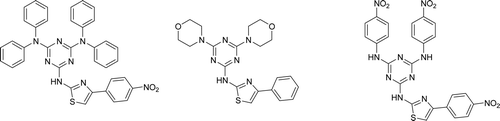
Abstract
Two novel series of hybrid class 4-chlorophenylthiazole-s-triazine were synthesized via nucleophilic substitution of 2,4,6-trichloro-1,3,5-triazine with distinguished alkenyl/alkyl/aryl/hetero alkyl–aryl amino and mercapto nucleophiles under nitrogen atmosphere. We identified that the spectrums of antibacterial activity of all tested compounds reveal promising and significant inhibition of gram-positive and gram-negative micro-organisms and the most active compounds, 31d and 32d, were found to be non-toxic in preliminary cytotoxicity assay. We also report that the Molinspiration and Osiris Property Explorer calculations have found a new lead 32d, which binds preferentially to the nuclear receptor to exhibit antibacterial potency.
Introduction
Subsequent development of antibiotics, or agents derived from them, has produced impressive reduction in the burden of disease imposed by bacterial infections. In the United States alone, the expenditure for bacterial and fungal diseases is more than $20 billion annuallyCitation1,Citation2. A summary report from Italian Ministry of research suggests that no single technology will represent the magic bullet for antibiotic discovery, but only the painstaking integration of a multidisciplinary team with profound knowledge of microbiology, chemistry and bioinformatics will ultimately lead to new antibacterial agents of medical relevance and commercial successCitation3. Indeed, World Health Organization and many experts also recommend that this area of research benefits tremendously through enhancing the efforts in the lead generation and optimization to develop new, cost-effective and innovative entities (http://www.idsociety.org/Content.aspx?id=5558)Citation4.
1,3,5-Triazine scaffold provides the basis for the design of biologically relevant molecules with widespread application in therapeutics. For example, these versatile compounds possess potent antimicrobial activities as antimalarialCitation5,Citation6, antiprotozoalCitation7, anticancerCitation8,Citation9, antiviralCitation10, antibacterial agentCitation11,Citation12 etc. In continuation of our endeavour programme in the area of s-triazines, particularly hybrid phenylthiazolyl-s-triazine derivatives (), two series A (1a–9a) and B (10b–18b) of s-triazines as novel antibacterial agents, and compounds with phenyl group showing potent antibacterial activity has been already reportedCitation13–15.
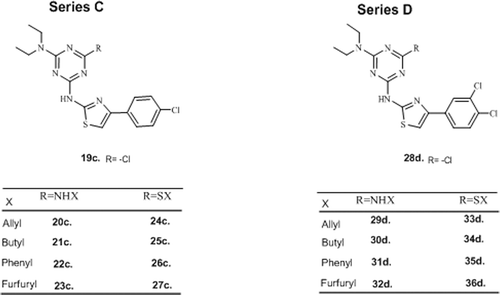
Bearing in mind the most popular hypotheses in the field of medicinal chemistry, the appropriate attachment of bulk groups (bulky flanking groups) to new bioactive chemical entities, such as halogen atoms, could lead to volumetric and conformational changes. More conspicuously, the possibility to establish halogen bonds or polar interactions may increase protein–ligand stability and subsequently, contribute to the binding affinity that will enable more selective drug candidates to be discoveredCitation16. Encouraged by these observations and in order to explore the scope of antibacterial potential of hybrid phenylthiazolyl-s-triazines in detail, here we incorporate 4-chloro and 3,4-dichloro to design a new class of diethylamino-chloro (series C) and diethylamino-dichloro (series D) phenylthiazolyl-s-triazine derivatives ().
Methods
All the chemicals used in the synthesis were of analytical grade and used without further purification. Melting points (m.p.) were determined on a Veego, model no. MPI open capillary melting point apparatus and boiling points (b.p.) were measured by 10–12-mm diameter test tube–inverted capillary tube and both were uncorrected. The completion of reaction was checked by thin layer chromatography using silica gel-GF254 (0.5-mm thickness, benzene, ethyl acetate and 0.1 M alcoholic KOH solvent system) and spots were visualized under ultraviolet radiation. The compounds reported in this study have been thoroughly characterized by their spectral data and elemental analysis ( and ). Fourier transform infrared readings (in KBr) were recorded on Perkin Elmer-Spectrum RX-I spectrophotometer. 1H NMR spectra were recorded on Bruker Avance II 400 NMR spectrometer in dimethyl sulfoxide (DMSO)-d6 using trimethylsilane as an internal standard (chemical shift in δ, ppm). Elemental analysis was carried out on a Vario EL III elemental analyzer.
Table 1. Some physicochemical properties and spectral data of diethylamino-chlorophenylthiazolyl-s-triazine derivatives (series C)
Table 2 . Some physicochemical properties and spectral data of diethyl amino dichlorophenylthiazolyl-s-triazine derivatives (series D).
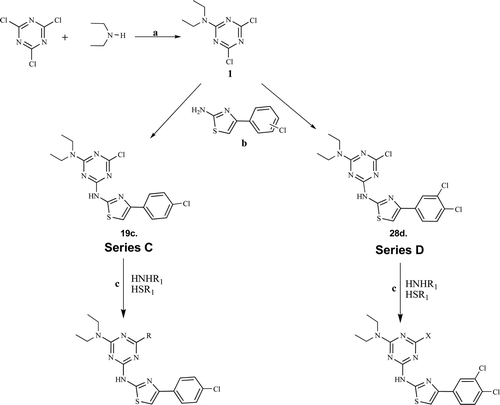
General procedure for synthesis of hybrid diethylamino chlorophenylthiazolyl-s-triazine derivatives, series C (19c–27c) and D (28d–36d)
As outlined in Scheme 1, 2-diethylamino-4,6-dichloro-1,3,5-triazine (α) was prepared as per published procedure using cyanuric chloride and diethyl amine17. The equimolar quantities of α (1 mol) and synthesized (4′-chlorophenyl)thiazolyl-2-amine (1 mol) mixture was heated to 40–45°C under aqueous NaHCO3–dioxane for 10 min with constant stirring, filtered immediately under vacuum to furnish (4′-chlorophenyl)thiazolyl-s-triazine (19c). Whereas (3′,4′-dichlorophenyl)thiazolyl-s-triazine (28d) was prepared under similar conditions using (3′,4′-dichlorophenyl)thiazolyl-2-amine (1 mol). These precursors (19c and 28d) were further intended for preparation of final compounds− 0.1 mmol each of them was added to various alkenyl/alkyl/aryl/hetero alkyl–aryl amines (0.1 mmol) and similar thiols (0.1 mmol) under nitrogen atmosphere in basic NaHCO3 (0.1 mmol) and 100 ml dioxane at 120°C for 6 h. The products were filtered, washed with water and purified by flash column chromatography (silica gel mesh size 100–200) using methanol/ethyl acetate (1:20 v/v) as eluent to yield pure diethylamino-chlorophenylthiazolyl-s-triazine derivatives (20c–27c) and diethyl amino dichlorophenylthiazolyl-s-triazine derivatives (29d–36d).
Antimicrobial assay
Finally, the purified products were tested for their minimum inhibitory concentration (MIC) against three representatives each from gram-positive viz. Lactobacillus casei (NCIM-2651), Bacillus cereus (NCIM-2458), Staphylococcus aureus (NCIM-2120) and gram-negative viz. Salmonella typhimurium (NCIM-2501), Escherichia coli (NCIM-2065), Klebsiella aerogenes (NCIM-2098) organisms by broth dilution methodCitation18. Penicillin G and streptomycin were used as reference antibacterial agents (). Solution of the test compounds and reference drugs were dissolved in DMSO. Nutrient broth (M002) was procured from Hi-Media Laboratories (Mumbai, India). The test compounds were dissolved in DMSO (200 μg/ml) and were serially diluted from 1 to 7 to obtain gradual dilution starting from 100, 50, 25, 12.5, 6.25 and 3.125 μg/ml. The inocula of test bacteria was adjusted to a volume of 0.5–2.5 × 105 colony-forming units/ml by matching with the turbidity of 0.5 McFarland reagent. A volume of 1 ml of the standardized broth culture was added to 1 ml of each serially diluted test tube. The capped tubes with cotton plugs were incubated at 35 ± 2°C for 20 h and compared with standardized 0.5 McFarland reagent.
Table 3 . MIC values of series C and D against gram-positive/negative bacteria.
Cytotoxicity test
To increase the chances for clinical success, assessment of toxicological studies on chemically diverse series is a necessary step in the drug discovery pathway. Nevertheless, the low therapeutic index of compounds, due to their general toxicity, may once more prevent their development as a drug. Consequently, it remains attractive to develop new derivatives possessing high efficacy and potency and higher therapeutic indices. We have reviewed our attention on the results of antibacterial screening as well as lead analogues 31d and 32d executed against brine shrimp assay that eventually led to preliminary assessment of their toxicity. Cytotoxicity test was carried out using brine shrimp assayCitation19 for the most active compounds 31d and 32d (). Samples were prepared by dissolving in DMSO to obtain a stock concentration of 1000 μg/ml, which was serially diluted six times to obtain a concentration of 15.63 μg/ml. Each concentration was tested for cytotoxicity in triplicates, using DMSO as control. Brine shrimp eggs (Artemia salina) were hatched in a hatching chamber filled with fresh sea water. Ten larvae of brine shrimps were transferred to each sample test tube using disposable pipettes. Survivors were counted after 24 h and the mortality at each concentration was determined.
Table 4 . Brine shrimp cytotoxicity assay for compounds 31d and 32d.
Molecular properties’ calculations
A viable drug candidate should have bioavailability, metabolic stability, toxicity and transport properties comparable to known drugs. These “drug-like” properties depend on the compound’s size, molecular flexibility, hydrophobicity and electronic bond distributions. The advanced computational design has sped up research by identifying new molecules with possible medical applications prior to laborious experiments and expensive preclinical studiesCitation20,Citation21. The intuitive and user-friendly open-access Molinspiration and Osiris Property Explorer programmes were used in this study to yield a matrix for each property.
Molinspiration calculations
Molinspiration methodology offers calculation of important molecular properties like logP (milogP), topological polar surface area (TPSA), number of hydrogen bond acceptors and donors, bioactivity score and possible molecular toxicity toolkit written in Java-based system. The statistical parameters rank milogP as one of the best methods available for logP prediction, whereas TPSA is also calculated based on the methodology as a sum of fragment contributions (http://www.molinspiration.com/cgi-bin)Citation22,Citation23. Method for the calculation of molecular volume developed at Molinspiration is based on group contributions. These have been obtained by fitting the sum of O- and N-centred polar fragment contributions to real three-dimensional (3D) volume for a training set of about 12,000, mostly drug-like molecules. The 3D molecular geometries for a training set were fully optimized by the semi-empirical AM1 method. In our studies, molecular properties of a total of 18 compounds from series C and D were valued, and the results were compared with the values obtained for standard drugs penicillin G ().
Table 5 . Molinspiration calculations of compounds.
Osiris calculations
Inhibition of one of the very important class of enzymes like Cytochrome P450 or production of unwanted metabolites might induce a variety of adverse drug reactions. The second stage of the process with online Osiris programme invokes the prediction of ADME-Tox liabilities as well as score of any entities with increasing success by using a combined electronic/structure docking procedure. According to the recent work on the drug design using Osiris-based calculation, it is now possible to predict activity and/or inhibition with increasing success in drug targets. The important features like solubility, toxicity and drug likeness can be predicted through these studies (http://www.organic-chemistry.org/prog/peo/). The outcome from these studies is successfully drawn in comparison to penicillin G ().
Table 6 . Osiris calculations of compounds.
Results
Our present study was undertaken to synthesize some novel phenylthiazolyl-s-triazine derivatives by nucleophilic substitution of cyanuric chloride (2,4,6-trichloro-1,3,5-triazine) with various amino and mercapto nucleophiles in basic dioxane medium under neat nitrogen atmosphere with simple standard work up procedures in good yields (). The first substitution is exothermic and, therefore, the temperature of the reaction mixture has to be maintained at 0°C. The substitution of the second chloride can be performed at room temperature. Finally, the third position is functionalized under reflux of the solvent. As a result, a careful control of the temperature during the substitution reactions will allow the synthesis of 2,4,6-trisubstituted-triazines by sequential and very selective addition of amines or thiols. The first substitution of chlorine in cyanuric chloride was carried out with diethyl amine at 0°C in aqueous dioxane medium followed by simple re-crystallization to facilitate isolation of pure product 1, in 81% yieldCitation17. The second substitution in 1 was carried out by 4-(4′-chloro)phenylthiazolyl-2-amine and 4-(3′,4′-dichloro)phenylthiazolyl-2-amine, respectively, at room temperature to afford 19c and 28d in good yields. These compounds were further reacted with various amino and mercapto nucleophiles by refluxing at 120°C under basic nitrogen dioxane environment to furnish targeted series C and D. The FTIR spectra of all C=N group vibrations were in the range of 1644–1682 cm−1. Absorption of−NH was confirmed in a broad range of 3287–3320 cm−1. The nuclear magnetic resonance spectra of the signals corresponding to−CH3 group appeared at 0.83–1.36 ppm. All compounds gave correct elemental analysis within ±0.4% of their respective theoretical values.
The synthesized phenylthiazolyl-s-triazine derivatives have been found to have significantly comparable antibacterial activity with that of standard antibiotic used. The heterocyclic entity 32d exhibited equipotent activity towards gram-positive (L. casei and S. aureus) organisms and 31d has marked equipotent activity against B. cereus when compared with standard drug penicillin G. The title compounds 23c, 26c, 30d and 34d have also shown equipotent activity against S. typhimurium when compared with penicillin whereas 22c, 29d, 31d and 32d showed more potent activity than penicillin. Moreover, in case of L. casei, compounds 22c, 23c and 31d were found to be more potent, on comparing with the standard drug streptomycin. The compound 32d was found to be more potent against B. cereus with the same reference standard streptomycin, as well as both 31d and 32d were found to be more potent than streptomycin in case of gram-positive S. aureus. The distinguished equipotent activities are also reported against different gram-positive bacteria by 23c, 24c, 26c, 28d, 29d and 30d when compared with the same antibiotic streptomycin. Clearly, in order to reach pocket, the title compounds 31d and 32d displayed the most interesting antibacterial activities against all gram-positive/negative bacteria used. However, it is particularly noteworthy that even though its significance is not clear, the novel class of these compounds have displayed no marked activity in case of K. aerogenes. Rest of compounds exhibited very weak to moderate antibacterial activities.
Discussion
Structure–activity relationships
The structure–activity relationship (SAR) study of the lead compound clearly indicates the presence of furfuryl-amino substitution in 23c and 32d as a core bioactive group against gram-positive micro-organisms. The supreme biological activity appears when the substituent is a phenyl-substituted amino or thiol derivative. Substitution of phenyl-amino group in 22c at fifth position on phenylthiazolyl-s-triazine enhanced the activity as compared to other s-triazines. In continuation, substitution with furfuryl-amino group in 23c also enhanced activity against B. cereus and S. aureus, respectively, whereas no prominent effect was observed against E. coli and K. aerogenes. Introduction of thioallyl group in 24c gives equipotent compound like streptomycin against S. aureus. There was further decrease in antibacterial activity if furfuryl-amino group was replaced by thiobutyl in 25c, thiophenyl in 26c and thiofurfuryl in 27c groups. By inserting allyl-amino group in 29d, which was equipotent with streptomycin against S. aureus, the effect of muzzling amino phenyl group would seem to predominate, except in the case of 35d. The free phenyl and furfuryl groups enhance the activity whereas free allyl and butyl groups interfere with the biological activity in the phenylthiazolyl-s-triazine. Perhaps the most intriguing starting point for a molecule upon which to build selectivity is the allyl type side chain as in case of 31d and 32d; the most intuitive efficacy was observed in compounds with this common substituted side chain. However, interestingly in allyl substitution all alone no remarkable evidence is reported and overall 3,4-dichlorophenylthiazolyl-s-triazine derivatives (series D) were found to be more active than their 4-chlorosubstituted (series C). As different alkenyl/alkyl/aryl/hetero alkyl–aryl substitution patterns with delocalized electronic structure were introduced in the s-triazine ring and hereby, a great variance in biological efficacy is predominant as evidenced in . For instance, in its infancy rest of the compounds demonstrated moderate-to-weak antibacterial efficacy against all gram positive/negative bacteria.
Cytotoxicity brine shrimp assay
In continuation of this study to explore preliminary therapeutic potential, the lead compounds 31d and 32d were further analyzed for toxicity results in terms of LC50 values, which were obtained from the best-fit line by plotting concentration verses percentage lethality. From the data in , it should be easy to see that brine shrimp assay for the most active compounds 31d and 32d postulated the non-toxicity of these compounds. Although in its infancy, molecular similarity matrices seem to have a lot to offer in the optimization of molecular structures for antibacterial effect and currently there is a great deal of research on these compounds for use as a medicinal agent in near future.
Molinspiration description
Molinspiration clearly depicts the calculated values of logP greater than 5, which is the lowest limit for the drugs to be able to penetrate through bio-membranes according to Lipinski’s rules. However, the lowest degree of lipophilicity among all the compounds was exhibited by compounds 19c, 20c and 23c, which is an indication for good water solubility (see supplemental file for details). The polar surface area (PSA) is calculated from the surface areas that are occupied by oxygen and nitrogen atoms and by hydrogen atoms attached to them. Molecules with PSA values of 140 or more are expected to exhibit poor intestinal absorption. The results state that all the compounds are having optimal TPSA values among which the highest degree of TPSA was achieved in the case of 23c and 32d. These identified entities can be characterized as permissible candidates for active drug absorption. All the compounds, excluding 35d and 36d have only one violation of the rule 5.
For the development of novel agent from hybrid chlorophenylthiazolyl-1,3,5-triazine, the identification of the presence of active structures is important, which can be drawn by sound knowledge of their physicochemical properties. Correlation results showed no single physicochemical parameter that triggers the biological activity of these phenylthiazolyl-s-triazines. For instance, the relationship between the average MIC (y) and most influencing physicochemical property – volume (x) – a simple regression was performed and a weak linear correlation was established as expressed by straight line Equation 1 ().
Figure 3. Straight-line correlation between average MIC and volume. MIC, minimum inhibitory concentration.
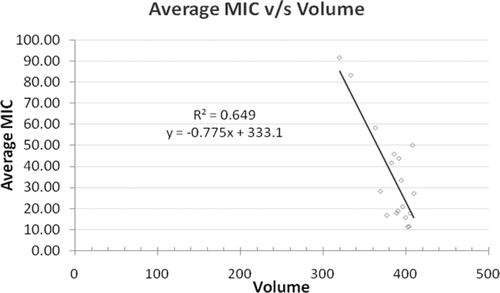
From this hallmark of MIC and volume, as well as bearing the potential of 3,4-dichlorosubstitution over 4-chlorosubstitution from earlier studies, it can be assumed that only optimized volume of chlorophenylthiazolyl-1,3,5-triazine is a general amplicon for biological activity. In this sequence, slight low correlation of natoms (r2 = 0.610) and comparatively weak for molecular weight (r2 = 0.389) was also observed. However, no correlation yet exists for logP (r2 = 0.208) in our studies, as this parameter has never been noteworthy for the most antimicrobial activities. For a standard drug penicillin G, the predicted set of simple molecular descriptors viz. logP (1.725), molecular weight (320.37), number of hydrogen bond acceptors (6), and number of hydrogen bond donors (2) were also found in agreement with the Lipinski rule of 5. The obtained set of molecular descriptors excluding logP, for novel compounds 31d and 32d by the same Molinspiration methodology, also support the Lipinski rule from such studies ().
The putative mechanism of this observed antibacterial action is brought about by predicted bioactivity for the most active compounds 31d and 32d. An understanding of the concepts discussed, preferred mechanism to underpin all this work is the availability of nuclear receptors. A major aspect of the utilization of this information will be the provision of these small molecules, which will recognize selected sequences, perhaps with the goal of switching off particular nuclear receptor as in bacterial chemotherapy. For some time, these identified entities have been known to bind preferentially to nuclear receptor to produce possible antibacterial action through the molecular mechanisms in terms of binding score of active compounds 31d (0.93) and 32d (1.47).
Osiris description
As a consequence of this analogue system, the compounds have been subjected to toxicity, drug likeness and score to model the possible therapeutic efficacy of compounds (). The present results also support the previous observations remarkably with well-behaved drug-likeness data to quantify the role played by various organic groups in promoting or interfering with the way a drug can associate to mimic the antibacterial efficacy. Activity of all test compounds and standard drug, penicillin G, were rigorously analyzed under four criteria of toxicological activities in areas of mutagenicity, tumorigenicity, irritation and reproductive action.
As seen in , it is also a hallmark in our studies that substitution of chlorine directly from triazine with distinguished alkenyl/alkyl/aryl/hetero alkyl–aryl amines or mercaptans and on other hand, clubbing of chlorine direct to phenylthiazole tends to drug-conform behaviour. In fact, chlorine is a moderate halogen-bond acceptor, the forming C–Cl bond is enough stable and has good binding affinity. Replacement of hydrogen with chlorine can provide a substantial alteration on the volumetric and shape issues. It was also described that subunits bearing chlorine can be accommodated in tight and deep cavities, as well as in hydrophobic pockets of the biological targetsCitation24. In the light of these features, 3,4-dichlorophenylthiazole may be best accommodated on the active site of targets to produce desired antibacterial action and justify their predominance over 4-chlorophenylthiazole. However, direct attachment of chlorine to triazine renders drug-likeness behaviour possibly through comparatively low logP value and supposed to be impermeable to biological membranes to exert pharmacological action. Therefore, successful attempt can be accomplished by replacement of third chloro group from 19c and 28d with these amines or thiols to generate series C and D. The efficacy of the given set of molecules in terms of threshold drug-likeness behaviour was predicted for 23c (4.13), 27c (4.12) and 32d (3.53), in contrast to the relative activity score of the standard drug penicillin (11.28). By analyzing, we can see that except in case of 29d (0.22), dichloro substitution to phenylthiazole conforms high drug behaviour over mono substitution. As predicted by this modelling, the best drug-conform behaviour in terms of drug score and drug likeness was also profound for the same 32d (0.22), in comparison to penicillin.
In order to reach clinical settings, drug has to cross various trials, where the odds of success are ever lower. For instance, plenty of new and novel candidates are being discovered each and every year; however, only six new antibiotics have been approved since 2003 by the US Food and Drug Administration (http://www.fda.gov/cder/approval/index.htm)Citation25. More advanced targeted drug delivery systems are nowadays available. In the light of this, lead identification is an essential exercise in the field of medicinal chemistry (http://www.nanomed.gazi.edu.tr)Citation26,Citation27. Nevertheless, the outcome of our wet-lab studies is in closer fitting with Molinspiration and Osiris property calculations. From all the studies performed, the compound 32d shows good inhibition against gram positive/negative bacteria and possess good characteristic as novel agent. Further, systematic modification studies around this lead can produce antibiotics with increased potency and spectrum in near future.
Conclusion
In summary, the preliminary in vitro antibacterial screening reported here indicates the antimicrobial potency of these novel synthesized compounds 19c–27c and 28d–36d against reference standard (penicillin G and streptomycin). The SAR observation has shown the insertion of furfuryl-amino or phenyl-amino group in mono-chloro-3,4-dichlorophenylthiazolyl-s-triazine (28d) to have more antibacterial potency than their allyl-amino, butyl-amino or any mercapto 3,4-dichlorophenylthiazolyl-s-triazine counterpart. The most effective compounds 31d and 32d were also found to be non-toxic in the brine shrimp lethality assay. The present studies also enable the execution of important physicochemical parameters like natoms, molecular weight and volume to reassemble compounds enriched in antibacterial activity. The above-mentioned antibacterial screening and the facts drawn allow us to find the new lead 32d, which may bind preferentially to nuclear receptor to produce possible antibacterial action. To elucidate the robustness, lead optimization work in chemotherapy is ongoing and will be reported subsequently.
Acknowledgements
The authors acknowledged the valuable contributions made to their work by Lonza, Switzerland for arranging a gift sample of cyanuric chloride for the work included in this paper and critical reading and supports from Archana Uppal, SGRRITS, Dehradun for structuring the manuscript. The authors are also thankful to an anonymous reviewer for carefully reading the manuscript and for his critical comments and valuable suggestions.
Declaration of interest
The authors declared no conflict of interest.
References
- News and analysis. Antibacterial and antifungal drug discovery challenge. Nature Biotechnology 2000;18:IT24–IT26.
- Cassell GH. Emergent antibiotic resistance: health risks and economic impact. Fems Immunol Med Microbiol 1997;18:271–274.
- Donadio S, Maffioli S, Monciardini P, Sosio M, Jabes D. Antibiotic discovery in the twenty-first century: current trends and future perspectives. J Antibiot 2010;63:423–430.
- Critically important antibacterial agents for human medicine for risk management strategies of non-human use, a report of a WHO working group consultation. Canberra, Australia: February, 2005, pp. 15–18.
- Melato S, Prosperi D, Coghi P, Basilico B, Monti D. A combinatorial approach to 2,4,6-trisubstituted triazines with potent antimalarial activity: combining conventional synthesis and microwave-assistance. Chem Med Chem 2008;3:873–876.
- Agarwal A, Srivastava K, Puri SK, Chauhan PMS. Syntheses of 2,4,6-trisubstituted triazines as antimalarial agents. Bioorg Med Chem Lett 2005;15:531–533.
- Baliani A, Bueno GJ, Stewart ML, Yardley V, Brun R, Barrett MP et al. Design and synthesis of a series of melamine-based nitroheterocycles with activity against Trypanosomatid parasites. J Med Chem 2005;48:5570–5579.
- Garaj V, Puccetti L, Fasolis G, Winum JY, Montero JL, Scozzafava A, Vullo D, Innocenti A, Supuran CT. Carbonic anhydrase inhibitors: novel sulfonamides incorporating 1,3,5-triazine moieties as inhibitors of the cytosolic and tumour-associated carbonic anhydrase isozymes I, II and IX. Bioorg Med Chem Lett 2005;15:3102–3108.
- Carta F, Garaj V, Maresca A, Wagner J, Avvaru B, Robbins A, Scozzafava A, McKenna R, Supuran CT. Sulfonamides incorporating 1,3,5-triazine moieties selectively and potently inhibit carbonic anhydrase transmembrane isoforms IX, XII and XIV over cytosolic isoforms I and II: solution and X-ray crystallographic studies. Bioorg Med Chem Lett. 2011;19:3105–3119.
- Xiong YZ, Chen FE, Balzarini J, De Clercq E, Pannecouque C. Non-nucleoside HIV-1 reverse transcriptase inhibitors. Part 11: structural modulations of diaryltriazines with potent anti-HIV activity. Eur J Med Chem 2008;43:1230–1236.
- Zhou C, Min J, Liu Z, Young Z, Deshazer H, Gao T, Chang YT, Kallenbach R. Synthesis and biological evaluation of novel 1,3,5-triazine derivatives as antimicrobial agents. Bioorg Med Chem Lett 2008;18:1308–1311.
- Srinivas K, Srinivas U, Bhanuprakash K, Harakishore K, Murthy USN, Rao VJ. Synthesis and antibacterial activity of various substituted triazines. Eur J Med Chem 2006;41:1240–1246.
- Singh UP, Singh RK, Bhat HR, Subhaschandra YP, Kumar V, Kumawat MK, Gahtori P. Synthesis and antibacterial evaluation of series of novel tri-substituted-s-triazine derivatives. Med Chem Res 2010.
- Gahtori P, Das A, Bhatt H. Synthesis and antibacterial assessment of N-[4-(4-substituted phenyl)-1,3-thiazol-2-yl]-1,3,5-triazin-2-amine. Indian J Pharm Sci 2009;71:79–82.
- Gahtori P, Singh A, Ghosh SK, Das A, Archana U. Synthesis of some substituted phenylthiazolyl 1,3,5-triazine derivatives. Asian J Chem 2010;23:1189–1192.
- Hernandes MZ, Cavalcanti SM, Moreira DR, de Azevedo Junior WF, Leite AC. Halogen atoms in the modern medicinal chemistry: hints for the drug design. Curr Drug Targets 2010;11:303–314.
- Thruston JT, Dudley JR, Kaiser DW, Henbleikner I, Schaefer FC, Holm-Hensen D. Cyanuric chloride derivatives. I. Aminochloro-s-triazines. J Am Chem Soc 1951;73:2981–2983.
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). EUCAST Definitive Document E.DEF 2.1, August 2000: Determination of antimicrobial susceptibility test breakpoints. Clin Microbiol Infect 2000;6:509–515.
- Chaudhary MI, Thomsen WJ. Bioassay techniques for drug development. Harwood Academic Publishers, 2001, pp. 9.
- Richards WG. Computer-aided drug design. Pure Appl Chem 1994;66:1589–1596.
- Dalafave DS. Design of druglike small molecules for possible inhibition of antiapoptotic BCL-2, BCL-W, and BFL-1 proteins. Biomed Eng Comput Biol 2010;2:11–21.
- Ertl P, Rohde B, Selzer P. Fast calculation of molecular polar surface area (PSA) as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J Med Chem 2000;43:3714–3717.
- Mannhold R, Poda GI, Ostermann C, Tetko IV. Calculation of molecular lipophilicity: State-of-the-art and comparison of log P methods on more than 96,000 compounds. J Pharm Sci 2009;98:861–893.
- Siegal G, Ab E, Schultz J. Integration of fragment screening and library design. Drug Discov Today 2007;12:1032–1039.
- Fox JL. The business of developing antibacterials. Nat Biotechnol 2006;24:1521–1528.
- Kim S, Lee J. Folate-targeted drug-delivery systems prepared by nano-comminution. Drug Dev Ind Pharm 2011;37:131–138.
- Lombardino JG, Lowe JA. A guide to drug discovery: The role of the medicinal chemist in drug discovery—then and now. Nat Rev Drug Discovery 2004;3:853–862.