Abstract
The new class of hybrid anticancer drugs were obtained by selective functionalization of the triazine scaffold. These were prepared by rearrangement of mono-, bis- and/or tris-(1,3,5-triazin-2-yl)-1,4-diazabicyclo[2.2.2]octanium chlorides leading to formation of 2-chloroethylamino fragments attached to 1,3,5-triazine via one, two or three piperazine rings respectively. Their inhibitory effect was found strongly dependent on the structure of substituents in triazine ring. The anti-proliferative activity of the hybrids evaluated in vitro by using mammalian tumour cells estimated as IC50 was in the range 0.62–139,78 µM. Both cytotoxicity and alkylating activity depended on the substituents of triazine ring, however, also the mono-functional analogues of nitrogen mustards, which are unable to form liaisons between two DNA strands, induced apoptosis and necrosis in the tested cells.
Introduction
The recent therapeutic approach in which drug candidates are designed to possess diverse pharmacological properties and act on multiple targets has stimulated development of the hybrid drugs. Such dual action drugs with two dissimilar drug molecules combined together by direct linking of the two molecular entities are already known and found to be useful tools in the therapy of complex diseases such as cardiovascular and inflammatory diseases and might be used as antibacterial agents useful in the treatment of drug resistant pathogensCitation1.
In several cases the collected data provide a conclusive molecular mechanism for the independent modes of action of both drug-like fragments, amplification of effects of fragments or even the extraordinary biological effects not attributed to any of the individual partner of the hybrid construct that make the emergence of drug resistance less likelyCitation2. Since cancer is a genetic disease with several mutations altering the replication system of the cell, the strategy based on the construction of hybrid anticancer drugs possessing different toxicity profiles seems to be very promising.
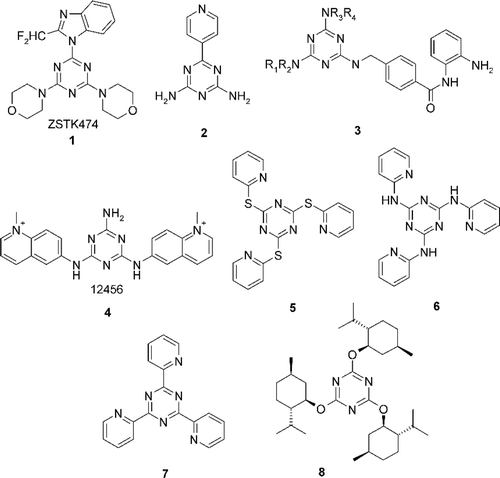
In the last decade, several new highly potent cytostatic agents, derived from 1,3,5-triazine, have been presented that are active as inhibitors of enzymes involved in the cell proliferation cycle (see ).
A melamine derivative, ZSTK474 [2-(2-difluoromethylbenzimidazol-1-yl)-4,6-dimorpholino-1,3,5-triazine] (1) has been found, which inhibits the growth of tumour cells with its molecular target identified as phosphatidylinositol 3-kinases (PI3K) with strong antitumour activity against human cancer xenografts without toxic effects on critical organsCitation3. For melamine 2 it has been suggested that its antimetastatic and antitumour activity is due in part to inhibition of angiogenesis, rather than direct anti-proliferative action on tumour cellsCitation4.
Melamine derivatives 3 were also found to be some of the best histone deacetylase inhibitors with anti-proliferative activities with IC50 values below the micromolar range. Some of these compounds can also significantly reduce tumour growth in human tumour xenograft models in mice. In general, derivatives of the triazine series show better profiles than their pyridine, pyrimidine, and purine analogues and moreover, these compounds are selective to the cancer cell line tested (HCT116) with a toxicity index that in some cases is over 100-times higher than that of a normal cell line (HMECCitation5). Melamine derivative 4 were also found to be the potent G-quadruplex ligand, which is shown to induce both telomere shortening and apoptosis in the human A549 cell line as a function of its concentration and time exposure. Several other melamine analogues were also considered as potential antitumour agents that block telomere replication stabilizing the telomeric G-rich single stranded DNA overhang into G-quadruplex and consistently inducing telomere erosion, which results in senescence after prolonged contact. They are also able to induce apoptotic or non-apoptotic cell death, alteration of cell cycle progression, and depression of telomerase activityCitation6.
A series of thiopyridine 5 and aminopyridine 6 substituted triazine derivatives has been found inducing G2/M arrest and apoptosis with a possible involvement of p53Citation7. Triazine 7 has been found to be efficient topoisomeraze inhibitorsCitation8 and terpene substituted triazine 8 is active as a cytostatic, but with an unknown molecular targetCitation9. Additionally, as a selective inhibitor, 1,3,5-triazine based molecules have been found to act on many different targets which include: HIV-1 reverse transcriptaseCitation10, estrogen receptor betaCitation11, glutathione S-transferaseCitation12, M. tuberculosis dihydrofolate reductaseCitation13, photosynthetic reaction centreCitation14, guanosine-5´-triphosphate binding siteCitation15, ATP competitive inhibitor of mammalian target of rapamycinCitation16 and urate oxidaseCitation17. Considering enzyme inhibition as an intrinsic property of the complete melamine structure, still remains vast potential of decoration of triazine scaffold by introducing “address” fragment increasing selectivity, components facilitating transport through the cell membranes, and last but not least, components of classic anticancer drugs with well-documented therapeutic competence. Therefore recent approaches involve development of hybrid cancer drugsCitation18 or application of 1,3,5-triazine as inductor of new specific molecular targetsCitation19. In these studies it has been attempted to introduce into 1,3,5-triazine scaffold one, two or three fragments bearing 2-chloroethylamine moiety characteristic for nitrogen mustardsCitation20,Citation21 and to confirm anti-proliferative activity of the obtained hybrids.
Materials and methods
General Information
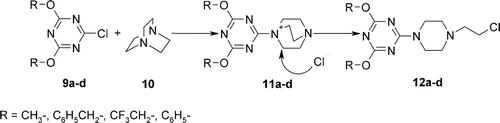
Thin layer chromatographies (TLC) were carried out on SiO2 (Merck; 60 Å F254) and spots located with: UV light (254 and 366 nm) and 1% ethanolic 4-(4´-nitrobenzyl)-pyridine (NBP). Melting points were determined on a Büchi apparatus, model 510. IR spectra were recorded as KBr pellets or film on a Infracord 137 E spectrometer. 1H-NMR, 13C-NMR, spectra were recorded on a Bruker Avance DPX 250 (250 MHz) spectrometer. Chemical shifts (ppm) are relative to TMS used as an internal standard. The multiplicity were marked as s = singlet, d = dublet, t = triplet, q = quartet, qu = quintet, m = multiplet. Triazines 9a-d were obtained from cyanuric chloride according to standard procedure describedCitation22.
2,4-Bis-methoxy-6-[4-(2-chloroethyl)-piperazin-1-yl]-[1,3,5]triazine (12a).
General procedure: 1,4-Diazabicyclo[2.2.2]octane (10) (DABCO) (1.12 g, 10 mmol) was added to a vigorously stirred solution of 2-chloro-4,6-dimethoxy-1,3,5-triazine (9a) (1.76 g, 10 mmol) in dichloromethane (20 mL), cooled to 5°C. The mixture was stirred at 5°C for 0.5 h and then under reflux condition for 1 h. Progress of reaction was monitored by TLC (Rf = 0 for salt 11a, Rf = 0.15 for 12a, DCM, 1% solution of (NBP) for visualization of spots). The organic layer was concentrated under evaporated reduced pressure. 2,4-Bis-methoxy-6-[4-(2-chloroethyl)-piperazin-1-yl]-[1,3,5]triazine (12a) was obtained (2.81 g, yield 98%), m.p. = 88–90˚C.
1H-NMR (CDCl3): 2.55 (t, 4H, J = 5.2 Hz); 2.77 (t, 2H, J = 7.5 Hz); 3.61 (t, 2H, J = 7.5 Hz); 3.87 (t, 4H, J = 5.2 Hz); 3.95 (s, 6H). 13C-NMR (CDCl3): 40.58; 43.13; 52.50; 54.20; 55.60; 59.41; 166.23; 172.06. IR (film/NaCl): 2952; 2840; 2808; 1584; 1536; 1472; 1368; 1308; 1280; 1255; 1190; 1135; 1040; 990. Anal. Calcd for C11H18ClN5O2: C, 45.92; H, 6.31; N, 24.34. Found: C, 45.81; H, 6.33; N, 24.39.
2,4-Bis-benzyloxy-6-[4-(2-chloroethyl)-piperazin-1-yl]-[1,3,5]triazine (12b)
Starting materials: 9b (1.64 g, 5 mmol), DABCO (1.12 g, 5 mmol). Product: 2,4-bis-benzyloxy-6-[4-(2-chloroethyl)-piperazin-1-yl]-[1,3,5]triazine (12b) (2.09 g, 95%), oil.
1H-NMR (CDCl3): 2.54 (t, 4H, J = 5.0 Hz); 2.76 (t, 2H, J = 7.5 Hz); 3.61 (t, 2H, J = 7.5 Hz); 3.88 (t, 4H, J = 5.0 Hz); 5.38 (s, 4H); 7.26–7.45 (m, 10H). 13C-NMR (CDCl3): 40.60; 43.23; 52.53; 59.44; 68.80; 127.94; 128.06; 128.25; 135.97; 166.35; 171.61. IR (film/NaCl): 3050; 2960; 2815; 2255; 1830; 1695; 1580; 1525; 1495; 1445; 1420; 1345; 1305; 1270; 1155; 1105; 1040; 995. Anal. Calcd for C23H26ClN5O2: C, 62.79; H, 5.96; N, 15.92. Found: C, 62.29; H, 5.71; N, 15.98.
2-[4-(2-Chloroethyl)-piperazin-1-yl]-4,6-bis-(2,2,2-trifluoroethoxy)-[1,3,5]triazine (12c)
Starting materials: 2-chloro-4,6-bis-(2,2,2-trifluoroethoxy)-[1,3,5]triazine (9c) (1.56 g, 5 mmol), DABCO (1.12 g, 5 mmol). Product: 2-[4-(2-chloroethyl)-piperazin-1-yl]-4,6-bis-(2,2,2-trifluoroethoxy)-[1,3,5]triazine (12c) (2.03 g, 96%), oil.
1H-NMR (CDCl3): 2.67 (t, 4H, J = 5.5 Hz); 2.88 (t, 2H, J = 8.0 Hz); 3.66 (t, 2H, J = 8.0 Hz); 3.94 (t, 4H, J = 5.5 Hz); 4.75 (qw, 4H, J = 7.5 Hz). 13C-NMR (CDCl3): 36.31; 40.08; 43.15; 52.26; 59.13; 63.30; 165.95; 170.67. IR (film/NaCl): 2980; 2940; 2870; 2820; 2250; 1720; 1670; 1590; 1525; 1420; 1375; 1300; 1270; 1160; 1130; 1070; 990; 950. Anal. Calcd for C13H16ClF6N5O2: C, 36.85; H, 3.81; N, 16.53. Found: C, 36.71; H, 3.75; N, 16.42.
2-[4-(2-Chloroethyl)-piperazin-1-yl]-4,6-diphenoxy-[1,3,5]triazine (12d)
Starting materials: 2-chloro-4,6-diphenoxy-1,3,5-triazine (9d) (1.50 g, 5 mmol), DABCO (1.12 g, 5 mmol). Product: 2-[4-(2-chloroethyl)-piperazin-1-yl]-4,6-diphenoxy-[1,3,5]triazine (12d) (1.73 g, 84%), m.p. = 138–140˚C.
1H-NMR (CDCl3): 2.48 (t, 4H, J= 7.5 Hz); 2.73 (t, 2H, J = 5.0 Hz); 3.57 (t, 2H, J = 5.0 Hz); 3.72 (t, 4H, J = 7.5 Hz); 7.12–7.37 (m, 10H). 13C-NMR (CDCl3): 40.53; 43.20; 52.48; 59.39; 121.58; 125.90; 129.04; 151.92; 166.42; 172.13. IR (film/NaCl): 2970; 2920; 2805; 1740; 1670; 1595; 1575; 1530; 1490; 1445; 1390; 1375; 1310; 1280; 1260; 1210; 1160; 1125; 1070; 1020; 995. Anal. Calcd for C21H22ClN5O2: C, 61.24; H, 5.38; N, 17.00. Found: C, 61.04; H, 5.41; N, 16.95.
2,4-Bis-[4-(2-chloroethyl)-piperazin-1-yl]-6-methoxy-[1,3,5]triazine (12e)
Starting materials: 2,4-dichloro-6-methoxy-1,3,5-triazine (9e, DCMT) (0.90 g, 5 mmol), DABCO (2.24 g, 10 mmol). Product: 2,4-bis-[4-(2-chloroethyl)-piperazin-1-yl]-6-methoxy-[1,3,5]triazine (12e) (2.02 g, 80%), m.p. = 278–281˚C.
1H-NMR (CDCl3): 2.53 (t, 8H, J = 5.1 Hz); 2.76 (t, 4H, J = 8.0 Hz); 3.63 (t, 4H, J = 8.0 Hz); 3.84 (t, 8H, J = 5.1 Hz); 3.87 (s, 3H). 13C-NMR (CDCl3): 38.99; 41.23; 51.10; 53.01; 57.97; 160.68; 164.01; 169.66. IR (film/NaCl): 3000; 2950; 2860; 2810; 2770; 2230; 1675; 1590; 1580; 1525; 1490; 1380; 1350; 1300; 1245; 1190; 1150; 1120; 1090; 1045; 995. Anal. Calcd for C16H27Cl2N7O: C, 48.81; H, 6.99; N, 23.44. Found: C, 48.54; H, 6.75; N, 23.40.
2,4,6-Tris-[4-(2-chloroethyl)-piperazin-1-yl]-[1,3,5]triazine (12f)
Starting materials: cyanuric chloride (0.92 g, 5 mmol), DABCO (3.36 g, 15 mmol). Product: 2,4,6-Tris-[4-(2-chloroethyl)-piperazin-1-yl]-[1,3,5]triazine (12f) (2.11 g, 81%), oil.
1H-NMR (CDCl3): 2.35–2.95 (m, 22H); 3.59-3.77 (m, 14H). 13C-NMR (CDCl3): 40.47; 42.69; 52.57; 59.26; 164.69. IR (film/NaCl): 3020; 2930; 2820; 2350; 1725; 1670; 1575; 1540; 1525; 1475; 1440; 1375; 1310; 1260; 1220; 1180; 1140; 1090; 1010; 950. Anal. Calcd for C21H36Cl3N9: C, 49.39; H, 7.16; N, 23.56. Found: C, 49.31; H, 7.11; N, 23.49.
Cytotoxicity determined by SRB method
The following established in vitro human cancer cell lines were applied: SW707 (colorectal adenocarcinoma), Jurkat (leukemia), A549 (lung cancer), LNCaP (prostate cancer) and T47D (breast cancer). All lines were obtained from the American Type Culture Collection (Rockville, Maryland, USA) and maintained at the Cell Culture Collection of the Institute of Immunology and Experimental Therapy, Wroclaw, Poland.
Twenty-four hours before addition of the tested agents, the cells were plated in 96-well plates (Sarstedt, USA) at a density of 104 cells per well in 100 μL of culture medium. The cells were cultured in the opti-MEM medium supplemented with 2 mM glutamine (Gibco, Warsaw, Poland), streptomycin (50 μg/mL), penicillin (50 U/mL) (both antibiotics from Polfa, Tarchomin, Poland) and 5% fetal calf serum (Gibco, Grand Island, USA). The cell cultures were maintained at 37°C in humid atmosphere saturated with 5% CO2.
Solutions of compounds were prepared by dilution of 1 mg of 12a,b,f in DMSO (100 μL) and diluted subsequently to 1000 μL with culture media or 12c,e in DMSO (1000 mL). Compound 12f was sonicated for 1 minute to enhance dilution. Compounds were tested in final concentration of: 100, 10, 1 and 01 μg/mL for 12a,c,e or 10, 1, 0.1 and 0.01 μg/mL for 12b,d,f in culture media.
SRB assay
The details of this technique were described by SkehanCitation23. The cytotoxicity assay was performed after 72-hour exposure of the cultured cells to varying concentrations (0.1–100 μg/mL) of the tested agents. The cells attached to the plastic were fixed by gently layering cold 50% TCA (trichloroacetic acid, Aldrich-Chemie, Germany) on the top of the culture medium in each well. The plates were incubated at 4°C for 1 h and then washed five times with tap water. The background optical density was measured in the wells filled with culture medium, without the cells. The cellular material fixed with TCA was stained with 0.4% sulforhodamine B (SRB, Sigma, Germany) dissolved in 1% acetic acid (POCh, Gliwice, Poland) for 30 minutes. Unbound dye was removed by rinsing (4×) with 1% acetic acid. The protein-bound dye was extracted with 10 mM unbuffered Tris base (POCh, Gliwice, Poland) for determination of optical density (at 540 nm) in a computer-interfaced, 96-well microtiter plate reader Multiskan RC photometer (Labsystems, Helsinki, Finland). Each compound in given concentration was tested in triplicates in each experiment, which was repeated 3–5 times.
The results of cytotoxic activity in vitro were expressed as an IC50 (μg/mL), i.e. the concentration of compound, which inhibits the proliferation of 50% of tumour cells as compared to the control untreated cells.
MCF-7 cultures
Stock cultures of breast MCF-7 cancer cells (purchased from the American Type Culture Collection, Rockville, MD) were maintained in continuously exponential growth by weekly passage in Dulbecco’s Modified Eagle’s Medium (Sigma) supplemented with 10% FBS(Sigma), 50 μg/mL streptomycin, 100 U/mL penicillin at 37°C in a humid atmosphere containing 5% CO2. Cells were cultivated in Costar flasks and subconfluent detached with 0.05% trypsin and 0.02% EDTA in a calcium-free phosphate buffered saline. The study was carried out using cells from passages 3 to 7, growing as monolayer in 6-well plates (Nunc) (5 × 105 cells per well and preincubated 24 hours without phenol red.
Determination of apoptotic index and cell vialibility
The compounds were dissolved in sterile water and used at concentrations of 1, 10, 50, 100 and 150 μM. Microscopic observations of cell monolayers were performed with a Nikon optiphot microscope. Wright-Giemsa staining was performed using the Fisher Leuko Stat Kit. Adherent MCF-7 cells grown in 6-well plates were stained after induction of apoptosis with a dye mixture (10 μM acridine orange and 10 μM ethidium bromide, prepared in phosphate buffered saline). At the end of each experimental time point, all of the media was removed and cells were harvested by incubation with 0.05% trypsin and 0.02% EDTA for 1 min and washed with the medium. Then, 250 μL of cell suspension was mixed with 10 μL of the dye mix and 200 cells per sample were examined by fluorescence microscopy, according to the following criteria:
viable cells with normal nuclei (a fine reticular pattern stained green in the nucleus and red-orange granules in the cytoplasm);
viable cells with apoptotic nuclei (green chromatin which is highly condensed or fragmented and uniformly stained by the acridine orange);
non-viable cells with normal nuclei (bright orange chromatin with organised structure);
non-viable cells with apoptotic nuclei (bright orange chromatin witch is highly condensed or fragmented).
Antitumour activity investigated compounds expressed as percentage of non-viable MCF-7 (summarized both apoptotic and necrotic) mammal tumour cells was shown in .
Table 1. Inhibitory effects of 12a–f on the growth of human tumour cell lines expressed as IC50 in μg/mL.
Determination of alkylating properties (Preussmann test)
The tested compounds (0.01 mmol) were dissolved in 2-metoxyethylether (200 μL) and solution of NBP in 2-metoxyethylether (5%, 200 μL) were added. The samples were heated at 100 ± 0.5°C for 1 h and then quickly cooled to 20°C. 2-Metoxyethylether (500 μL) and piperidine (100 μL) were added to the samples to give a total volume of 1 mL. The final concentration of the tested compounds was 10 μM. After 90 s, the absorbance was measured at λ = 560 nm in a quartz cell (1 cm). 2-Metoxyethylether was used as a reference solvent.
Ethidium bromide (EtBr) assay
Each well of 96-well plate was loaded with Tris buffer containing ethidium bromide (0.1 M Tris, 1 M NaCl, pH 8, 0.5 mM EtBr final concentration, 100 μL). To each well was added 15 μg plasmid. pBR322 as water solution (0.05 μg/μL). Then, to each well was added chlorambucil (CHL) or compound 12a–f (1 μL of a 1 mM solution in water, 10 μM final concentrations). After incubation at 25°C for 30 min, the fluorescence of each well was read on a Multilabel Reader Victor 3V (ex.: 355 nm, em.: 615 nm) in duplicate experiments with two control wells (no drug = 100% fluorescence, no DNA = 0% fluorescence). Fluorescence readings are reported as % fluorescence relative to the controls.
Statistical analysis
In all experiments, the mean values for three independent assays ± standard deviations (S.D) were calculated. The results were submitted to statistical analysis using the Student’s test. Differences were considered significant when p < 0.05. Mean values, the standard deviations and the number of measurements in the group are presented in the figures.
Results and discussion
As exemplified above, the appropriate modification of 1,3,5-triazine ring system could lead to numerous novel and diverse target-specific inhibiors of enzymes. Thus, the diversity of action mode of triazine based inhibitors and presence of three reactive centres which could be used for the selective functionalization of the triazine scaffold have paved the way to a new class of hybrid anticancer drugs with a lowered toxicity and broader antitumour spectrumCitation24. Therefore, while designing the structure of new hybrid anticancer drugsCitation25 we attempted to attach one, two, or three chloroethylamine groups to surround triazine substituted respectively with one (), two or three piperazine rings ().
Scheme 2. Synthesis 1,3,5-triazine derivatives bearing two 2-chloroethylamino residues 12e and three 2-chloroethylamino residues 12f.
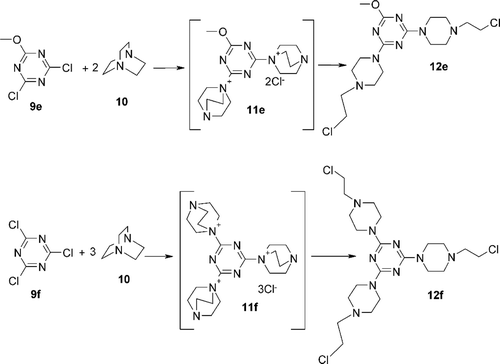
In order to prepare such constructs we made use of the observationCitation26,Citation27 that some N-triazinylammonium chlorides 11a-f easy accessible from a broad range of triazines 9a–d and 1,4-diazabicyclo[2.2.2]octane (DABCO) (10) rearranged with a formation of 12a–f bearing 2-chloroethylamino fragment. Most N-triazinylammonium salts 11 were obtained in almost quantitative yield.
The rearrangement of 11a–f to 12a–f proceeds at room temperature relatively slowly and is accelerated in non-aqueous media and at elevated temperatures.
The anti-proliferative activity of the hybrids 12a–f has been evaluated in vitro by using six tumour cell lines. The colorimetric tests were performed in 96-well culture plate and the activity of the cytotoxic drugs presented as IC50 values were determined after 72 h of drug exposures ().
Inhibitory effects of 12a–f was found strongly dependent on the structure of substituents in triazine ring. Unexpectedly, for five tumour cell lines the crosslinking ability, which obligatory required the presence of two or tri chloroethylamino group were found not crucial for activity. Inhibitory effects of 12b,d, bearing only single chloroethylamino group, expressed as IC50 in the range 0.62–3.45 μg/mL on the growth of human tumour cell lines has been found much stronger than bi- and trifunctional analogues 12e,f (IC50 in the range 4.50–6.22 μg/mL or totally negative). This suggests that cell proliferation may be inhibited by mono-alkylation involving chloroethylamino functionality or by the other mechanisms involving, unknown as yet, inhibitory effect of triazine structure.
In the further studies inhibition of proliferation, apoptotic index and cell vialibility was determined in vitro on breast MCF-7 cancer cells. The obtained results were compared with the anti-proliferative effect of chlorambucil and summarized in .
Table 2. Viability of MCF-7 cells treated for 24 h with different concentrations of chlorambucil (CHL) and compounds 12a–f.
All the compounds 12a–f showed cytotoxic and anti-proliferative effect. The concentration which inhibits 50% of colony formation was in the range 18.70–139.78 µM. It is worthy to notice that in the case MCF-7 cancer the activity increased with the amount of 2-chloroethylamino fragments. The analogue 12f with IC50 = 18.70 µM has three such fragments and is the most active against the investigated cells. It is more active than chlorambucil with IC50 = 29.14 µM, the therapeutics with alkylating properties belonging to the first class of cytostatics used for cancer therapyCitation28.
Our other aim was to determine the in vitro alkylating activity of the novel mustards 12a–f. For this purpose an in vitro Preussmann testCitation29 was used. All of the tested compounds demonstrate their alkylating activity toward NBP molecule. Alkylating activity results are presented in .
Table 3. Alkylating activity of compounds 12a–f on comparison to chlorambucil; NBP test results.
Only compound 12c has a low alkylating activity (+) and is less active than chlorambucil, the other ones are more active and can be included in the group of high (++) alkylating activity.
One can see a correlation between the alkylating activity of the tested compounds and their cytotoxicity on the breast cancer MCF-7 cell line. Both cytotoxicity and alkylating activity increase with the amount of 2-chloroethylamino fragments.
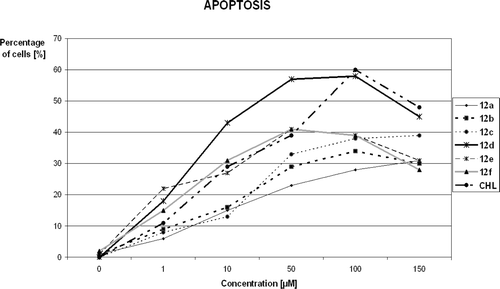
The analyzed triazines 12a–f, analogously to standard chlorambucil (CHL), inhibited tumour cell growth by exerting direct anti-proliferative affects with the cytotoxic (apoptosis/necrosis) consequences. The investigations showed that all of new compounds induced concentration-dependent apoptosis of MCF-7 cells. The percentage of apoptotic and necrotic MCF-7 cells after treatment with 50 μM solutions of CHL and compounds 12a–f is shown in .
Figure 2. Percentage of apoptotic and necrotic MCF-7 cells after treatment with 50 μM solutions of chlorambucil (CHL) and compounds 12a–12f. 100% = {apoptotic(%) + necrotic(%) + viable cells}.
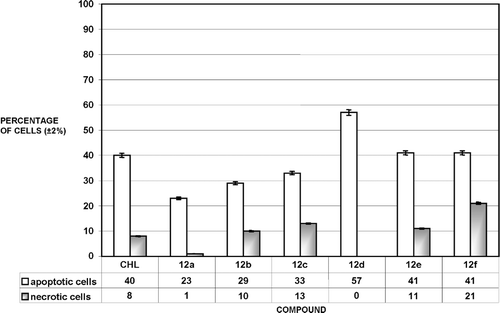
Apoptosis is the main way of the cell death at this concentration for all 12a–f.
Interestingly, the apoptotic cell death was mainly observed for every of them in the range of concentration from 1 to 100 µM, as we can see in . These results also show that necrosis is predominant cell death for all of compounds at concentration 150 µM. The ethidinium displacement assay showed that triazines 12a–e can bind to DNA although relatively weaker than chlorambucil ().
Table 4. DNA binding effect of compounds 12a–f.
Conclusions
New dual action drugs combining together by direct linking melamine based potential inhibitors of enzymes with mustard alkylating groups were obtained. The method opened access to mono-, di- and trifunctional derivatives. In case of five tumour cell lines (breast cancer T47D, prostate cancer LNCaP, colorectal cancer SW707, lung cancer A549 and Jurkat lymphoblastic leukemia) the strongest inhibition of proliferation was observed for triazines 12b,d substituted with single chloroethylamino fragment only. These exclude formation of bifunctional lesions and cross-link of nucleobases within the DNA duplex, but could be attributed to the additional inhibitory activity of triazine scaffold.
However, the experiments on the breast cancer MCF-7 cell line shown that in this case bi- trifunctional analogues 12e,f of nitrogen mustards, which are capable to form liaisons between two DNA strands, induced apoptosis and necrosis in the tested cells more effectively than mono-functional 12a-d. The data from ethidium displacement assay suggests that DNA binding may be implicated in the cytotoxicity of the compounds, but there also might be other possible targets and more complex mechanism of action of triazine NM derivatives, so it strongly suggests the need for further studies.
Declaration of interest
This studies were supported by the grant N N405 355537 donated by M.S.H.E. The skilful assistance of dr Malgorzata Rusak is gratefully acknowledged.
References
- He J, Anderson MH, Shi W, Eckert R. Design and activity of a ‘dual-targeted’ antimicrobial peptide. Int J Antimicrob Agents 2009;33:532–537.
- (a) Feyen F, Cachoux F, Gertsch J, Wartmann M, Altmann KH. Epothilones as lead structures for the synthesis-based discovery of new chemotypes for microtubule stabilization. Acc Chem Res 2008;41:21–31 ; (b) Hulsman N, Medema JP, Bos C, Jongejan A, Leurs R, Smit MJ, et al. Chemical insights in the concept of hybrid drugs: the antitumor effect of nitric oxide-donating aspirin involves a quinone methide but not nitric oxide nor aspirin. J Med Chem 2007;50:2424–2431.
- for review see (a)Ben Abid F, Gazzah A, Ousbane A, Gutierrez M, Brain E. Les alkylants. Oncologie 2007;9:751–757 ; (b) Di Francesco AM, Hargreaves RH, Wallace TW, Mayalarp SP, Hazrati A, Hartley JA, et al. The abnormal cytotoxicities of 2,5-diaziridinyl-1,4- benzoquinone-3-phenyl esters. Anticancer Drug Des 2000;15:347–359; (c) Vedejs E, Naidu BN, Klapars A, Warner DL, Li VS, Na Y, Kohn H. Synthetic enantiopure aziridinomitosenes: Preparation, reactivity, and DNA alkylation studies. J Am Chem Soc 2003;125:15796–15806 and references cited therein.
- Maeda M, Ligo M, Tsuda H, Fujita H, Yonemura Y, Nakagawa K et al. Antimetastatic and antitumor effects of 2,4-diamino-6-(pyridine-4-yl)-1,3,5-triazine (4PyDAT) on the high lung metastatic colon 26 tumor in mice. Anticancer Drug Des 2000;15:217–223.
- Paquin I, Raeppel S, Leit S, Gaudette F, Zhou N, Moradei O et al. Design and synthesis of 4-[(s-triazin-2-ylamino)methyl]-N-(2-aminophenyl)-benzamides and their analogues as a novel class of histone deacetylase inhibitors. Bioorg Med Chem Lett 2008;18:1067–1071.
- Riou JF, Guittat L, Mailliet P, Laoui A, Renou E, Petitgenet O et al. Cell senescence and telomere shortening induced by a new series of specific G-quadruplex DNA ligands. Proc Natl Acad Sci usa 2002;99:2672–2677 ; (b) Gomez D, Aouali N, Londono-Vallejo A, Lacroix L, Megnin-Chanet F, Lemarteleur T, et al. Resistance to the short term anti-proliferative activity of the g-quadruplex ligand 12459 Is Associated with telomerase overexpression and telomere capping alteration. J Biol Chem 2003;278:50554–50562.
- Mandal S, Bérubé G, Asselin E, Mohammad I, Richardson VJ, Gupta A et al. A novel series of potent cytotoxic agents targeting G2/M phase of the cell cycle and demonstrating cell killing by apoptosis in human breast cancer cells. Bioorg Med Chem Lett 2007;17:4955–4960.
- Chandra M, Sahay AN, Pandey DS, Tripathi RP, Saxena JK, Reddy VJM, et al. Potential inhibitors of DNA topoisomerase II. Ruthenium (II) polypyridyl and pyridyl azine complexes; potential DNA cleaving agents. J Organomet Chem 2004;689:2256–2267.
- Kaminski ZJ, Kolesinska B, Markowicz SW. Synthesis and cytostatic properties of monoterpene derivatives of cyanuric and isocyanuric acids. Acta Pol Pharm 2004;61 Suppl:29–32.
- Das K, Clark AD Jr, Lewi PJ, Heeres J, De Jonge MR, Koymans LM et al. Roles of conformational and positional adaptability in structure-based design of TMC125-R165335 (etravirine) and related non-nucleoside reverse transcriptase inhibitors that are highly potent and effective against wild-type and drug-resistant HIV-1 variants. j Med Chem 2004;47:2550–2560.
- Henke BR, Consler TG, Go N, Hale RL, Hohman DR, Jones SA et al. A new series of estrogen receptor modulators that display selectivity for estrogen receptor beta. j Med Chem 2002;45:5492–5505.
- Prade L, Huber R, Bieseler B. Structures of herbicides in complex with their detoxifying enzyme glutathione S-transferase - explanations for the selectivity of the enzyme in plants. Structure 1998;6:1445–1452.
- Li R, Sirawaraporn R, Chitnumsub P, Sirawaraporn W, Wooden J, Athappilly F et al. Three-dimensional structure of M. tuberculosis dihydrofolate reductase reveals opportunities for the design of novel tuberculosis drugs. j Mol Biol 2000;295:307–323.
- Lancaster CR, Michel H. Refined crystal structures of reaction centres from Rhodopseudomonas viridis in complexes with the herbicide atrazine and two chiral atrazine derivatives also lead to a new model of the bound carotenoid. j Mol Biol 1999;286:883–898.
- Kim YJ, Sackett DL, Schapira M, Walsh DP, Min J, Pannell LK et al. Identification of 12Cysbeta on tubulin as the binding site of tubulyzine. Bioorg Med Chem 2006;14:1169–1175.
- Verheijen JC, Richard DJ, Curran K, Kaplan J, Yu K, Zask A. 2-Arylureidophenyl-4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)triazines as highly potent and selective ATP competitive mTOR inhibitors: optimization of human microsomal stability. Bioorg Med Chem Lett 2010;20:2648–2653 ; (b) Venkatesan AM, Dehnhardt CM, Santos ED, Chen Z, Dos Santos, O, Ayral-Kaloustian S, et al. Bis(morpholino-1,3,5-triazine) derivatives: Potent adenosine 50-triphosphate competitive phosphatidylinositol-3-kinase/mammalian target of rapamycin inhibitors: Discovery of compound 26 (PKI-587), a highly efficacious dual inhibitor. J Med Chem 2010;53:2636–2645.
- Retailleau P, Colloc’h N, Vivarès D, Bonneté F, Castro B, El-Hajji M et al. Complexed and ligand-free high-resolution structures of urate oxidase (Uox) from Aspergillus flavus: a reassignment of the active-site binding mode. Acta Crystallogr d Biol Crystallogr 2004;60:453–462.
- Kumar R, Gupta L, Pal P, Khan S, Singh N, Katiyar SB et al. Synthesis and cytotoxicity evaluation of (tetrahydro-beta-carboline)-1,3,5-triazine hybrids as anticancer agents. Eur J Med Chem 2010;45:2265–2276.
- Moreau D, Jacquot C, Tsita P, Chinou I, Tomasoni C, Juge M et al. Original triazine inductor of new specific molecular targets, with antitumor activity against nonsmall cell lung cancer. Int J Cancer 2008;123:2676–2683.
- Nieto Y. DNA-binding agents. Cancer Chemother Biol Response Modif 2005;22:163–203.
- Kaldor JM, Day NE, Hemminki K. Quantifying the carcinogenicity of antineoplastic drugs. Eur J Cancer Clin Oncol 1988;24:703–711.
- Kamiński ZJ, Kolesińska B, Kinas R, Jastrza˛bek K, Kolesińska J, The method of manufacturing of trisubstituted triazine derivatives. Pl Pat. Appl P-360–728.
- (a) SRB test according to: Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst 1990;82:1107–1112 ; (b) MTT test according to: Marcinkowska E, Kutner A, Radzikowski C. Cell differentiating and anti-proliferative activity of side-chain modified analogues of 1,25-dihydroxyvitamin D3. J Steroid Biochem Mol Biol 1998;67:71–78.
- Petrelli A, Valabrega G. Multitarget drugs: the present and the future of cancer therapy. Expert Opin Pharmacother 2009;10:589–600; (b) Wei D, Jiang X, Zhou L, Chen J, Chen Z, He C, et al. Discovery of multitarget inhibitors by combining molecular docking with common pharmacophore matching. J Med Chem 2008;51:7882–7888.
- Nieto Y. DNA-binding agents. Cancer Chemother. Biol Response Modif 2005;22:163–203.
- Kolesińska B, Kamiński ZJ. Transformation of Tertiary Amines into alkylating reagents by treatment with 2-chloro-4,6-dimethoxy-1,3,5-triazine. A synthetic application of side-reaction accompanying coupling by means of 4-(4,6-dimethoxy-[1,3,5]triazin-2-yl)-4-methyl-morpholin-4-ium chloride (DMTMM). Pol J Chem 2008;82:2115–2123.
- Kolesińska B, Kamiński ZJ. The umpolung of substituent effect in nucleophilic aromatic substitution. A new approach to the synthesis of N,N-disubstituted melamines (triazine triskelions) under mild reaction conditions. Tetrahedron 2009;65:3573–3576.
- Zon G. Cyclophosphamide analogues. Prog Med Chem 1982;19:205–246.
- Preussmann R, Schneider H, Epple F. Identification of alkylating agents. II. Identification of different classes of alkylating agents by a modification of the color reaction with 4-(4-nitrobenzyl)-pyridine (NBP). Arzneimittelforschung 1969;19:1059–1073.