest.
References
- Boveries A, Cadenas E. Cellular sources and steady-state levels of reactive oxygen species. In Oxygen, Gene Expression and Cellular Functions. Clerch LB, Massaro DJ, (eds.) Marcel Dekkar: New York, 1977; pp. 1–25.
- Halliwell B, Gutteridge JM. Free Radical in Biology and Medicine, 2nd Edn; Oxford University Press: Clerandon: Oxford, 1989; pp. 1–20.
- Braughler JM, Duncan LA, Chase RL. The involvement of iron in lipid peroxidation. Importance of ferric to ferrous ratios in initiation. J Biol Chem 1986;261:10282–10289.
- Abid MR, Schoots IG, Spokes KC, Wu SQ, Mawhinney C, Aird WC. Vascular endothelial growth factor-mediated induction of manganese superoxide dismutase occurs through redox-dependent regulation of forkhead and IκB/NF-κB. J Biol Chem 2004;279:44030–44038.
- Schiavone JR, Hassan HM. The role of redox in the regulation of manganese-containing superoxide dismutase biosynthesis in Escherichia coli. J Biol Chem 1988;263:4269–4273.
- Li S, Yan T, Yang JQ, Oberley TD, Oberley LW. The role of cellular glutathione peroxidase redox regulation in the suppression of tumor cell growth by manganese superoxide dismutase. Cancer Res 2000;60:3927–3939.
- Sorg O. Oxidative stress: A theoretical model or a biological reality? c r Biol 2004;327:649–662.
- Chow HS, Lynch JJ 3rd, Rose K, Choi DW. Trolox attenuates cortical neuronal injury induced by iron, ultraviolet light, glucose deprivation, or AMPA. Brain Res 1994;639:102–108.
- Saunders RD, Dugan LL, Demediuk P, Means ED, Horrocks LA, Anderson DK. Effects of methylprednisolone and the combination of α-tocopherol and selenium on arachidonic acid metabolism and lipid peroxidation in traumatized spinal cord tissue. J Neurochem 1987;49:24–31.
- Lamson DW, Brignall MS. Antioxidants in cancer therapy: Their actions and interactions with oncologic therapies. Altern Med Rev 1999;4:304–329.
- Flamm ES, Demopoulos HB, Seligman ML, Poser RG, Ransohoff J. Free radicals in cerebral ischemia. Stroke 1978;9:445–447.
- Gey KF. On the antioxidant hypothesis with regard to arteriosclerosis. Bibl Nutr Dieta 1986;37:53–91.
- Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: Meta-analysis of randomised trials. Lancet 2003;361:2017–2023.
- Ziakas GN, Rekka EA, Gavalas AM, Eleftheriou PT, Kourounakis PN. New analogues of butylated hydroxytoluene as anti-inflammatory and antioxidant agents. Bioorg Med Chem 2006;14:5616–5624.
- Mattson MP. Excitotoxic and excitoprotective mechanisms: Abundant targets for the prevention and treatment of neurodegenerative disorders. Neuromolecular Med 2003;3:65–94.
- Stavrovskaya IG, Kristal BS. The powerhouse takes control of the cell: Is the mitochondrial permeability transition a viable therapeutic target against neuronal dysfunction and death? Free Radic Biol Med 2005;38:687–697.
- Hille R. Structure and Function of xanthine oxidoreductase. Eur J Inorg Chem 2006;10:1913–1926.
- Pacher P, Nivorozhkin A, Szabó C. Therapeutic effects of xanthine oxidase inhibitors: Renaissance half a century after the discovery of allopurinol. Pharmacol Rev 2006;58:87–114.
- Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 2007;357:1121–1135.
- Li C, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol, Cell Physiol 2002;282:C227–C241.
- Fong JJ, Rhoney DH. NXY-059: Review of neuroprotective potential for acute stroke. Ann Pharmacother 2006;40:461–471.
- Joe B, Vijaykumar M, Lokesh BR. Biological properties of curcumin-cellular and molecular mechanisms of action. Crit Rev Food Sci Nutr 2004;44:97–111.
- Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin”: From kitchen to clinic. Biochem Pharmacol 2008;75:787–809.
- Park SY, Kim DS. Discovery of natural products from Curcuma longa that protect cells from β-amyloid insult: A drug discovery effort against Alzheimer’s disease. J Nat Prod 2002;65:1227–1231.
- Wahlström B, Blennow G. A study on the fate of curcumin in the rat. Acta Pharmacol Toxicol (Copenh) 1978;43:86–92.
- Ammon HP, Wahl MA. Pharmacology of Curcuma longa. Planta Med 1991;57:1–7.
- Rashmi R, Santhosh Kumar TR, Karunagaran D. Human colon cancer cells differ in their sensitivity to curcumin-induced apoptosis and heat shock protects them by inhibiting the release of apoptosis-inducing factor and caspases. FEBS Lett 2003;538:19–24.
- Hsu CH, Cheng AL. Clinical studies with curcumin. Adv Exp Med Biol 2007;595:471–480.
- Pan MH, Huang TM, Lin JK. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos 1999;27:486–494.
- Sharma RA, Steward WP, Gescher AJ. Pharmacokinetics and pharmacodynamics of curcumin. Adv Exp Med Biol 2007;595:453–470.
- Robinson TP, Ehlers T, Hubbard IV RB, Bai X, Arbiser JL, Goldsmith DJ et al. Design, synthesis, and biological evaluation of angiogenesis inhibitors: Aromatic enone and dienone analogues of curcumin. Bioorg Med Chem Lett 2003;13:115–117.
- Ohtsu H, Xiao Z, Ishida J, Nagai M, Wang HK, Itokawa H et al. Antitumor agents. 217. Curcumin analogues as novel androgen receptor antagonists with potential as anti-prostate cancer agents. J Med Chem 2002;45:5037–5042.
- Liang G, Yang S, Jiang L, Zhao Y, Shao L, Xiao J et al. Synthesis and anti-bacterial properties of mono-carbonyl analogues of curcumin. Chem Pharm Bull 2008;56:162–167.
- Liang G, Li X, Chen L, Yang S, Wu X, Studer E et al. Synthesis and anti-inflammatory activities of mono-carbonyl analogues of curcumin. Bioorg Med Chem Lett 2008;18:1525–1529.
- Bandgar BP, Patil SA, Korbad BL, Nile SH, Khobragade CN. Synthesis and biological evaluation of β-chloro vinyl chalcones as inhibitors of TNF-α and IL-6 with antimicrobial activity. Eur J Med Chem 2010;45:2629–2633.
- Woo HB, Shin WS, Lee S, Ahn CM. Synthesis of novel curcumin mimics with asymmetrical units and their anti-angiogenic activity. Bioorg Med Chem Lett 2005;15:3782–3786.
- Sedlacek HH, Czech J, Naik R, Kaur G, Worland P, Losiewisc M, Parker B, Carlson B, Smith A, Senderowisc A, Sausville EA. Review of chemistry, pharmacology, and antitumor spectrum. Int J Oncol 1996;9:1143–1168.
- Murthi KK, Dubay M, McClure C, Brizuela L, Boisclair MD, Worland PJ et al. Structure-activity relationship studies of flavopiridol analogues. Bioorg Med Chem Lett 2000;10:1037–1041.
- Hampson LJ, Arden C, Agius L, Ganotidis M, Kosmopoulou MN, Tiraidis C et al. Bioactivity of glycogen phosphorylase inhibitors that bind to the purine nucleoside site. Bioorg Med Chem 2006;14:7835–7845.
- Naik RG, Kattige SL, Bhat SV, Alreja NJ, De Souza NJ, Rupp RH. An antiinflammatory cum immunomodulatory piperidinylbenzopyranone from dysoxylum binectariferum: Isolation, structure and total synthesis. Tetrahedron 1988;44:2081–2086.
- Thimmegowda NR, Nanjunda Swamy S, Kumar CS, Kumar YC, Chandrappa S, Yip GW et al. Synthesis, characterization and evaluation of benzimidazole derivative and its precursors as inhibitors of MDA-MB-231 human breast cancer cell proliferation. Bioorg Med Chem Lett 2008;18:432–435.
- Ziakas GN, Rekka EA, Gavalas AM, Eleftheriou PT, Kourounakis PN. New analogues of butylated hydroxytoluene as anti-inflammatory and antioxidant agents. Bioorg Med Chem 2006;14:5616–5624.
- Gaspar A, Garrido EM, Esteves M, Quezada E, Milhazes N, Garrido J et al. New insights into the antioxidant activity of hydroxycinnamic acids: Synthesis and physicochemical characterization of novel halogenated derivatives. Eur J Med Chem 2009;44:2092–2099.
- Bandgar BP, Patil SA, Gacche RN, Korbad BL, Hote BS, Kinkar SN et al. Synthesis and biological evaluation of nitrogen-containing chalcones as possible anti-inflammatory and antioxidant agents. Bioorg Med Chem Lett 2010;20:730–733.
- Khobragade CN, Bodade RG, Shinde MS, Jaju DR, Bhosle RB, Dawane BS. Microbial and xanthine dehydrogenase inhibitory activity of some flavones. J Enzyme Inhib Med Chem 2008;23:341–346.
- Dengler WA, Schulte J, Berger DP, Mertelsmann R, Fiebig HH. Development of a propidium iodide fluorescence assay for proliferation and cytotoxicity assays. Anticancer Drugs 1995;6:522–532.